Photodynamic therapy (PDT) involves the combination of a light source and a photosensitizing agent to induce tissue damage via the generation of singlet oxygen. Although topical PDT has been approved for other indications, its use in facial photodamage is uncertain.
AimsTo assess the efficacy and safety of PDT in facial skin photoaging.
MethodsAll randomized clinical trials (RCTs) evaluating the efficacy and safety of any form of topical PDT for the treatment of facial photodamage (dermatoheliosis) or photoaging in patients older than 18 years, were included. Photodynamic-therapy using any topical photosensitizing agent at any dose, and with any light-source, were considered. Comparators were chemical exfoliation, intense pulsed light (IPL), light emitting diodes (LED), dermabrasion or microdermabrasion, ablative or non-ablative lasers, injectables, surgery, placebo and/or no treatment.
A systematic search in PubMed, Embase, Lilacs, Google Scholar and RCT's registry databases, was performed.
ResultsSearch was conducted up to May 4th 2016. Four authors independently selected and assessed methodological quality of each RCT. According to inclusion criteria, twelve studies were included (6 aminolevulinate (ALA) trials and 6 methyl aminolevulinate (MAL) trials), but the majority of them had methodological constraints particularly in randomization description and patients/outcome assessors blindness.
Discussion and conclusionsOverall results indicated that PDT either with ALA or with MAL was effective and safe for facial photodamage treatment, but high quality of evidence was found mainly for MAL studies.
La terapia fotodinámica (TF) incluye una combinación de una fuente de luz y un agente fotosensibilizante para inducir daño tisular a través de la generación de oxígeno singlete. Aunque la TF se ha aprobado para otras indicaciones, su uso en el fotodaño facial resulta incierto.
ObjetivoValorar la eficacia y seguridad de la TF en el fotoenvejecimiento de la piel del rostro.
MétodosSe incluyeron todos los ensayos clínicos aleatorizados (ECA) que evalúan la eficacia y seguridad de cualquier forma de TF tópica para el tratamiento del fotodaño facial (dermatoheliosis) o fotoenvejecimiento en pacientes mayores de 18 años. Se consideró la TF que utiliza cualquier dosis de agente fotosensibilizante, así como cualquier fuente lumínica. Los comparadores fueron: exfoliación química, luz pulsada intensa (IPL), diodo emisor de luz (LED), dermoabrasión o microdermoabrasión, láseres ablativos o no ablativos, inyectables, cirugía, placebo y/o ausencia de tratamiento.
Se llevó a cabo una búsqueda sistemática en las bases de datos de los registros de PubMed, Embase, Lilacs, Google Scholar y ECA.
ResultadosLa búsqueda se realizó hasta el mes de mayo de 2016. Cuatro autores seleccionaron y valoraron de manera independiente la calidad metodológica de cada ECA. Con arreglo a los criterios de inclusión, se incluyeron 12 estudios (6 ensayos sobre aminolevulinato [ALA] y 6 sobre metiloaminolevulinato [MAL]), aunque la mayoría de ellos contenían limitaciones metodológicas, particularmente en cuanto a la descripción de la aleatorización y la valoración a ciegas de los asesores de los pacientes/resultados.
Discusión y conclusionesLos resultados generales indicaron que la TF, tanto con ALA como con MAL, era una terapia efectiva y segura para el tratamiento del fotodaño facial, aunque se encontró evidencia de alta calidad principalmente en los estudios realizados sobre MAL.
The interplay of intrinsic (age-related decline of cutaneous cellular functions and/or genetic predisposition) and extrinsic factors (exposure to ultraviolet (UV) radiation, smoking or environmental changes) all lead to visible skin changes that result from an abnormal water distribution in tissue, (or a lack of hygroscopic substances), from an increase in skin pH, and from a prevailed oxidative cell metabolism that overwhelms local antioxidant activity. Those changes as a whole are usually referred as photodamage or actinic damage.1,2 Such disturbances result in a dry appearance of the skin, in an increase in skin surface pH and in a continuous production of reactive oxygen species (ROS) in mitochondria due to an oxidative cell metabolism and a decrease in antioxidant activity.3,4 Keratinocyte functional disturbances also occur due to a decreased mitotic activity and a 50%-increase in keratinocyte-migration time from the basal cell layer to the stratum corneum and an increase in cell-cycle duration.5,6 Skin aging is also accompanied by spinous cell layer atrophy and dermo-epidermal junction flattening which both contribute to skin fragility.6
Aged skin is also characterized by an overall collagen synthesis reduction via the diminution of procollagen production, a down regulation of the transforming growth factor-b (TGF-b) type II receptor (a major regulator of dermal extracellular matrix (ECM) synthesis), and by a disturbed TGF-b activity that also stimulates fibroblast proliferation.7–10 Skin collagen is also affected by UV-induced matrix metalloproteinases (MMP)11 such as MMP-1 (fibroblast collagenase), MMP-9 (gelatinase) and MMP-3 (stromelysin),10,12 and solar elastosis seems to be a consequence of an increased production of elastic fibers and elastin degradation by MMP-12 (human macrophage metalloelastase).10,13–15
Photodynamic therapy (PDT) is a selective therapeutic modality that combines an oxygen rich environment and a light source that stimulates a photosensitizing agent to produce singlet oxygen which is highly toxic to the cells.16,17 Porphyrins and particularly hematoporphyrins (e.g.: photofrin) were the first intravenous substances used for PDT, characterized by their long-term accumulation in target tissue that required rigorous photoprotection for several weeks after administration.17
In 1990 new topical porphyrins such as 5-aminolevulinic acid (ALA) or its methyl ester (MAL) emerged, which could both easily penetrate the epidermis and produce short-term circumscribed photosensitivity.18 More recently, hexylester 5-aminolevulinate (HAL) has been proposed to induce formation of high concentrations of PpIX in neoplastic tissue, but its use is still experimental.19
These molecules intervene in heme biosynthesis intracellular pathway, by inducing the formation of a photoactive porphyrin known as protoporphyrin IX (PpIX), which is an efficient photosensitizer that accumulates particularly in photodamaged skin.17
PDT requires either an incoherent/coherent light source that should be ideally specific to the chromophore/photosensitizer used. Incoherent light devices include a continuous-wave red light (635nm), blue light (417nm) and intense-pulsed light (IPL),20 whereas lasers are among the most used coherent light equipment.20
As photosensitizers can either localize in lysosomes, mitochondria, Golgi apparatus, endoplasmic reticulum, and plasma membranes, PDT effects are a consequence of how PS interact with cells within the target tissue/organ or tumor.21,22 Moreover, it seems that PDT direct DNA cellular damage can occur via modifications of guanine moiety and through strand breaks at uracil and thymine sites, whereas indirect DNA disruption is explained by deactivation of repairing enzymes by free radicals and singlet oxygen production.23
In addition, skin effects of topical PDT include solar elastosis improvement and neocollagenesis via the induction of expression of collagen type I/III production, MMP-1,-3,-9 and-12 down-regulation, and TGF β up-regulation.10,24 Photodynamic therapy with MAL has also been reported to increase dermal thickness and to improve collagen, elastic tissue and perifollicular fibrosis in treated skin.25,26
Several procedures have been used for actinic damage treatment (e.g.: chemical exfoliation, topical retinoids, lasers, intense pulsed light (IPL) and LEDs (light emitting diodes)).24,27–34 However, and according to a systematic review, there is limited scientific evidence to support the preferential use of any of these therapies in photodamaged skin.35
Up to know, topical PDT use in the treatment of photodamage is still off-label. Therefore, as uncertainty still remains in this field, this paper aims to assess published scientific evidence to establish the efficacy and safety of such therapy.
