Screening to detect latent tuberculosis infection (LTBI) is essential before patients with moderate to severe psoriasis start treatment with biologics and vigilance will continue to be needed during and after such treatment. The most recently analyzed statistics from the BIOBADADERM registry show a 20.5% prevalence of LTBI in psoriasis patients treated with biologics in Spain. Various screening protocols are in effect in different countries according to their levels of endemic TB and bacillus Calmette-Guérin (BCG) vaccination, and there is no consensus on a gold-standard approach to the diagnosis of LTBI. Tuberculin skin testing (TST) continues to be the diagnostic method of choice in spite of its limited sensitivity, mainly in immunocompromised patients. Additional problems include the TST's well-established lack of specificity, errors in application, subjectivity in the interpretation of results (which must be read during a second visit), and lack of privacy; the main advantages of this test are its low cost and ease of application. Most cost-benefit studies are therefore inclined to favor using interferon-γ release assays to detect LTBI because they minimize false positives (especially in BCG-vaccinated individuals), thereby eliminating the extra costs and side effects of unnecessary chemoprophylaxis. We review the methods used for LTBI screening in psoriasis patients who are candidates for biologic therapy. Additionally, given the fact that most guidelines do not currently consider it necessary to screen patients about to start conventional systemic therapy, we discuss the reasons underlying the need for such screening.
Los pacientes con psoriasis moderada-grave que van a iniciar tratamiento con agentes biológicos deben ser monitorizados para la detección de infección tuberculosa latente antes, durante y después del tratamiento. En el último informe publicado de BIOBADADERM la prevalencia de infección latente por M.tuberculosis (ILMT) alcanzaba el 20,5% de los pacientes psoriásicos tratados con agentes biológicos en nuestro país. En la actualidad no existe un método diagnóstico gold standard que permita una aplicación sistemática y consensuada, con variaciones en los diferentes países según el grado de endemicidad y vacunación con BCG. La prueba de tuberculina (PT) continúa siendo el método de elección para el diagnóstico de infección, pero presenta importantes limitaciones en su sensibilidad (principalmente en pacientes inmunodeprimidos). Esta situación, junto a su falta de especificidad conocida, errores en su administración, la subjetividad en la interpretación de los resultados, la necesidad de una segunda visita para la lectura y la ausencia de privacidad hacen de ella una prueba limitada, cuyas principales ventajas resultarían su bajo coste y fácil realización. Por eso la mayoría de los estudios de coste beneficio se inclinan por el método IGRA para el diagnóstico de la ILMT, ya que minimiza los falsos positivos (especialmente en población vacunada), eliminando costes extra y efectos secundarios de la quimioprofilaxis antituberculosa. Valoramos la aplicabilidad en pacientes psoriásicos candidatos a terapia biológica y discutimos la necesidad de su realización previamente a terapia sistémica convencional, puesto que la mayoría de las guías actuales no consideran imprescindible su realización.
Tuberculosis (TB) infection occurs when a person comes into contact with airborne pathogens that originate from “open” lung lesions, i.e., lesions connected to the exterior via a bronchus draining a tuberculous cavity. Other routes of contagion are possible but are no longer of epidemiological importance. Infected humans are thus the only important reservoir of the Mycobacterium tuberculosis bacterium responsible for the persistence of the TB pandemic. Primary TB infection generates an immune response, which in most cases halts the multiplication of the bacillus, enabling control of the infection and subsequent cure. However, when the immune system fails to destroy all the bacteria, some of these remain in a latent state in the host's cells and the host develops what is known as latent tuberculosis infection (LTBI). Affected individuals normally have no clinical manifestations or objective findings on examination.
