Melanomas in the elderly account for 40% of diagnosed melanomas and account for 60.2% of deaths.1 Sentinel lymph node biopsy (SLNB) is currently recommended as a staging method for intermediate-thickness melanoma (Breslow thickness, ≥0.8mm). The ASCO guidelines state that SLNB may be recommended at all ages after discussion of the potential benefits and risks of harm.2
Data from the Surveillance, Epidemiology and End Results Program (SEER) suggest that the incidence of thick melanoma is significantly higher in patients over 60 years of age and that mortality is higher than in other age groups. The age group with the highest percentage of death from melanoma is patients aged 75–84 years. In addition, a study analysing 3 different cohorts – including the SEER cohort – with more than 300,000 patients found that age predicts a worse melanoma-specific survival.3,4 SLNB remains controversial and the peculiarities in the elderly have not been thoroughly studied.
The primary objective of our study was to determine if there is a difference in SLNB status predictors in two different age groups, younger and older than 75 years old. A secondary objective was to describe the difference in complications in both groups.
A cohort, retrospective, single centre, longitudinal, observational study was performed with all the patients that have underwent SNLB from January 2008 to December 2020. Inclusion criteria were the following: All consecutive adult patients with cutaneous melanoma that underwent SLNB. The cohort was divided in two age groups for age related analysis, younger and older than 75 years old. The study protocol was approved by institutional review board and informed consent was obtained from all patients.