MethodsSearch strategiesWe aimed to identify all relevant published or unpublished RCTs regardless of language. Searches were performed during the last 10 years, and updated up to 4 May 2016. Search terms with results are depicted in Supplementary Material 1. Trial registries were scanned up to December 15, 2015, using the search terms: “photodamage”, “photoaging”, “photodynamic therapy” and “photodynamic rejuvenation” (Supplementary Material 2). We also checked for relevant references in included and excluded studies. If full text of the articles was not available, we tried to contact authors.
We did not perform a separate search for adverse effects of the specified intervention. However, we examined data on adverse effects from all included studies.
Inclusion criteriaAll parallel randomized controlled trials (RCTs) of PDT for facial photodamage (dermatoheliosis)/photoaging and/or photodynamic rejuvenation that included adult participants diagnosed with facial photodamage (dermatoheliosis) or photoaging, and who have been treated with any PDT-based rejuvenation procedure, were included. Any photodynamic-based intervention for photodamage (dermatoheliosis) or photoaging was considered.
Types of outcomes measuresPrimary endpoints included: (1) the proportion of participants who had an improvement/failure in global facial photodamage; (2) quality of life (QoL) changes measured through any validated and recognized generic or disease-specific instrument; (3) any adverse events, safety and tolerability.
Secondary outcomes corresponded to: (1) the proportion of participants who had an improvement/failure in facial hyperpigmentation, wrinkles, sallowness, erythema or roughness according to a validated and/or recognized generic or disease-specific instrument; (2) change in facial cosmetic appearance; (3) participant satisfaction; (4) pain evaluation after the photodynamic-based procedure, and (5) cost effectiveness studies, if available.
Data extraction and synthesisFour authors (V.R., J.M., A-P F., G. S.) checked the searched titles and abstracts. Potentially relevant studies were selected for full-text review. All authors independently assessed whether each study met the predefined selection criteria, and differences were resolved by discussion. The same four authors performed data extraction in pre-established data collection formats and independently assessed the risk of bias of included studies as low, high or unclear according to a domain-based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions36 (e.g.: ‘sequence generation’; ‘allocation concealment’; ‘blinding’; ‘outcome data’; ‘selective reporting’ and other sources of bias) that could put it at high risk of bias (e.g. baseline imbalance).
Statistical issuesWe planned to include only parallel designed trials. Dichotomous outcomes data were presented as reported in each individual study. However, if sufficient data were available, outcomes data were presented as relative risks (RR) with their associated 95% confidence intervals (CIs) and analyzed them in the Review Manager (RevMan) software, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark), using the Mantel–Haenszel test, unless we stated otherwise.
We re-analyzed the data originally described according to an intention to treat (ITT) principle, whenever possible. If study authors conducted a per-protocol analysis, we evaluated potential imbalances in the dropout rate between the trial arms to determine bias. If treatment by allocation population was unavailable, we used an available case population and reported this accordingly.
We planned to evaluate clinical, statistical, and methodological heterogeneity. Statistical heterogeneity was planned to be tested by using the I2 statistic.36 If substantial heterogeneity (I2 greater than 60%) was found for the primary outcomes, we planned to explore reasons for heterogeneity. As at least ten studies suitable for meta-analysis were needed for funnel plot asymmetry testing,37 we were unable to perform such test. Also, as we were unable to identify an adequate number of homogenous studies (n≥3),38 we did not perform a meta-analysis but we summarized the data for each trial qualitatively. The number of studies was also inadequate to perform subgroup and sensitivity analysis.