According to the latest epidemiological data from the World Health Organization (WHO), which includes information up to 2014, 6.1 million TB cases were reported in 2013.1 Of these, 5.7 million cases were newly diagnosed, while the remaining 0.4 million were cases under treatment. In Spain, the reported prevalence of TB is 16 cases per 100000 inhabitants (including human immunodeficiency virus [HIV] coinfection cases), and the estimated incidence for 2013 was 13 cases per 100000 inhabitants. These data correspond to cases of active TB and illustrate the magnitude of the problem. It is more difficult, however, to determine the prevalence of LTBI, as there are no standardized systems for recording cases. Figures vary according to the level of endemism, with rates ranging from 0.03% in low-burden countries, such as the United States, to 4.2% in high-burden countries, such as South Africa.2 The figures over the past 10 years have varied only minimally.
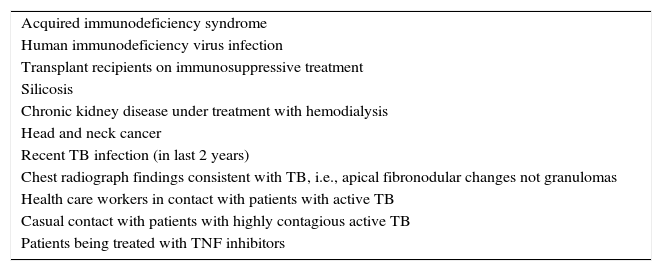
The risk of a person with LTBI developing active TB is variable and ranges between 0.1% (in healthy individuals without risk factors) to approximately 10% (in patients with HIV coinfection). Adequate screening for latent infection can therefore reduce the incidence of active TB. As mentioned, patients with inadequately treated active TB are the main source of infection.3 The main factors that favor TB transmission include “severe disease in the index patient, long periods of exposure to the index patient, and poor ventilation and poor exposure to UV light during proximity to the index patient”.4 A detailed description of intrinsic M tuberculosis– and host-related factors is beyond the scope of this article, but suffice it to say that in dermatology, LTBI prevention and treatment is of interest to dermatologists working with patients with moderate to severe psoriasis under treatment with biologics—and tumor necrosis factor (TNF) inhibitors in particular—as these patients are among the high-risk groups for infection (Table 1).
High-Risk Groups for Active TB.
| Acquired immunodeficiency syndrome |
| Human immunodeficiency virus infection |
| Transplant recipients on immunosuppressive treatment |
| Silicosis |
| Chronic kidney disease under treatment with hemodialysis |
| Head and neck cancer |
| Recent TB infection (in last 2 years) |
| Chest radiograph findings consistent with TB, i.e., apical fibronodular changes not granulomas |
| Health care workers in contact with patients with active TB |
| Casual contact with patients with highly contagious active TB |
| Patients being treated with TNF inhibitors |
Abbreviations: TB, tuberculosis; TNF, tumor necrosis factor.
In this review, we describe the main techniques currently available for diagnosing LTBI, update the existing LTBI screening algorithm, analyze current screening practices in psoriasis patients in low- and high-TB-burden countries, and explore the possibility of extending screening recommendations for psoriasis patients to patients who are being treated with or are candidates for nonbiologic systemic therapy.
Diagnosing LTBIIndividuals with LTBI at risk of developing active TB must be identified in order to administer appropriate prophylactic treatment. Screening for LTBI, however, should not be applied indiscriminately, but rather reserved for patients in whom it will bring clear benefits, as a positive result mandates treatment.
Tuberculin skin testing (TST) is the traditional method for diagnosing LTBI, although new, immune-based in vitro blood tests, known as interferon-γ release assays (IGRAs), have gained ground in recent years. It should be noted, however, that there is no gold-standard approach for detecting LTBI. Current tests are all immune-based, i.e., they involve the participation and response of memory T cells to antigens with varying levels of specificity. The main disadvantage of both TST and IGRAs, which are described in the sections below, is that they cannot differentiate between LTBI and active TB.