The following clinical and histologic characteristics were selected as independent variables: sex, age, anatomic location, histologic subtype, Breslow thickness, ulceration, regression, lymphovascular invasion, mitosis, SLNB location, and SLNB complications.
Statistical analyses were performed using SPSS software ver. 22.0 (IBM®, Armonk, NY, USA). Group comparisons were performed using the Fisher test or Mann–Whitney U-test, Pearson chi-square test or independent samples T test as necessary. Multivariate logistic regression analysis with backward stepwise selection was performed to assess associations (p<0.1).
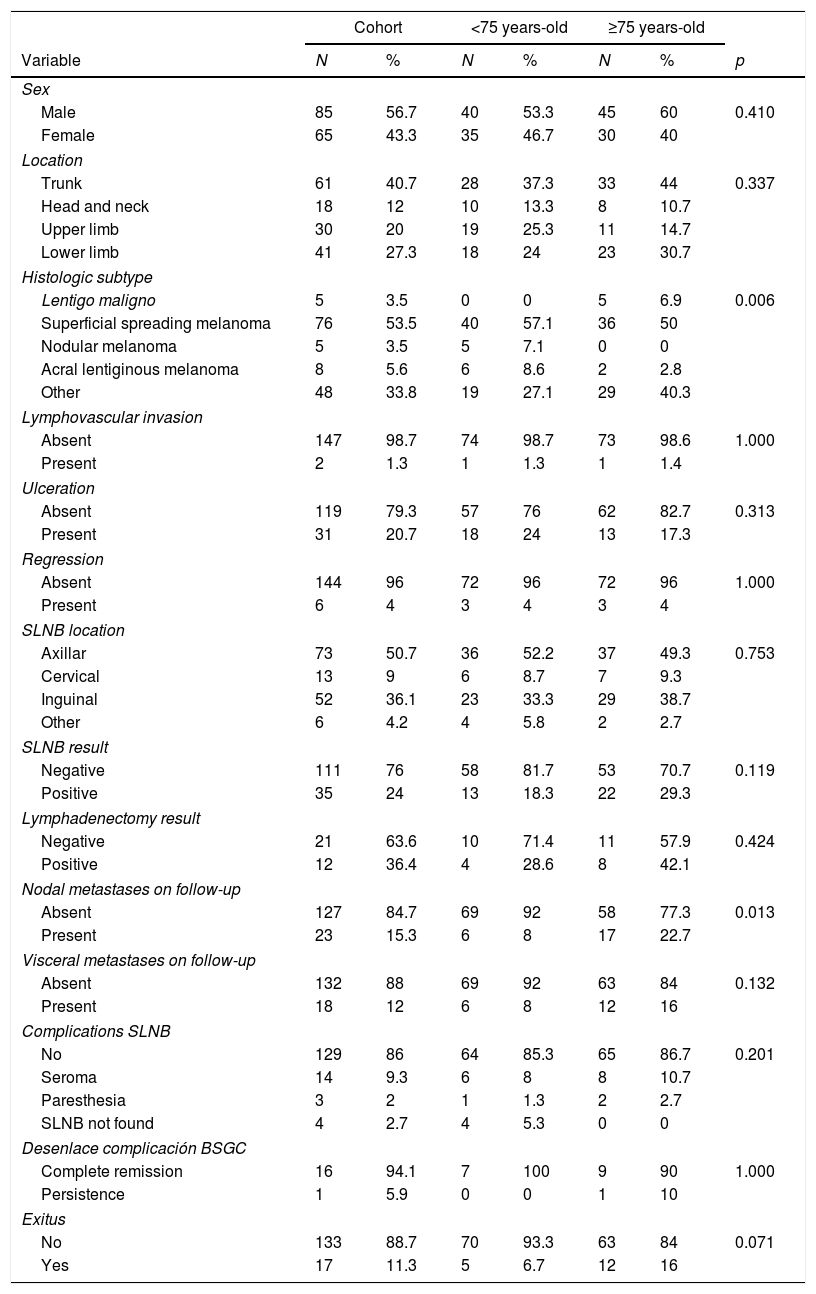
Patient characteristics are summarized in Table 1. The sample includes a total of 150 patients who have underwent SLNB. The SLNB was identified in 146 patients (97.3%). The mean age of the participants was 70.6 years old. Positive SLNB was more frequent in ≥75-year-old group (29.3% vs. 18.3%). Although this result was not statistically significant (p=0.114).
Clinical and histopathological characteristic of study patients.
| Cohort | <75 years-old | ≥75 years-old | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | p |
| Sex | |||||||
| Male | 85 | 56.7 | 40 | 53.3 | 45 | 60 | 0.410 |
| Female | 65 | 43.3 | 35 | 46.7 | 30 | 40 | |
| Location | |||||||
| Trunk | 61 | 40.7 | 28 | 37.3 | 33 | 44 | 0.337 |
| Head and neck | 18 | 12 | 10 | 13.3 | 8 | 10.7 | |
| Upper limb | 30 | 20 | 19 | 25.3 | 11 | 14.7 | |