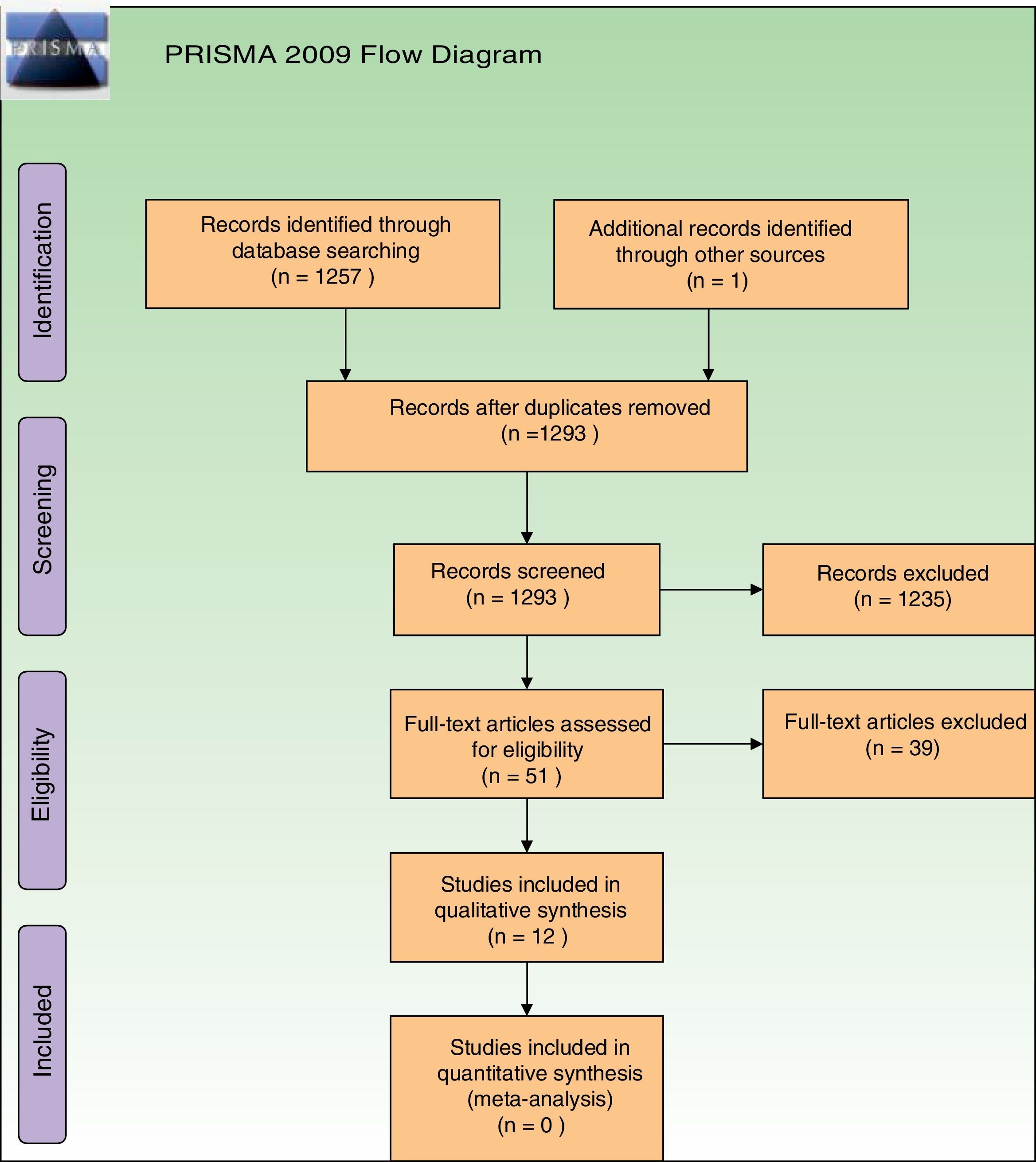
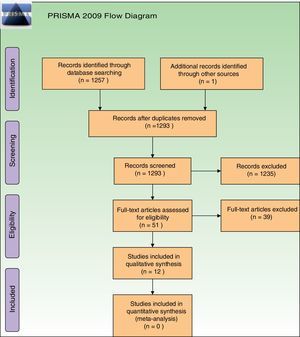
ResultsStudies descriptionAfter removal of duplicates our searches retrieved 1293 references. Further to titles and abstracts examination, we excluded 1235 references from the review. We obtained full-text copies of the remaining 51 records for further evaluation, and after evaluation, 39 studies were excluded. Reasons for their exclusion are depicted in Supplementary Material 3. Exclusions were made only after assessment of the full-text reports. The most frequent reason for exclusion was that they were non-RCTs. Regarding ongoing studies, we only identified one ongoing study, which is in the recruiting phase (Supplementary Material 3). Re-analysis of retrieved data was not necessary.
All retrieved studies were published in English. Thirteen references to 12 studies were finally included, as one same trial had two reports25,39 (Fig. 1). A total of 286 participants (135 patients from ALA studies and 151 from MAL studies) were evaluated. Among these, 30 were men and 212 women, but in 2 studies, gender of participants was not specified28,40 (Supplementary Material 4). The age of the participants ranged from 35 to 82 years.
Among all 12 included randomized trials, 6 studies used ALA and 6 MAL, as chromophores. Only 2 studies used a placebo as control39,41 and 10 had an active control treatment arm. Studies were published from year 2004 until 2015. Four studies were conducted in the USA,28,42–44 four in South America,39–41,45 2 in Spain,27,46 1 in Denmark,47 and one in China.48
The range number of participants included in the individual studies varied from 4 to 60 participants. Ten studies had a split-face design whereas 2 had a full-face design. All patients were diagnosed with mild to severe facial photodamage.
The included studies evaluated the following interventions: (1) IPL vs IPL+5-ALA in a split-face design28,44,48; (2) laser fractional resurfacing alone vs laser fractional resurfacing+MAL+red light in a split-face design46; (3) MAL+red light with 1h incubation vs MAL+red light with 3h incubation in a split-face design27; (4) IPL waveband from 530 to 750nm and short pulse durations (J/cm2, 2–2.5ms, delay 10ms)+0.5% liposome encapsulated vs IPL (waveband from 400 to 720nm), three passes were performed (3.5J/cm2, 30ms pulse duration), also in a split-face design47; (5) blue light at different incubation times+5-ALA in a split-face design42; (6) IPL vs IPL+5-ALA in a full-face design40; (7) MAL+red light with 3h incubation vs placebo+red light with 3h incubation in a split-face design39; (8) MAL with 1h incubation+red light vs MAL with 1h incubation+blue light in a split-face design43; (9) MAL+red light vs MAL+red light+1.5mm length micro-needling in a split-face design45; and (10) MAL+2h daylight exposure vs placebo+2h daylight exposure in a full-face design.41
The majority of included studies (except two39,41) did not specify primary and secondary outcomes.
Facial photodamage assessment was performed by the use of several original or modified scales such the Griffith's, Dover's, Fitzpatrick's, or arbitrary scales. Four studies reported that they had applied a “modified version” of another previous developed scale,39,41,45,48 but authors did not provide details of how and if their “modified version” was tested prior to its use. Only one study assessed intervention's effects on quality of life (QoL).41
A large proportion of the trials (n=9) assessed intervention-associated adverse events a priori, either through questionnaires that rated the treatment tolerability, or as skin reactions, such as erythema, dryness, edema, oozing, vesiculation, crusting, pigmentation disturbances, skin atrophy, scarring and desquamation or scaling.28 In addition, several included studies assessed secondary outcomes, but overall, the methods used for measurement and the timing of the assessments were not uniform or clear.
