While Janus kinase inhibitors (JAKi) have shown efficacy in the treatment of dermatologic conditions, a subset of patients may not achieve symptomatic control. In such situations, our approach may involve either switching to an alternative treatment or considering the addition of a different drug.
Given the limited literature available on the safety profile of combining JAKi with systemic immunomodulatory therapies to treat dermatologic conditions,1,2 we conducted a retrospective review of patients undergoing simultaneous treatment in our department at a tertiary teaching hospital.
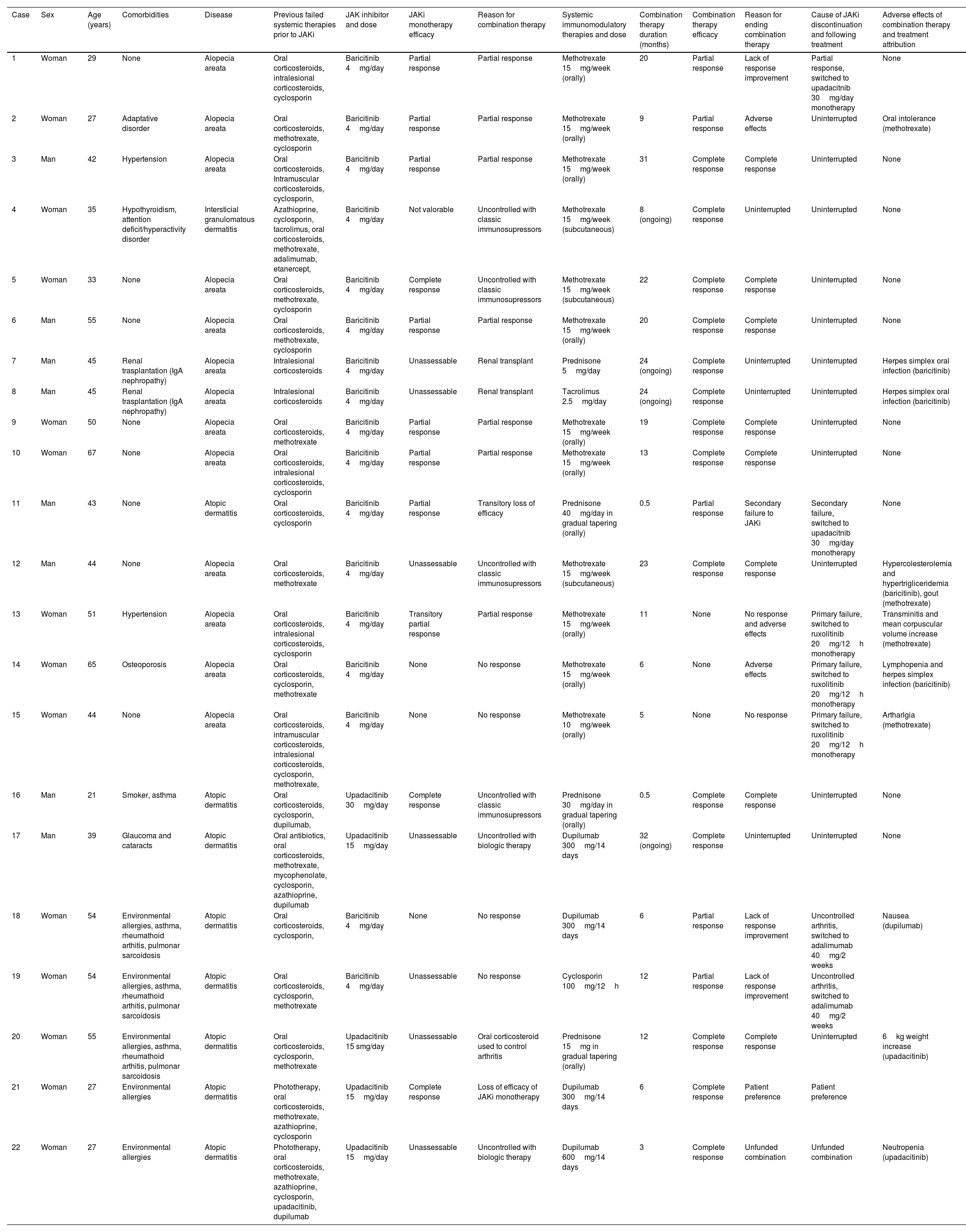
We identified a total of 18 patients (mean age, 43 years; 7 men and 11 women) treated with 22 different combination therapies. Twelve of these patients, 12 were prescribed a JAKi for alopecia areata (AA), 5 for atopic dermatitis (AD), and 1 for interstitial granulomatous dermatitis (IGD), in this case as an off-label treatment. A total of 15 patients received a median 21-month regimen of baricitinib 4mg/day (range, 3–43 months). In 4 cases the given treatment was upadacitinib at a dose of 15mg/day (in one case at 30mg/day) for a median 20 months (range, 12–30 months).