| Lower limb | 41 | 27.3 | 18 | 24 | 23 | 30.7 | |
| Histologic subtype | |||||||
| Lentigo maligno | 5 | 3.5 | 0 | 0 | 5 | 6.9 | 0.006 |
| Superficial spreading melanoma | 76 | 53.5 | 40 | 57.1 | 36 | 50 | |
| Nodular melanoma | 5 | 3.5 | 5 | 7.1 | 0 | 0 | |
| Acral lentiginous melanoma | 8 | 5.6 | 6 | 8.6 | 2 | 2.8 | |
| Other | 48 | 33.8 | 19 | 27.1 | 29 | 40.3 | |
| Lymphovascular invasion | |||||||
| Absent | 147 | 98.7 | 74 | 98.7 | 73 | 98.6 | 1.000 |
| Present | 2 | 1.3 | 1 | 1.3 | 1 | 1.4 | |
| Ulceration | |||||||
| Absent | 119 | 79.3 | 57 | 76 | 62 | 82.7 | 0.313 |
| Present | 31 | 20.7 | 18 | 24 | 13 | 17.3 | |
| Regression | |||||||
| Absent | 144 | 96 | 72 | 96 | 72 | 96 | 1.000 |
| Present | 6 | 4 | 3 | 4 | 3 | 4 | |
| SLNB location | |||||||
| Axillar | 73 | 50.7 | 36 | 52.2 | 37 | 49.3 | 0.753 |
| Cervical | 13 | 9 | 6 | 8.7 | 7 | 9.3 | |
| Inguinal | 52 | 36.1 | 23 | 33.3 | 29 | 38.7 | |
| Other | 6 | 4.2 | 4 | 5.8 | 2 | 2.7 | |
| SLNB result | |||||||
| Negative | 111 | 76 | 58 | 81.7 | 53 | 70.7 | 0.119 |
| Positive | 35 | 24 | 13 | 18.3 | 22 | 29.3 | |
| Lymphadenectomy result | |||||||
| Negative | 21 | 63.6 | 10 | 71.4 | 11 | 57.9 | 0.424 |
| Positive | 12 | 36.4 | 4 | 28.6 | 8 | 42.1 | |
| Nodal metastases on follow-up | |||||||
| Absent | 127 | 84.7 | 69 | 92 | 58 | 77.3 | 0.013 |
| Present | 23 | 15.3 | 6 | 8 | 17 | 22.7 | |
| Visceral metastases on follow-up | |||||||
| Absent | 132 | 88 | 69 | 92 | 63 | 84 | 0.132 |
| Present | 18 | 12 | 6 | 8 | 12 | 16 | |
| Complications SLNB | |||||||
| No | 129 | 86 | 64 | 85.3 | 65 | 86.7 | 0.201 |
| Seroma | 14 | 9.3 | 6 | 8 | 8 | 10.7 | |