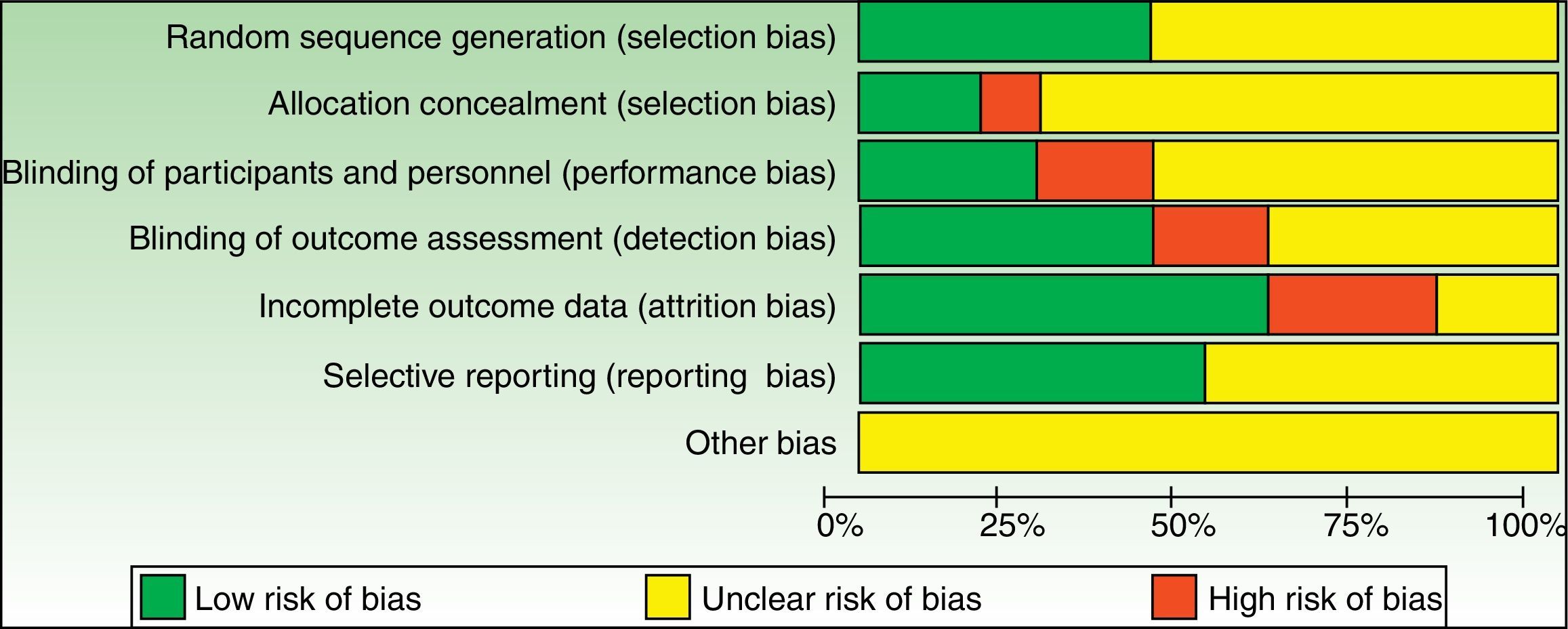
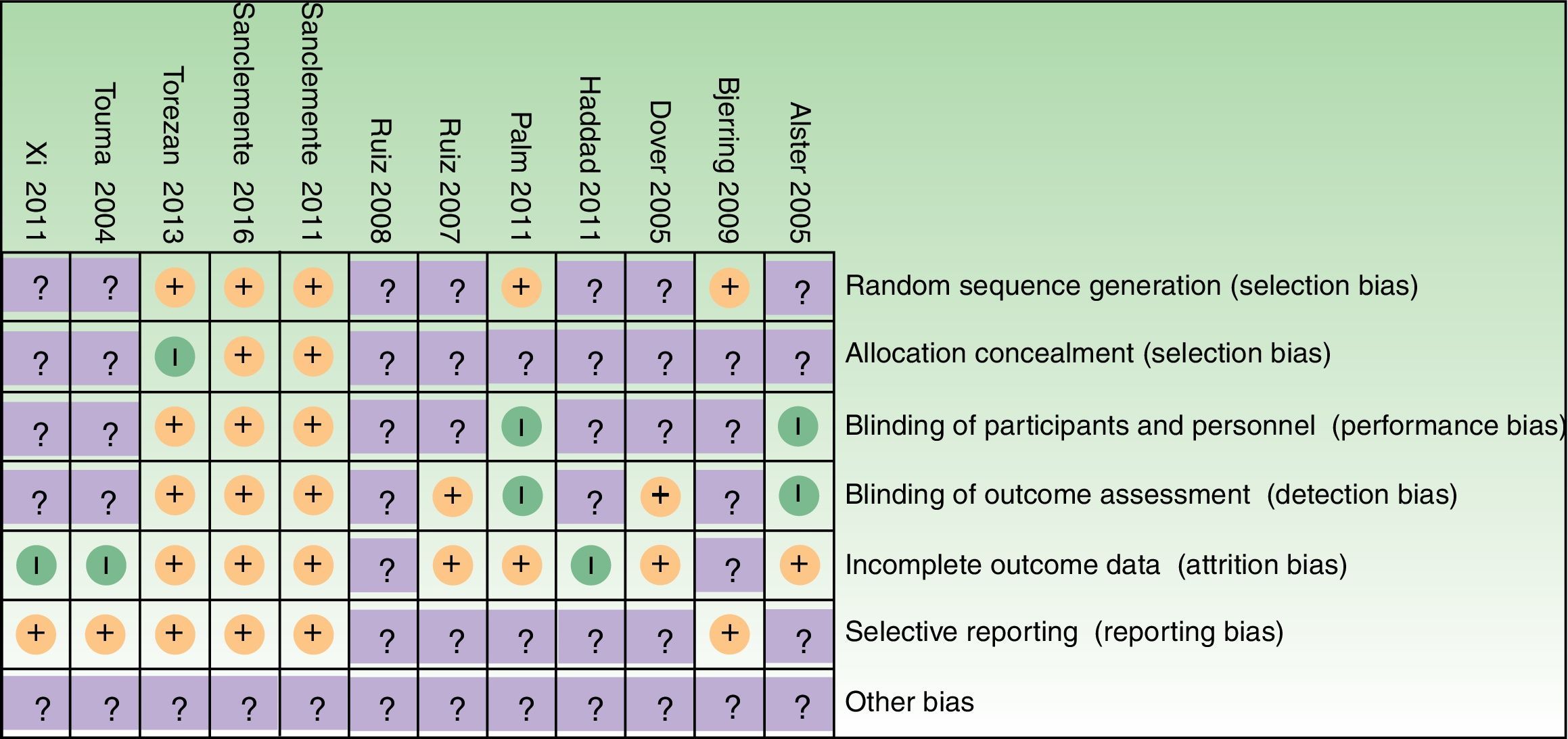
Risk of bias AssessmentRisk of bias for each included study and graphs are depicted in Supplementary Material 4, and Figs. 2 and 3, respectively. As one of the reviewers (GS) was the main author of 2 studies, both studies were assessed only by the other 3 assessors.