The systemic therapies used in combination regimens were varied: methotrexate (12), prednisone (4), dupilumab (4), oral tacrolimus (1) and cyclosporine (1). The decision to combine treatments was based on several factors. The most common reason was a partial response to JAKi, where methotrexate was added in all cases, at a mean dose of 15mg per week. This combination was used for a median 18 months. The second most frequent reason for combining therapies was a lack of response to JAKi monotherapy. In 2 cases, methotrexate was added, and in another, the combination included dupilumab 300mg every 14 days and cyclosporine 100mg twice a day for 6 months (cases #18 and #19; Table 1). Another reason for prescribing the combination therapy involved a patient with a kidney transplant, who was already on tacrolimus 2.5mg/day plus oral prednisone 5mg/day (cases #7 and #8; Table 1). Other reasons for prescribing the combination therapy included a transient relapse of atopic dermatitis, for which oral prednisone 40mg/day was prescribed and tapered over 2 weeks, and a secondary loss of efficacy following the down titration of upadacitinib to 15mg/day due to adverse effects, for which a 6-month regimen of dupilumab 300mg every other week was prescribed. In 6 cases, patients who did not achieve complete response with classical immunosuppressive treatments or biologic therapies were initiated on JAKi. Two cases of AA and 1 of IGD who had been on methotrexate at doses of 15mg/week for a median time of 16 months (range, 7–33 months) were prescribed baricitinib 4mg/day. There was 1 patient with atopic dermatitis who had been on oral prednisone 30mg/day for 1 month prior to starting upadacitinib 30mg/day (case #16; Table 1) and another atopic patient who had been on dupilumab 300mg every other week for 1 month and was added upadacitinib 15mg/day to control a very severe flare (case #17; Table 1).
Patients on a combination therapy with a JAK inhibitor and other immunomodulatory treatment.
| Case | Sex | Age (years) | Comorbidities | Disease | Previous failed systemic therapies prior to JAKi | JAK inhibitor and dose | JAKi monotherapy efficacy | Reason for combination therapy | Systemic immunomodulatory therapies and dose | Combination therapy duration (months) | Combination therapy efficacy | Reason for ending combination therapy | Cause of JAKi discontinuation and following treatment | Adverse effects of combination therapy and treatment attribution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Woman | 29 | None | Alopecia areata | Oral corticosteroids, intralesional corticosteroids, cyclosporin | Baricitinib 4mg/day | Partial response | Partial response | Methotrexate 15mg/week (orally) | 20 | Partial response | Lack of response improvement | Partial response, switched to upadacitnib 30mg/day monotherapy | None |
| 2 | Woman | 27 | Adaptative disorder | Alopecia areata | Oral corticosteroids, methotrexate, cyclosporin | Baricitinib 4mg/day | Partial response | Partial response | Methotrexate 15mg/week (orally) | 9 | Partial response | Adverse effects | Uninterrupted | Oral intolerance (methotrexate) |
| 3 | Man | 42 | Hypertension | Alopecia areata | Oral corticosteroids, Intramuscular corticosteroids, cyclosporin, | Baricitinib 4mg/day | Partial response | Partial response | Methotrexate 15mg/week (orally) | 31 | Complete response | Complete response | Uninterrupted | None |
| 4 | Woman | 35 | Hypothyroidism, attention deficit/hyperactivity disorder | Intersticial granulomatous dermatitis | Azathioprine, cyclosporin, tacrolimus, oral corticosteroids, methotrexate, adalimumab, etanercept, | Baricitinib 4mg/day | Not valorable | Uncontrolled with classic immunosupressors | Methotrexate 15mg/week (subcutaneous) | 8 (ongoing) | Complete response | Uninterrupted | Uninterrupted | None |
| 5 | Woman | 33 | None | Alopecia areata | Oral corticosteroids, methotrexate, cyclosporin | Baricitinib 4mg/day | Complete response | Uncontrolled with classic immunosupressors | Methotrexate 15mg/week (subcutaneous) | 22 | Complete response | Complete response | Uninterrupted | None |
| 6 | Man | 55 | None | Alopecia areata | Oral corticosteroids, methotrexate, cyclosporin | Baricitinib 4mg/day | Partial response | Partial response | Methotrexate 15mg/week (orally) | 20 | Complete response | Complete response | Uninterrupted | None |
| 7 | Man | 45 | Renal trasplantation (IgA nephropathy) | Alopecia areata | Intralesional corticosteroids | Baricitinib 4mg/day | Unassessable | Renal transplant | Prednisone 5mg/day | 24 (ongoing) | Complete response | Uninterrupted | Uninterrupted | Herpes simplex oral infection (baricitinib) |