Tuberculin Skin TestingTST is performed with an extract obtained from filtrates of sterilized concentrated cultures of the tubercle bacillus. The antigen used is purified protein derivative (PPD), and in Spain, the RT-23 variant is used, with Tween 80 as a stabilizer.5,6
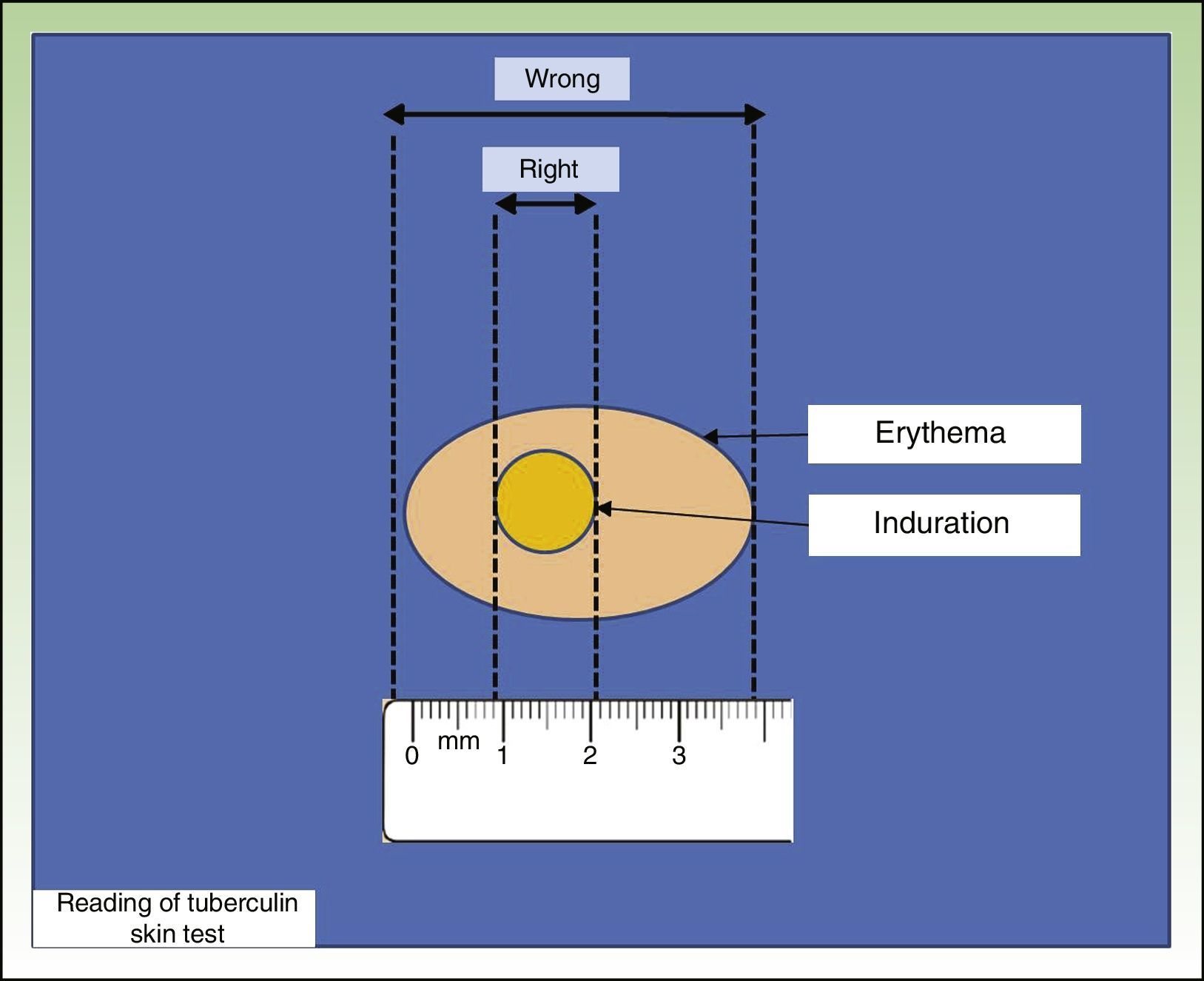
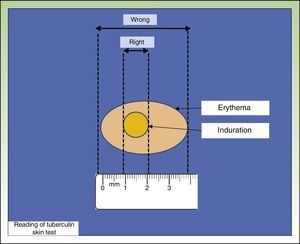
The most widely used TST technique is the Mantoux technique, which involves injecting 0.1 mL of PPD at a dose of 2 tuberculin units into the inner surface of the forearm. The injection should trigger the appearance of a wheal, which disappears after a short time. Results are read after 48 to 72hours by measuring the diameter of the induration perpendicular to the long axis of the forearm (Fig. 1). Another technique is the multipuncture test, which is also performed on the forearm but using tines or pins dipped in tuberculin. This test, however, is considered inadequate, as the amount of tuberculin that actually penetrates the skin is unknown.7,8
The main disadvantage of TST is that the proteins used are not specific to M tuberculosis, as they are shared by the TB bacillus used in the bacillus Calmette-Guérin (BCG) vaccine and by other species of environmental mycobacteria. As a result, the test has reduced specificity.