We assessed the global risk of bias for each included study, and we considered two studies to be at “low risk of bias” as both met all criteria across all domains in the Cochrane “Risk of bias” assessment tool (plausible bias unlikely to seriously alter the results).39,41 We rated the remaining 10 studies as at “unclear risk of bias” (plausible bias that raises some doubt about the result) because one or more criteria were assessed as unclear (Fig. 4).
An important number of included studies (9) provided insufficient detail to enable us to make accurate judgements in respect to the domain “concealment of the allocation sequence”.27,28,40,42–44,46–48 Indeed, intervention allocations masking through sequentially numbered, opaque, sealed envelopes or pharmacy-controlled allocation was only performed in 2 studies.39,41 Similarly, the “blinding of outcome assessors” domain was unclear in 5 studies,27,40,42,47,48 and only two studies described blinding of study participants and personnel in sufficient detail.39,41 Also, baseline characteristics of patients or groups were only described in 3 studies.39,41,43
Incomplete data of patients lost to follow-up, and subsequent per-protocol analyses were other important sources of potential bias in a number of the included studies. In addition, trial protocol was provided in only two studies.39,41 Judgements were amended as required, after contact with trial investigator.45 In some other studies authors replied that they no longer had information regarding trial methods42 and for other studies, we did not obtain a reply from authors.
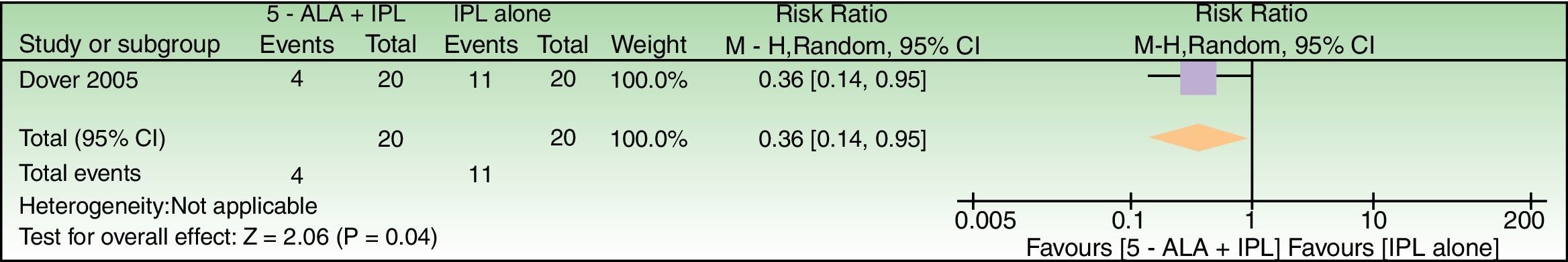
Effects of interventions5-ALAAlthough 6 studies evaluated the effect of 5-ALA as chromophore, either insufficient data or just baseline vs post-treatment comparisons limited relative risks calculations which could be performed only for 3 studies28,47,48 (Figs. 4–6).
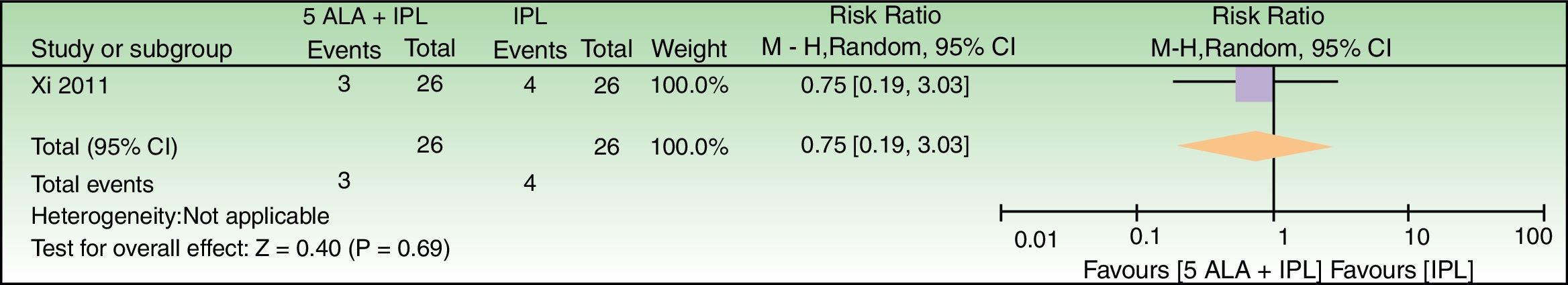
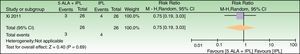
5-ALA+IPL vs IPL alone, outcome: 3.1 Failure to improve according to a global score of photoaging (analysis was performed with n=26 patients (52 splitfaces)). The 2 patients that were excluded were labeled as not improved in the 5-ALA+IPL intervention group. Review Manager (RevMan) software, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark).
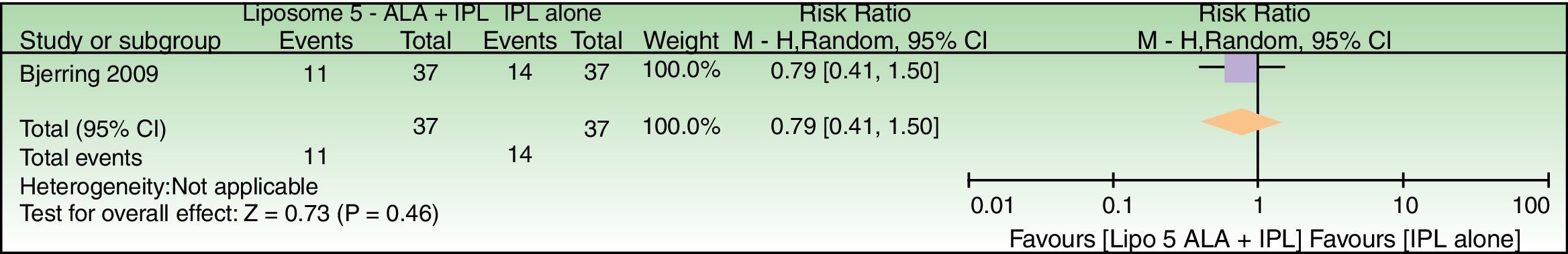
Forest plot of comparison: liposome encapsulated 5-ALA+IPL vs IPL alone, outcome: 5.1 Failure to improve periorbital wrinkles. n=37 subjects (74 splitfaces). Review Manager (RevMan) software, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark).
