Cutaneous squamous cell carcinoma (cSCC) represents between 20% and 50% of malignant cutaneous tumors. Most are cured with surgery, presenting a subgroup of them with poor prognosis despite surgical treatment, due to local recurrence (LR), in-transit metastases (ITM), nodal metastases (NM), distant metastases (DM), or disease-specific death (DSD). Staging systems stratify tumor prognosis and optimize its treatment (Table 1).
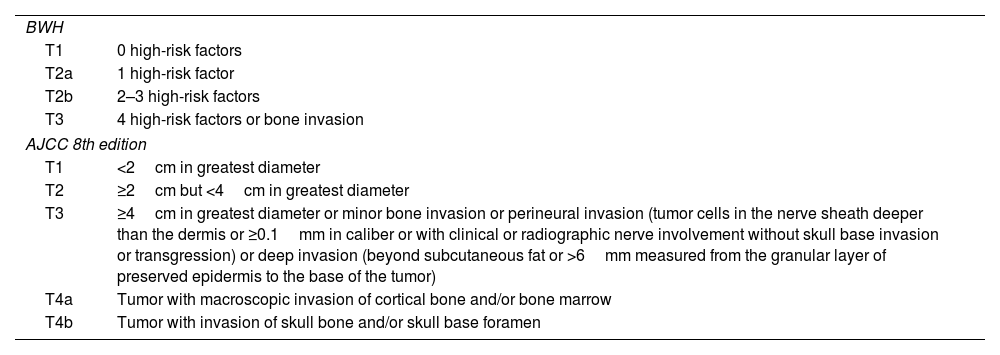
CEC classification according to BWH and AJCC.
| BWH | |
| T1 | 0 high-risk factors |
| T2a | 1 high-risk factor |
| T2b | 2–3 high-risk factors |
| T3 | 4 high-risk factors or bone invasion |
| AJCC 8th edition | |
| T1 | <2cm in greatest diameter |
| T2 | ≥2cm but <4cm in greatest diameter |
| T3 | ≥4cm in greatest diameter or minor bone invasion or perineural invasion (tumor cells in the nerve sheath deeper than the dermis or ≥0.1mm in caliber or with clinical or radiographic nerve involvement without skull base invasion or transgression) or deep invasion (beyond subcutaneous fat or >6mm measured from the granular layer of preserved epidermis to the base of the tumor) |
| T4a | Tumor with macroscopic invasion of cortical bone and/or bone marrow |
| T4b | Tumor with invasion of skull bone and/or skull base foramen |
High-risk factors in BWH classification are tumor diameter≥2cm. Poor histological differentiation. Perineural invasion of nerves≥0.1mm in caliber. Tumor invasion beyond subcutaneous fat (excluding bone invasion, which directly classifies as T3).
AJCC: American Joint Committee on Cancer; BWH: Brigham and Women's Hospital; CEC: Cutaneous Squamous Cell Carcinoma.
Although lymphovascular invasion (LVI) is associated with DSD and DM in cSCC, it is excluded from staging systems because there is insufficient data for its independent prognostic significance.1 Some authors describe an increased risk of metastasis in cSCC with LVI.2–4 Other authors have not shown a statistically significant association.5
Moore et al. (n=193) conducted a prospective study concluding that cSCC cases of the head and neck with LVI+ were statistically significantly associated with NM (p<0.0001).2
Gupta et al.4 conducted a retrospective multicenter cohort study to define risk factors in low-risk cSCC (specifically in the BWH T2a stage) that are associated with NM, DM, and DSD. They concluded that 1 major criterion (tumor diameter≥40mm, poor differentiation, invasion beyond subcutaneous tissue, large-caliber perineural invasion) and ≥1 minor criteria (subcutaneous fat tissue invasion, moderate differentiation, small-caliber perineural invasion, or lymphovascular invasion) were predictive of worse prognosis, with a cumulative incidence of poor prognosis events of 8% (95%CI, 5.1–13.7) in T2a cSCC vs 2.8% (95%CI, 1.9–4.1) in other T2a tumors.
Kus et al.3 (n=10,707) conducted the first multicenter study to evaluate the impact of LVI on cSCC prognosis. They included retrospective cohorts of cSCC patients from Brigham and Women's Hospital (BWH) and Cleveland Clinic Foundation, with cSCC diagnosed between March 1999 and October 2020. The statistical analysis was performed by separating low- (T1, T2a) and high-risk (T2b, T3) tumors according to BWH. They analyzed a total of 10,707 cSCC: 78 presented LVI+. In low-risk stages, the authors found a higher cumulative incidence of LR (LVI+: 12.3%; LVI−: 1.1%; p<0.01), metastases, whether in-transit, nodal, or distant (LVI+: 4.2%; LVI−: 0.4%; p<0.01), and DSD (LVI+: 16.2%; LVI−: 0.4%; p<0.01). In high-risk stages, LVI+ tumors presented higher rates of metastases at 5 years (LVI+: 28.5%; LVI−: 16.8%; p=0.06) and DSD (LVI+: 25.3%; LVI−: 13.9%; p=0.03), without any differences being reported in LR (LVI+: 16.3%; LVI−: 15.8%; p=0.11).3
In low-risk cSCC, LVI+ becomes more important, presenting 12, 10, and 41 times more risk of LR, metastases, and DSD, respectively, vs LVI− cSCC. By presenting at most one of the risk factors established in the staging systems, in LVI+ with poor prognosis events, LVI should acquire more importance in staging in the absence of other factors.
We consider that staging systems should include LVI as a predictor of poor prognosis in cSCC.