In Spain, the TST is considered positive when a person who has not received the BCG vaccine develops an induration of 5mm or larger. Interpretation of results in BCG-vaccinated individuals is more complicated due to the need to distinguish between effects attributable to the vaccine and to TB infection. In such cases, an induration larger than 5mm is considered to indicate a positive result if the individual is living with or in frequent contact with a TB patient or if he or she has chest radiograph changes suggesting former or untreated TB infection. All other BCG-vaccinated individuals need to be treated, because, although it is thought that the larger the induration, the greater the chance that the response is due to the presence of TB infection, the effect of the vaccine cannot be proven with certainty.9,10
An induration of any size in a severely immunocompromised patient (e.g., an HIV-infected patient, transplant recipient, patient receiving biologic or corticosteroid therapy) is indicative of a positive result.
TST is known to cause a booster effect that can lead to erroneous interpretation of results. This effect, which corresponds to the induction or re-establishment of waned reactivity in a previously infected patient, can be misinterpreted as a new skin test conversion. The booster effect is typically seen in individuals over 55 years of age and/or in BCG-vaccinated persons, and in such cases, a test with a negative result should be repeated after 7 to 10 days; the result of the second test is considered definitive.5,6
Previous BCG vaccination and use of antigens that are not specific to M tuberculosis (i.e., antigens shared with nontuberculous mycobacteria) are the main causes of false-positive results in TST. Nontuberculous mycobacteria, however, are only an important cause of false positives in countries with a high prevalence of these bacteria and a low prevalence of M tuberculosis infection.10 The impact of the BCG vaccine depends on time since vaccination and number of doses administered.
Interpretation of results can also be complicated by errors in administering the test, resulting in bruising or injection site infections. Other causes of false-negative results during testing and reading of results include concurrent viral infection, live virus vaccination, immunosuppressed states or use of immunosuppressive drugs, and immune response in very elderly patients.
In general, although the TST continues to be the method of choice for diagnosing infection, its sensitivity is considerably reduced, particularly in immunodepressed patients. This reduced sensitivity limits the usefulness of the test. Additional problems include the test's lack of specificity, errors in administration, subjectivity in the interpretation of results, need for a second visit to read the results, and lack of privacy. However, the test does have important advantages, in particular its low cost and ease of administration.
Interferon-γ Release AssaysIGRAs are based on the detection of interferon-γ in the blood. This cytokine has a fundamental role in the control of TB infection and its release is triggered by the in vitro stimulation of T cells sensitized to M tuberculosis–specific antigens.
The antigens used in this test are ESAT-6 (early secretory antigenic target-6) and CFP-10 (culture filtrate protein 10), which are encoded by genes in the RD1 (region of difference 1) of the M tuberculosis genome.11,12 These antigens are more specific than PPD, as they are not shared by any of the strains used in the BCG vaccine or by the majority of nontuberculous mycobacteria; exceptions are Mycobacterium marinum, Mycobacterium kansasii, Mycobacterium szulgai, and Mycobacterium flavencens.13
There are 2 commercially available IGRAs: QuantiFERON TB Gold In-Tube (QFT-GIT) (Cellestis/Qiagen), which is based on the enzyme-linked immunoabsorbent assay (ELISA), and T-SPOT.TB (T-Spot) (Oxford Immunotec), which is based on the enzyme-linked immunospot (ELISPOT) technique. Both tests were recently approved for sale in Europe and have also finally received clearance from the US Food and Drug Administration for the diagnosis of LTBI. The QFT-GIT incorporates a third antigen in addition to ESAT-6 and CFP-10: TB 7.7.