In general, there was a tendency to less treatment failure in ALA-treated split-faces. Also, we were unable to determine primary and secondary endpoints in all 5-ALA studies.
The effect of ALA in actinic keratosis, as well as in facial photodamage was assessed in 2 studies.40,42
Investigator assessment of changes in facial photodamage and participant's photodamage evaluation were performed through digital standardized photographs in 3 studies,40,44,47 and two studies,28,48 respectively.
Partial or full sponsoring of ALA trials by the pharmaceutical industry was detected in 4 studies28,40,42,48 and in 2 studies,44,47 sponsoring information was lacking.
Photodamage results according to the timeline at which outcomes were assessed in ALA studies varied from 1 month after last session28,42, 2 months after last session,40 1–2 months after last session,48 3 months after last session,47 and at 1, 3 and 6 months, after last session.44
Regarding patient related outcomes, subject satisfaction was evaluated in 4 out of 6 ALA studies.28,42,47,48
In general, more erythema, edema and desquamation were reported in the ALA-treated side of the face.
5-ALA-related adverse eventsHerpes simplex reactivation and post-inflammatory hyperpigmentation were reported in Touma et al.42 and in Xi et al.48 studies, respectively.
A summary of findings table of 5-ALA results according to GRADE standards is depicted in Table 1.
Summary of findings.
| 5-ALA for facial photodamage? |
|---|
| Patient or population: Facial Photodamage |
| Setting: |
| Intervention: 5-ALA |
| Comparison: Any intervention |
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with any intervention | Risk with 5-ALA | |||||
| Failure to improve according to a Global Photodamage scale | 550 per 1000 | 198 per 1000 (77–523) | RR 0.36 (0.14–0.95) | 40 (1 RCT) | –a | As this review assessed the quality of evidence of individual trials and a meta-analysis could not be performed, a full recommendation cannot be made. |
| Failure to improve according to a global score of photoaging | 154 per 1000 | 115 per 1000 (29–466) | RR 0.75 (0.19–3.03) | 52 (1 RCT) | –b,c | As this review assessed the quality of evidence of individual trials and a meta-analysis could not be performed, a full recommendation cannot be made. |
| Failure to improve periorbital wrinkles | 378 per 1000 | 299 per 1000 (155–568) | RR 0.79 (0.41–1.50) | 74 (1 RCT) | –d | As this review assessed the quality of evidence of individual trials and a meta-analysis could not be performed, a full recommendation cannot be made. |
The method of sequence generation was not reported. The method used for allocation concealment was not described. It was a single-blinded (investigator) study. Patient's satisfaction outcome could have been influenced by participants unblinding. A blinded investigator evaluated photodamage improvement but tolerability assessment was performed by an unblinded investigator. All split-faces were included in the analysis and follow-ups were performed in all patients. Patient's satisfaction through photographs evaluation was not specified in the methods section, but was included in the abstract and in the discussion section of the manuscript. Telangiectasia and erythema results were only depicted in the discussion section. Sample size calculation was not specified. The power of the study might have led to non-statistical significant differences in some outcomes at different time-points. Fluence changes might have influenced the results. Baseline characteristics of groups were not included.
The method of sequence generation was not reported. The method used for allocation concealment was not described. Although the study was labeled as double-blind, it was unclear who was also blinded besides the outcome assessors. A blinded “independent” investigator evaluated outcomes but it was unclear if assessments were performed clinically or through the photographs taken. Measures used to assure outcome assessor's blinding were not included in the article. An ITT analysis was not performed. Two patients withdrew from the study: one due to an allergy to IPL, but it was unclear which side of the face (or whole face) was affected. In the other excluded patient, it was unclear if not meeting study requirements was related to the type of intervention received. The exclusion of these 2 patients in the analysis might have influenced the results due to the low power of the study. Selective reporting was not detected. This was an industry-sponsored trial with positive results, with scarce specific data on potential conflicts of interest. Sample size calculation was not specified. Variations in IPL parameters according to individual features might have influenced final results. Baseline characteristics of groups were not included.
An ITT analysis was not performed. Two patients withdrew from the study. The exclusion of these 2 patients in the analysis might have influenced the results due to the low power of the study.
Sample size calculation was not specified. The low power of the study might have led to non-statistical significant differences in outcomes when contralateral comparisons were made.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio.
GRADE Working Group grades of evidence:
High quality: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Although 6 studies evaluated the effect of MAL as chromophore, the lack of contralateral comparisons or insufficient data allowed the calculation of relative risks only for 4 out of 6 studies (Figs. 7–10).
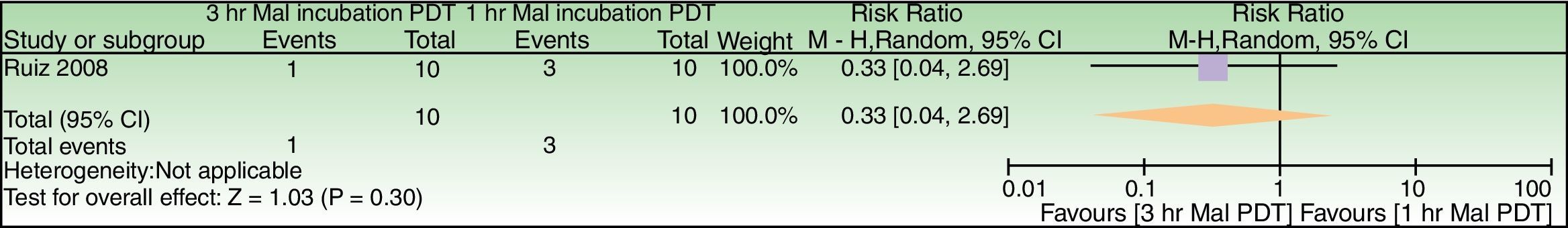
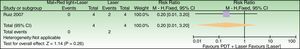
Forest plot of comparison: 3h MAL incubation+red light vs 1h MAL incubation+red light, outcome: 4.1 Failure to improve fine lines. The excluded patient was labeled as a failure in the 1h MAL incubation group. n=10 subjects (20 split-faces). Review Manager (RevMan) software, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark).
