The diagnosis of cutaneous melanoma is complex. Histopathology is the gold standard for diagnosis of melanocytic lesions, although its intraobserver concordance and interobserver concordance are low. In addition, it depends on the experience of the pathologist and can therefore be prone to diagnostic error.1 It seems necessary to incorporate new tools for the evaluation of melanocytic lesions.
Recently published studies on the use of artificial intelligence for the diagnosis of cutaneous lesions have generated much interest. In 2017, Esteva et al.2 published the results of a revolutionary study in which they evaluated the ability to distinguish between benign melanocytic lesions and melanoma and between seborrheic keratosis and cutaneous carcinoma after training with 129 450 images of cutaneous lesions from a deep convolutional neural network (DCNN). The diagnostic accuracy of the approach was compared with that of 21 dermatologists. The reliability of both the DCNN and the dermatologists was comparable. Haenssle et al.3 published the results of a study (“man vs machine”) in which they compared the diagnostic accuracy of the DCNN Google Inception-v4 (trained with >100 000 images) with that of 58 dermatologists, 30 of whom were experts in dermoscopy. The authors used a series of 100 dermoscopy images and worked in 2 stages: the first, with no clinical information; and the second, with additional clinical images and information. They measured sensitivity, specificity, and the area under the curve (AUC). The DCNN reached an average AUC that was significantly superior to the dermatologists in both phases of the study (0.86 vs 0.79; P<.01). In phase 2, the dermatologists improved their sensitivity, reaching 88.9%. Even so, the DCNN obtained greater specificity (82.5% versus 75.7%) and was superior to most dermatologists. One of the main drawbacks of the DCNN is the number of images necessary to “train” it (>100 000), which makes it difficult and costly. Fujisawa et al.4 trained a DCNN to distinguish between 14 diagnoses using only 4860 images of benign and malignant cutaneous tumors and compared its diagnostic accuracy with that of 13 dermatologists and 9 dermatology residents. The DCNN had an accuracy of 92.4%, which was significantly higher than that of the dermatologists and residents (85.3% and 74.4%; P<.001).
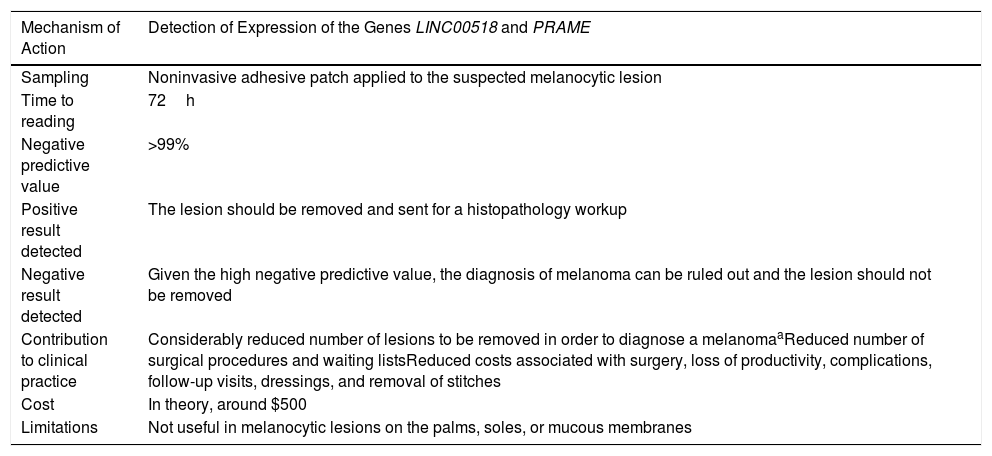
The recently developed pigmented lesion assay (PLA) (Table 1) is a noninvasive gene expression assay consisting of an adhesive patch that makes it possible to rule out cutaneous melanoma with a negative predictive value >99%, which is considerably higher than that of histology (83%). Given its high negative predictive value, the PLA reduces the number of lesions removed to diagnose a melanoma (usually between 8 and 20). Hornberger et al.5 compared the costs of the PLA with those of a standard biopsy procedure, histologic diagnosis, medical supervision, and follow-up. With a cost of $500, the authors concluded that the PLA can reduce the costs associated with the diagnosis of a lesions suspected of being a melanoma by 47%. The PLA would reduce the number of surgical procedures, undiagnosed melanomas, and costs.
Characteristics of the Pigmented Lesion Assay.
| Mechanism of Action | Detection of Expression of the Genes LINC00518 and PRAME |
|---|---|
| Sampling | Noninvasive adhesive patch applied to the suspected melanocytic lesion |
| Time to reading | 72h |
| Negative predictive value | >99% |
| Positive result detected | The lesion should be removed and sent for a histopathology workup |
| Negative result detected | Given the high negative predictive value, the diagnosis of melanoma can be ruled out and the lesion should not be removed |
| Contribution to clinical practice | Considerably reduced number of lesions to be removed in order to diagnose a melanomaaReduced number of surgical procedures and waiting listsReduced costs associated with surgery, loss of productivity, complications, follow-up visits, dressings, and removal of stitches |
| Cost | In theory, around $500 |
| Limitations | Not useful in melanocytic lesions on the palms, soles, or mucous membranes |
Abbreviations: LINC00518, long intergenic non–protein-coding RNA 518; PRAME, preferentially expressed antigen in melanoma.
The number of lesions to be removed for diagnosing a melanoma was between 8 and 30, depending on the series analyzed.
Source: Gerami et al.6
The diagnosis of melanocytic lesions is complex. New technological tools based on artificial intelligence and gene expression promise to simplify this process and reduce the number of diagnostic and therapeutic errors.
Please cite this article as: Morgado-Carrasco D, Fustà-Novell X, Bosch-Amate X, Giavedoni P. FR - Nuevas tecnologías que prometen revolucionar el diagnóstico del melanoma cutáneo. Actas Dermosifiliogr. 2020;111:329–330.