Both tests offer procedural advantages over TST, and are significantly more specific in BCG-vaccinated individuals.14,15 In addition, they incorporate controls to test for anergy and rule out false negatives. They can also be repeated immediately and do not cause the booster effect as they do not induce an immune response. Loss of patients is also avoided as patients do not need to return for a second visit, as they do with TST. Finally, IGRAs are easier to standardize and use in the laboratory, and they protect patient privacy. Their main disadvantage compared with TST is their cost.
The QFT-GIT is performed by incubating 1mL of peripheral blood with heparin in 3 tubes: 1) a nil control tube containing blood from the patient, 2) a positive control tube with phytohemagglutinin to measure the patient's lymphoproliferative response, and 3) a tube containing the specific antigens: ESAT-6, CFP-10, and TB 7.7. The 3 tubes are incubated for 16 to 18hours at a temperature of 37°C and the concentration of interferon (IFN) γ is measured in international units (IU) per milliliter by ELISA. Results are interpreted using a standard curve in a software program supplied by the manufacturer.
In the T-Spot, unlike the QFT-GIT, peripheral blood mononuclear cells must be separated prior to stimulation, and as mentioned, the presence of interferon-γ is determined by ELISPOT not ELISA. The ELISPOT assay involves counting the number of spot-forming cells, whereby each spot corresponds to the footprint of a single IFN-γ–secreting T cell. Technically, the T-Spot requires more blood, has longer preparation times, and is more difficult to conduct. However, it appears to offer greater sensitivity.
Unfortunately, IGRAs may yield indeterminate results due to low IFN-γ production in the positive control (mitogen) or high response to the nil control.
IGRAs have a specificity of over 95% in areas with a low incidence of active TB, and this is not affected by BCG vaccination. According to several studies, T-Spot has higher specificity (90%) than both QFT-GIT and TST (80% in both cases).16,17
Biologic Therapy and Screening for LTBIIn this section, we are going to look at the current situation regarding screening for LTBI in patients with moderate to severe psoriasis who are candidates for biologic therapy, the incidence of LTBI in this population in Spain, and the current position of the WHO regarding LTBI screening. We also provide a brief analysis of studies that have analyzed the diagnostic agreement between IGRAs and TST, and conclude with an easy-to-apply algorithm.
The need to screen for LTBI in patients with moderate to severe psoriasis became a key issue in 2005, when biologics were introduced into the therapeutic arsenal for this condition. Drawing on experiences with rheumatological patients, in their first consensus document on the evaluation and treatment of moderate to severe psoriasis,18 the Spanish Psoriasis Group, under the auspices of the Spanish Academy of Dermatology and Venereology, considered 3 situations related to screening for LTBI in this setting (Table 2).
Screening Algorithm for LTBI20
| Negative TST Negative booster effect Normal chest radiograph findings | Start biologic therapy |
| Positive TST and/or positive booster effect Normal chest radiograph findings | Diagnose LTBI Start chemoprophylaxis Delay initiation of biologic therapy for 1 month |
| Positive TST and/or booster effect (and BCG vaccination >10 years ago in vaccinated individuals) Abnormal chest radiograph findings | Diagnose active TB Start TB treatment Delay initiation of biologic therapy for 6 month |
TST was repeated yearly in patients with a negative result.
After thorough history taking with relevant search for history and risk factors.