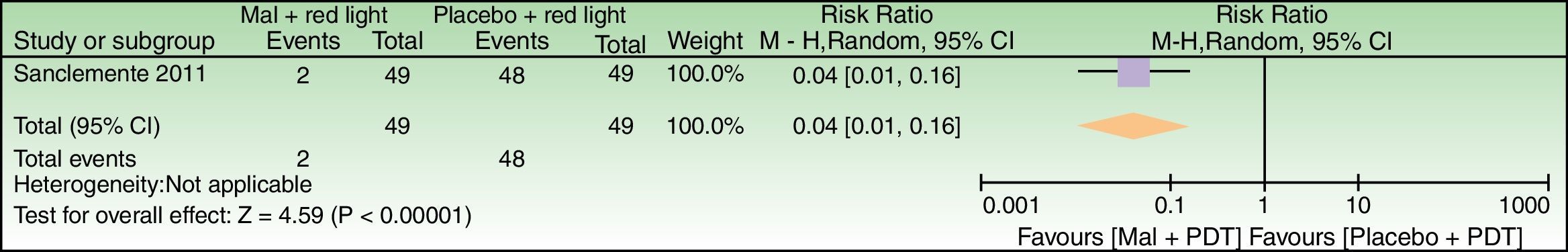
Forest plot of comparison: 6 MAL+red light vs placebo+red light, outcome: 6.1 Global Photodamage failure to improve. n=98 split-faces. The subject who discontinued treatment due to an adverse reaction was labeled as a failure in the MAL+red light group. Review Manager (RevMan) software, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark).
We also were unable to differentiate primary and secondary endpoints in 4 out of 6 studies.27,43,45,46
The effect of MAL, actinic keratosis, and in facial photodamage simultaneously was assessed in 2 studies.43,45 Investigator assessment of photodamage outcomes was performed through digital standardized photographs in 1 study.43
In general, timelines for photodamage outcome evaluation in MAL trials ranged from 1 to 3 months after last session.
Partial or full sponsoring of trials by the pharmaceutical industry was detected in 3 studies.39,41,43 Sponsoring details were not described in 2 studies,27,46 and one study was developed without any sponsor.45
In respect to patient satisfaction, such outcome was evaluated in 3 studies with better satisfaction scores in the MAL treated split-face.39,43,46 In addition, just 1 out of the 12 studies (ALA and MAL) included a quality of life outcome.41
Overall, erythema, edema and desquamation were more frequently reported in the side of the face that included MAL as chromophore in all studies, except in one study43 in which no statistical differences between the use of blue vs red light were found, but also mild discomfort was more frequent with red-light exposure.
MAL-related adverse eventsHerpes simplex reactivation was reported in two studies whereas post-inflammatory hyperpigmentation was described in 3 studies.39,41,46 A severe infection related to the concomitant use of microdermabrasion+PDT was reported in 1 out of 10 patients in Torezan et al.’s45 study and a severe allergic reaction not related to PDT was reported in another study.39
A summary of findings table of MAL results according to GRADE standards is depicted in Table 2.
Summary of findings.
| MAL for Facial Photodamage |
|---|
| Patient or population: Facial Photodamage |
| Setting: |
| Intervention: MAL |
| Comparison: any intervention |
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with Any intervention | Risk with MAL | |||||
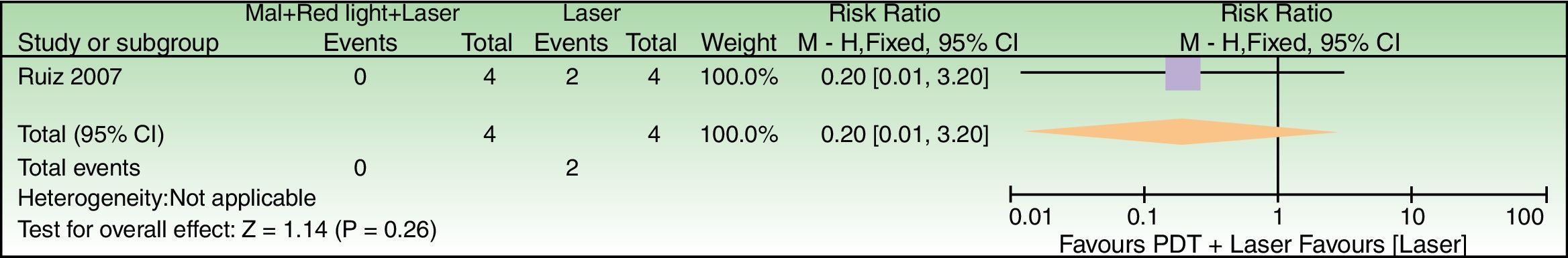
| Failure to achieve good cosmetics results | 500 per 1000 | 100 per 1000 (5–1000) | RR 0.20 (0.01–3.20) | 8 (1 study) | –a,b | As this review assessed the quality of evidence of individual trials and a meta-analysis could not be performed, a full recommendation cannot be made. |
| Failure to improve fine lines | 300 per 1000 | 99 per 1000 (12–807) | RR 0.33 (0.04–2.69) | 20 (1 study) | –c,d | As this review assessed the quality of evidence of individual trials and a meta-analysis could not be performed, a full recommendation cannot be made. |
| Global Photodamage failure to improve | 980 per 1000 | 39 per 1000 (10–157) | RR 0.04 (0.01–0.16) | 98 (1 study) | – | As this review assessed the quality of evidence of individual trials and a meta-analysis could not be performed, a full recommendation cannot be made. |
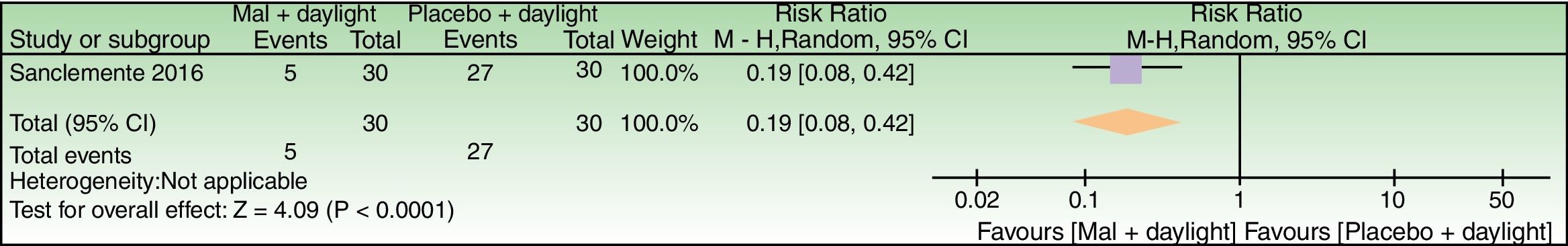
| Global Photodamage failure to improve | 900 per 1000 | 171 per 1000 (72–378) | RR 0.19 (0.08–0.42) | 60 (1 study) | – | As this review assessed the quality of evidence of individual trials and a meta-analysis could not be performed, a full recommendation cannot be made. |
The method of sequence generation was not reported. The method used for allocation concealment was not described. Measures used for blinding were not specified. It was not clear if patients were blinded for satisfaction assessment. Quote: “A blinded investigator evaluated each side of the perioral area”. All split-faces were included in the analysis. Safety outcome was not specified in the methods section, but was included in the analysis. Only superficial wrinkles were evaluated but other photodamage features were not included. Baseline characteristics of groups were not included. Potential conflicts of interests and financial support were not described.