| 8 | Man | 45 | Renal trasplantation (IgA nephropathy) | Alopecia areata | Intralesional corticosteroids | Baricitinib 4mg/day | Unassessable | Renal transplant | Tacrolimus 2.5mg/day | 24 (ongoing) | Complete response | Uninterrupted | Uninterrupted | Herpes simplex oral infection (baricitinib) |
| 9 | Woman | 50 | None | Alopecia areata | Oral corticosteroids, methotrexate | Baricitinib 4mg/day | Partial response | Partial response | Methotrexate 15mg/week (orally) | 19 | Complete response | Complete response | Uninterrupted | None |
| 10 | Woman | 67 | None | Alopecia areata | Oral corticosteroids, intralesional corticosteroids, cyclosporin | Baricitinib 4mg/day | Partial response | Partial response | Methotrexate 15mg/week (orally) | 13 | Complete response | Complete response | Uninterrupted | None |
| 11 | Man | 43 | None | Atopic dermatitis | Oral corticosteroids, cyclosporin | Baricitinib 4mg/day | Partial response | Transitory loss of efficacy | Prednisone 40mg/day in gradual tapering (orally) | 0.5 | Partial response | Secondary failure to JAKi | Secondary failure, switched to upadacitnib 30mg/day monotherapy | None |
| 12 | Man | 44 | None | Alopecia areata | Oral corticosteroids, methotrexate | Baricitinib 4mg/day | Unassessable | Uncontrolled with classic immunosupressors | Methotrexate 15mg/week (subcutaneous) | 23 | Complete response | Complete response | Uninterrupted | Hypercolesterolemia and hypertrigliceridemia (baricitinib), gout (methotrexate) |
| 13 | Woman | 51 | Hypertension | Alopecia areata | Oral corticosteroids, intralesional corticosteroids, cyclosporin | Baricitinib 4mg/day | Transitory partial response | Partial response | Methotrexate 15mg/week (orally) | 11 | None | No response and adverse effects | Primary failure, switched to ruxolitinib 20mg/12h monotherapy | Transminitis and mean corpuscular volume increase (methotrexate) |
| 14 | Woman | 65 | Osteoporosis | Alopecia areata | Oral corticosteroids, cyclosporin, methotrexate | Baricitinib 4mg/day | None | No response | Methotrexate 15mg/week (orally) | 6 | None | Adverse effects | Primary failure, switched to ruxolitinib 20mg/12h monotherapy | Lymphopenia and herpes simplex infection (baricitinib) |
| 15 | Woman | 44 | None | Alopecia areata | Oral corticosteroids, intramuscular corticosteroids, intralesional corticosteroids, cyclosporin, methotrexate, | Baricitinib 4mg/day | None | No response | Methotrexate 10mg/week (orally) | 5 | None | No response | Primary failure, switched to ruxolitinib 20mg/12h monotherapy | Artharlgia (methotrexate) |
| 16 | Man | 21 | Smoker, asthma | Atopic dermatitis | Oral corticosteroids, cyclosporin, dupilumab, | Upadacitinib 30mg/day | Complete response | Uncontrolled with classic immunosupressors | Prednisone 30mg/day in gradual tapering (orally) | 0.5 | Complete response | Complete response | Uninterrupted | None |
| 17 | Man | 39 | Glaucoma and cataracts | Atopic dermatitis | Oral antibiotics, oral corticosteroids, methotrexate, mycophenolate, cyclosporin, azathioprine, dupilumab | Upadacitinib 15mg/day | Unassessable | Uncontrolled with biologic therapy | Dupilumab 300mg/14 days | 32 (ongoing) | Complete response | Uninterrupted | Uninterrupted | None |
| 18 | Woman | 54 | Environmental allergies, asthma, rheumathoid arthitis, pulmonar sarcoidosis | Atopic dermatitis | Oral corticosteroids, cyclosporin, | Baricitinib 4mg/day | None | No response | Dupilumab 300mg/14 days | 6 | Partial response | Lack of response improvement | Uncontrolled arthritis, switched to adalimumab 40mg/2 weeks | Nausea (dupilumab) |
| 19 | Woman | 54 | Environmental allergies, asthma, rheumathoid arthitis, pulmonar sarcoidosis | Atopic dermatitis | Oral corticosteroids, cyclosporin, methotrexate | Baricitinib 4mg/day | Unassessable | No response | Cyclosporin 100mg/12h | 12 | Partial response | Lack of response improvement | Uncontrolled arthritis, switched to adalimumab 40mg/2 weeks | |
| 20 | Woman | 55 | Environmental allergies, asthma, rheumathoid arthitis, pulmonar sarcoidosis | Atopic dermatitis | Oral corticosteroids, cyclosporin, methotrexate | Upadacitinib 15 smg/day | Unassessable | Oral corticosteroid used to control arthritis | Prednisone 15mg in gradual tapering (orally) | 12 | Complete response | Complete response | Uninterrupted | 6kg weight increase (upadacitinib) |
| 21 | Woman | 27 | Environmental allergies | Atopic dermatitis | Phototherapy, oral corticosteroids, methotrexate, azathioprine, cyclosporin | Upadacitinib 15mg/day | Complete response | Loss of efficacy of JAKi monotherapy | Dupilumab 300mg/14 days | 6 | Complete response | Patient preference | Patient preference | |