Source: Adapted from the 2009 Consensus Document on the Evaluation and Treatment of Moderate to Severe Psoriasis of the Spanish Academy of Dermatology and Venereology.18
The current situation regarding LTBI in patients with moderate to severe psoriasis in Spain is quite well known thanks to a study published by Sánchez Moya et al.,19 who reported on statistics from the 2013 BIOBADADERM registry. The study reported a prevalence of 20.5% for LTBI in 1425 patients with moderate to severe psoriasis, 56% of whom were receiving biologics and 44% of whom were receiving conventional systemic treatment.
Screening for LTBI was performed in 83% of these patients, but adherence to screening protocol recommendations was observed in just 51% of cases.
The authors attributed this low adherence to the introduction of IGRAs in many of the participating centers and to the fact that the finding was based only on TST screening. The study highlights the need to modify guidelines for the management of moderate to severe psoriasis and emphasizes the importance of adherence to established protocols.
In its recently published guidelines on the management of LTBI,21 the WHO highlights 3 very interesting points:
- •
“Individuals should be asked about symptoms of TB before being tested for LTBI. Chest radiography must be done if efforts are intended also for active TB case finding. Individuals with TB symptoms or any other radiological abnormality should be investigated further for active TB and other conditions (strong recommendation, very low quality of evidence).”
- •
“Either TST or IGRA can be used to test for LTBI in high-income and upper middle-income countries with estimated TB incidence less than 100 per 100 000 (strong recommendation, very low quality of evidence).”
- •
“IGRA should not replace TST in low-income and other middle-income countries (strong recommendation, very low quality of evidence).”
Considering the above, and the relatively recent introduction of new LTBI screening methods—IGRAs—now seems an opportune moment to revise the situation. Studies of diagnostic agreement between TST and IGRA have shown clear differences between high- and low-TB-burden countries due to BCG vaccination and reinfection rates.22,23
- •
In countries with a high incidence of TB and BCG vaccination, TST overestimates the diagnosis of LTBI, leading to unnecessary prophylactic treatment and associated adverse effects,24,25 while IGRAs do not offer significant differences with respect to control groups, with similar rates detected in psoriasis patients and the general population.
- •
In moderately endemic countries with low vaccination rates, TST also overestimates LTBI compared with IGRAs.26
- •
Finally, in low-burden countries with low BCG vaccination rates, better diagnostic agreement has been found between the 2 tests, but TST continues to overestimate the prevalence of LTBI compared with IGRAs.27,28
One particularly interesting recent study in this area is that by Gisondi et al.,29 which evaluated the prevalence of LTBI in patients with psoriasis being considered for biologic therapy. The authors compared rates with those observed in health care workers and patients with rheumatoid arthritis or Crohn disease. The findings complemented data from the Italian psoriasis registry (Psocare),30 which had established a prevalence rate of 8.2%. All the patients in the study by Gisondo et al. were screened for LTBI using an IGRA (QFT-GIT), but 88 of the patients simultaneously underwent screening with TST. The rates detected were 8.2% for patients with psoriasis, 8.8% for health care workers, 9% for patients with rheumatoid arthritis, and 7% for patients with Crohn disease. None of the patients diagnosed with LTBI developed active TB after treatment with appropriate chemoprophylaxis. IGRA testing was repeated after a mean (SD) of 31 (1.7) months—the authors do not explain why they chose this exact period—and revealed reversion in 25% of patients diagnosed and treated for LTBI and conversion in 0.8% of cases. Agreement was low between the QFT-GIT and TST (κ=0.15), although the authors were clearly in favor of using the IGRA, as it can be repeated as often as the dermatologist considers necessary without inducing a booster effect or sensitization. New studies, however, are required to help explain the fluctuations seen in the serial tests performed.