Neither sample size calculation nor statistical tests used in analysis, were specified. The low power of the study might have led to non-statistical significant differences.
The method of sequence generation was not reported. The method used for allocation concealment was not described. It was unclear if the study was single or double-blinded. A blinded investigator evaluated photodamage improvement through baseline vs post-treatment patient's photographs but blinding of side effects assessment was not specified. Measures used to assure outcome assessor's blinding were not described. Nine out of ten patients completed follow-ups. No intention to treat analysis (ITT) was specified. Safety outcome was not specified in the methods section, but was included in the results section of the manuscript. Side-effects outcomes were measured as ordinal variables but in the analysis section these were treated statistically as quantitative variables. A qualitative comparison of clinical facial photodamage improvement was performed from baseline vs post-treatment in the same split-face, but there were neither contralateral comparisons, nor statistical comparisons for this outcome. Baseline characteristics of groups were not included.
Sample size calculation was not specified. The lack of an ITT analysis could have an impact in efficacy results due to the small sample size of the study. Similarly, the low power of the study might have led to non-statistical significant differences in all outcomes.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio.
GRADE Working Group grades of evidence:
High quality: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
To the best of our knowledge, this is the first published systematic review (SR) to assess and examine the evidence for the efficacy and safety of PDT in facial photodamage, and although the full protocol of this SR was not published (just its abstract), it is available upon request to correspondence author.
Two high quality studies suggest that MAL-PDT is effective in the treatment of facial photodamage, but low to moderate quality of evidence shows that PDT with ALA seems to be also effective. Although adverse events (AEs) were reported inadequately in most studies, non-serious reported AEs include herpes simplex recurrence and post-inflammatory hyperpigmentation, particularly in studies that included participants with darker skin (3 out of 4 studies with PIH reports).39,41,46,48
The main objectives of a randomized clinical trial are to assess efficacy and safety of an intervention. In this respect, concealment and blinding are key domains in the assessment of risk of bias.49–51 In particular, the methods used to generate the allocation sequence and how the sequence was concealed, are the most important and sensitive indicators that bias has been minimized in a clinical trial, as participants and investigators are masked to the upcoming assignment, and therefore could not “manipulate” it. Also, standardization of outcomes and objective measurement are key elements not only for clinical decision making, but also to establish health policies.52–54
Overall, the evidence provided by this review was limited mainly due to outcome inconsistencies, the lack of description of randomization methods/sequence generation, and a lack of a double-blind trial design. Moreover, the high heterogeneity of studies did not allow a quantitative synthesis of the evidence found. However, there was a tendency for having a better result when PDT with any chromophore was used, when compared to the use of a light source alone.
Most of the included studies in this review were performed before the requirement of trial registration. As a result of insufficient data and the lack of reporting, we were “forced” to downgrade the quality of evidence to “unclear risk of bias”, as we appraised these studies according to the information contained within the full text of each article. However, more unbiased judgment elements could have been obtained if we were able to get a reply from all authors, but such information could only be gathered from one main author.
In this review, it is unlikely that we have missed studies with an important sample size as we performed a rigorous systematic search of published and unpublished literature and we also tried to contact leading experts. Nonetheless, we cannot absolutely rule out the influence of publication bias. Unfortunately we were unable to reliably assess the presence of publication bias, given the small number of included studies. Also, we attempted to lower potential biases in the evaluation of two MAL trials39,41 performed by one of the reviewers of this systematic review by limiting their assessment only by the other 3 assessors.
The applicability of evidence found by this review corresponds to what is usual in clinical practice,4,55 as included trials covered the usual age spectrum of participants who seek a dermatologist for photodamage therapeutic options. However, it is important to bear in mind that depending on the severity of facial photodamage, patients over 70 years old might not be the best candidates for PDT as they often require more invasive procedures such as resurfacing with ablational lasers and/or surgery. Also, as all included studies had participants with Fitzpatrick's SPT from I through IV, the applicability of the evidence in SPT V-VI is limited. Similarly, results applicability in men could be affected, as the majority of included participants, were women.
In conclusion, the findings in our review showed that MAL PDT was effective for the treatment of facial photodamage, but also have highlighted that only a small number of published trials have followed the Consolidated Standards of Reporting Trials (CONSORT-Statement) and the criteria of evidence-based medicine. Such standards are necessary to heighten evidence strength and quality in future trials design. Also, as there is a relative paucity of long-term evaluations of the effect of PDT in facial photodamage and in quality of life, such knowledge gaps deserve further research.
SponsoringThis work was supported by the Group of Investigative Dermatology-GRID of the Universidad de Antioquia, Medellin, Colombia and by the Fundacion Dermabase.
Conflict of interestDr. Gloria Sanclemente has participated in advisory boards and has received honoraria and scientific meeting support from Galderma Laboratories.
Dr. Veronica Ruiz Cañas has received scientific meeting support from Galderma Laboratories.
Dr. Jenny Marcela Miranda Orozco has received scientific meeting support from Galderma Laboratories.
Dr. Alba Patricia Ferrín Bastidas has received scientific meeting support from Galderma Laboratories.
Paola Andrea Ramirez has nothing to disclose
Gilma Hernandez has nothing to disclose.