| 22 | Woman | 27 | Environmental allergies | Atopic dermatitis | Phototherapy, oral corticosteroids, methotrexate, azathioprine, cyclosporin, upadacitinib, dupilumab | Upadacitinib 15mg/day | Unassessable | Uncontrolled with biologic therapy | Dupilumab 600mg/14 days | 3 | Complete response | Unfunded combination | Unfunded combination | Neutropenia (upadacitinib) |
The mean duration of combination therapy was 14.8 months (with 4 patients still undergoing combined treatment to date). Complete responses were achieved in 8 patients diagnosed with AA and 3 cases of AD, representing 61.1%. However, 3 patients (17%), all diagnosed with AA (case #13 and case #15; Table 1) showed no significant improvement after a mean duration of 7 months of combination therapy with baricitinib and methotrexate at 15mg/week. In 2 of these cases, adverse effects—mild lymphopenia and transaminitis—were also observed, leading to the interruption of both treatments and the initiation of another JAK inhibitor.
A total of 12 distinct adverse events (AEs) were reported in 8 different patients. Two-thirds of these AEs occurred in 5 patients who were on methotrexate 15mg/week plus baricitinib 4mg/day to treat alopecia areata. These events appeared after a mean 9 months (range, 1–23 months) of using the combination therapy. The reported AEs included oral intolerance (1), arthralgia (1), gout episode (1), relapse of oral herpes simplex (1), dyslipidemia (1), mild lymphopenia (810cells/10^9/L) (1), transaminitis (alanine transaminase, 87.6U/L; aspartate transaminase, 50.99U/L) (1), and an increase in mean corpuscular volume to 106fL (1).
In addition, 1 patient with AD on baricitinib 4mg/day and dupilumab experienced several days of nausea after the first dose of dupilumab. Another patient, treated for 6 months with upadacitinib 30mg/day and prednisone 10mg, had a 6kg weight gain, leading to a 50% down titration of the JAKi. Although a patient with AD on upadacitinib 15mg/day and intensified dupilumab therapy (case #22; Table 1) developed mild neutropenia (1030cells/10^9/L), this patient had a prior history of neutropenia and had already down titrated upadacitinib by 50%. Finally, a renal transplant recipient experienced a recurrence of oral herpes 12 months into baricitinib while on immunosuppressive therapy with tacrolimus and prednisone (case #7 and case #8; Table 1).
In 7 patients who achieved complete responses, the classic immunosuppressant was withdrawn. In contrast, in 4 cases, both treatments were discontinued and replaced with another JAK inhibitor due to primary treatment failure. Three patients with AA who had been on baricitinib and methotrexate were switched to ruxolitinib 20mg twice daily. Additionally, 2 patients were switched from baricitinib 4mg/day to upadacitinib 30mg/day—one due to a stationary response in an AA patient, and the other due to secondary failure of the JAK inhibitor in a patient with AD (case #1 and case #11; Table 1).
Based on our experience, combining JAKi with systemic immunomodulatory therapy seems to be a viable strategy for a substantial proportion of patients, particularly those unable to achieve complete responses in monotherapy or with specific comorbidities. Of note, side effects were observed in <50% of patients, all of which were classified as mild. Only 2 patients discontinued the combination therapy, switching to another JAKi in monotherapy, not due to the severity of the events but because a viable alternative was readily available. Of note, no adverse events of special interest, such as venous thromboembolism, pulmonary embolism, major adverse cardiovascular events, neoplasms, serious infections, or non-melanoma skin cancers were reported at the follow-up.
While this combination therapy introduces new avenues for managing challenging cases of inflammatory skin conditions, the validation of our observations requires further prospective studies with larger cohorts and longer follow-ups.
Declaration of generative AI and AI-assisted technologies in the writing processDuring the preparation of this work the authors used ChatGPT to optimize the word count of the article and orthographics. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflicts of interestClara Muntaner-Virgili declared to have received support for attending meetings from Lilly, and Sanofi.
Clara Torrecilla-Vall-llossera declared to have received support for attending congresses from Lilly, Sanofi, and LEO pharma.
Montserrat Bonfill-Orti declared to have received honoraria as a speaker for Lilly, Abbvie, LEO pharma, and Sanofi.
Ignasi Figueras-Nart declared to have received honoraria as speaker and advisor for Lilly, Abbvie, and Sanofi.