The reasons for the discrepancies between IGRAs and TST have not been clearly elucidated, but may include variations in psoriasis severity, BCG vaccination status, and use (or not) of concomitant immunosuppressive therapy. Patients with moderate to severe psoriasis exhibit a lower M tuberculosis–specific immune response and in general greater sensitivity to TST. Finally, immunosuppressive therapy can affect TST but not IGRA results.31
Most cost-benefit studies tend to weigh in favor of the use of IGRAs to diagnose LTBI, as these tests minimize false positives (particularly in vaccinated populations) and eliminate the extra costs and adverse effects associated with TB chemoprophylaxis.23
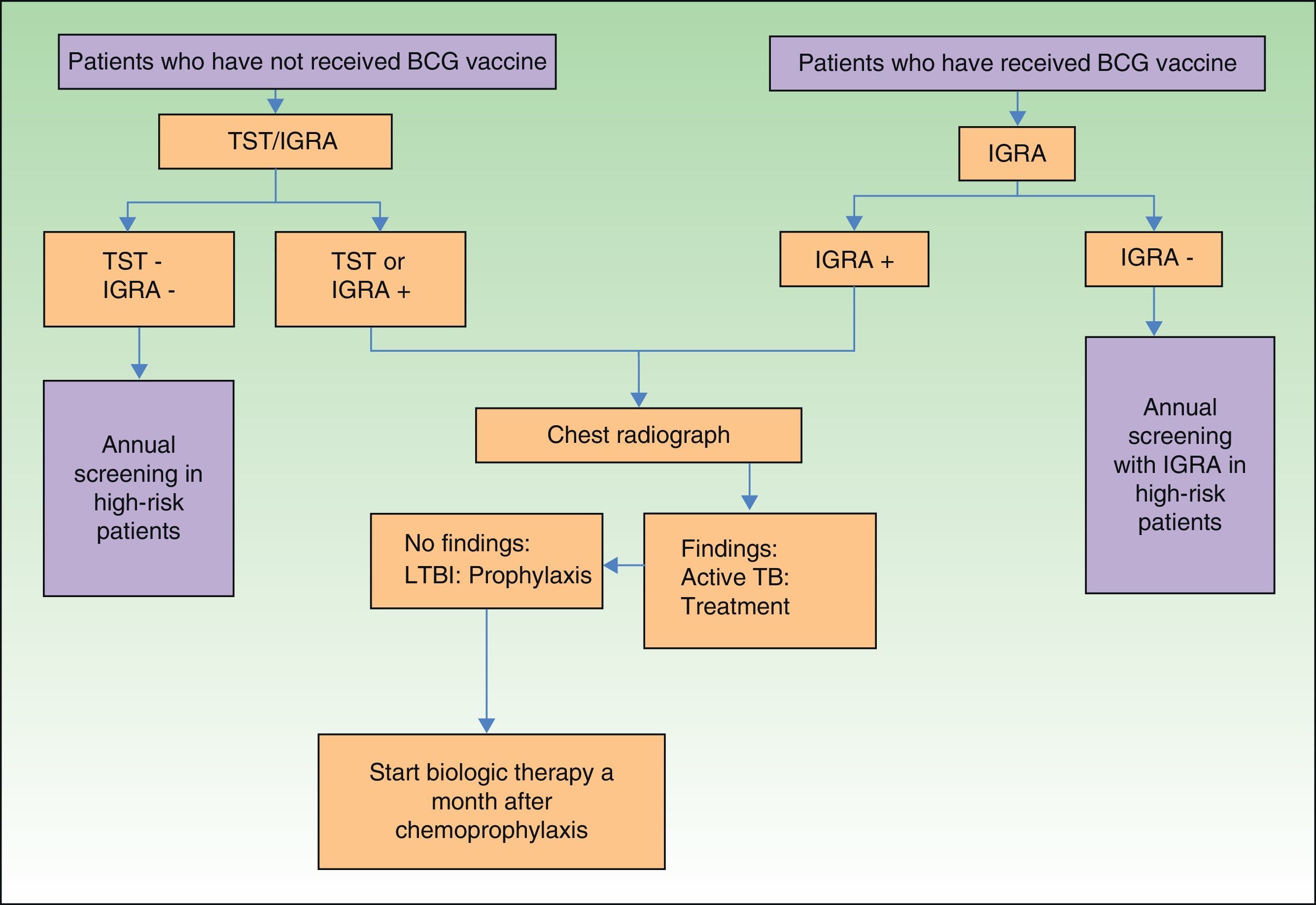
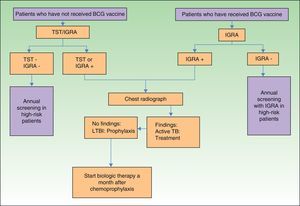
In view of the above, and considering that not all hospitals in Spain have access to IGRAs, we have designed an algorithm (Fig. 2) for screening for LTBI in patients with moderate to severe psoriasis in our setting.
Systemic Treatment and Screening for LTBI: Other ConsiderationsWhile the need for LTBI screening in patients who are candidates for biologic therapy is fully acknowledged, there are reasonable doubts regarding the need for such testing in patients transitioning from topical to systemic therapy. Not all systemic drugs or phototherapy have the same mechanism of action, and only methotrexate and ciclosporin, due to their immunosuppressive action, are associated with a notable increased risk of viral, fungal, and bacterial infections, including LTBI of course.
Both Spanish18 and American32 guidelines on the diagnostic and therapeutic management of psoriasis recommend screening for LTBI prior to initiating treatment with ciclosporin or methotrexate. However, they do not recommend screening in the case of acitretin or phototherapy, as these have not been found to be associated with an increased risk of TB reactivation. These recommendations are supported by the results of the recent prevalence study of LTBI in patients with psoriasis by Gisondi et al.,27 discussed in the previous section.
In their study based on the latest figures from the BIOBADADERM registry, Sánchez-Moya et al.19 reported no cases of active TB in patients treated with conventional systemic agents, suggesting possibly a lower risk of LTBI in such cases. The findings of such studies and lessons drawn from previous experiences perhaps explain why certain guidelines, such as the European S3-guidelines,33 do not consider it necessary to screen for LTBI before initiating treatment with any of the 4 classical treatments used in the management of moderate to severe psoriasis.
While beyond the scope of our study, we believe it important to draw attention to one aspect highlighted by several authors: the inclusion of oral corticosteroids in treatment regimens that require screening for LTBI prior to initiation, particularly when doses of over 20mg/d are to be used for longer than 2 weeks in areas with a high prevalence of TB.34–36 In a review on the evidence regarding screening for infectious diseases before initiation of systemic immunosuppressive treatment in patients with autoimmune bullous diseases, Keith et al.36 recommended assessing individual risk in order to choose the most appropriate method. The authors also stressed the fact that patients with autoimmune bullous diseases may require multiple immunosuppressive therapies over time.
Patients with moderate to severe psoriasis also typically need multiple treatments over time, and it would therefore seem reasonable to screen for LTBI in all patients about to start systemic therapy, although, as we have mentioned, there is no consensus on this matter.
ConclusionsWe have provided an update of the methods currently used to screen for LTBI and their application in routine dermatology practice. IGRAs are now widely used in the diagnosis of TB due to the absence of cross-reactions with the BCG vaccine and other nontuberculous mycobacteria. Compared with TST, they are influenced less by immunosuppression, although not all authors would agree,37 and can be repeated over time without causing a booster effect. Accordingly, they are ideal for monitoring for LTBI in patients with moderate to severe psoriasis who are candidates for biologic therapy. Their cost, however, continues to be their main disadvantage. Although consensus is lacking regarding the need for LTBI screening before the initiation of conventional systemic treatments in patients with moderate to severe psoriasis, the fact that these patients also need multiple treatments, including biologics, throughout the course of their disease, makes screening highly recommendable. LTBI screening should also be considered for other systemic or autoimmune diseases that require treatments such as systemic corticosteroids, as these are also associated with higher rates of LTBI reactivation.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martínez-López A, Rodriguez-Granger J, Ruiz-Villaverde R. Despistaje de tuberculosis latente en el paciente con psoriasis moderada grave candidato a terapia sistémica y/o biológica. Actas Dermosifiliogr. 2016;107:207–214.