| Paresthesia | 3 | 2 | 1 | 1.3 | 2 | 2.7 | |
| SLNB not found | 4 | 2.7 | 4 | 5.3 | 0 | 0 | |
| Desenlace complicación BSGC | |||||||
| Complete remission | 16 | 94.1 | 7 | 100 | 9 | 90 | 1.000 |
| Persistence | 1 | 5.9 | 0 | 0 | 1 | 10 | |
| Exitus | |||||||
| No | 133 | 88.7 | 70 | 93.3 | 63 | 84 | 0.071 |
| Yes | 17 | 11.3 | 5 | 6.7 | 12 | 16 | |
| Mean | St. dev. | Mean | St. dev. | Mean | St. dev | p | |
|---|---|---|---|---|---|---|---|
| Age | 70.6 | 15.9 | 58.4 | 13.2 | 82.8 | 5.6 | (<0.001) |
| Breslow index | 2.6 | 2.1 | 2.6 | 2.1 | 2.6 | 2.0 | 0.914 |
| Mitosis | 3.8 | 6.1 | 4.0 | 5.7 | 3.6 | 6.4 | 0.707 |
SLNB: sentinel node biopsy, St. dev: standard deviation.
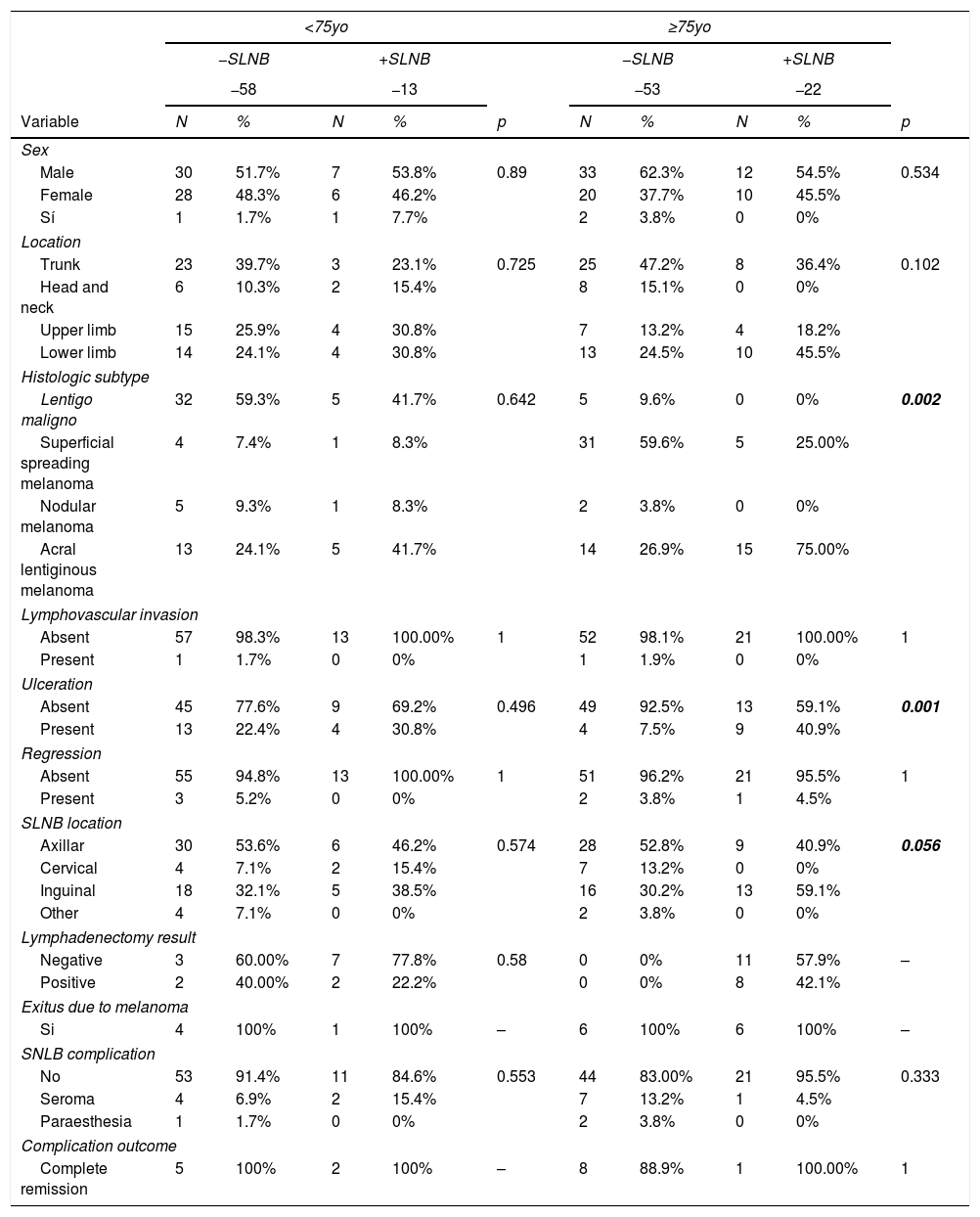
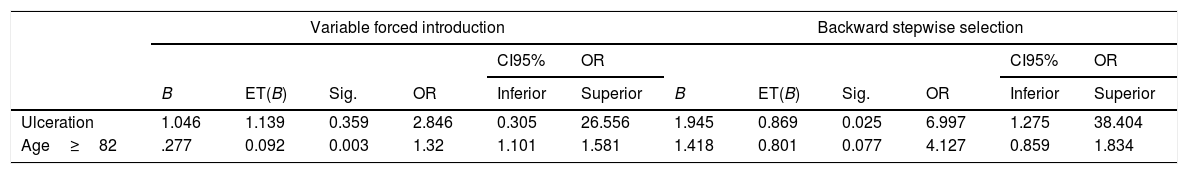
Bivariate and multivariate analysis are summarized in Tables 2 and 3. The logistic regression multivariate analysis showed that for the ≥75-year-old group, ulceration was associated with SNLB positivity with an Odds Ratio of 13.3 (95%CI 2.7–65.0). Also, the risk grows when age increases, specifically the odds increase by 19% for each year over 82-year-old on the stepwise selection model. As secondary objective regarding complications, 86% of the patients did not experience any, and seroma, affecting 9.6% of the patients was the commonest with no difference among both age groups.
Superior: Bivariate studies between study category and outcome using X2, Fisher, Mann–Whitney U or independent samples T test as necessary.
| <75yo | ≥75yo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −SLNB | +SLNB | −SLNB | +SLNB | |||||||
| −58 | −13 | −53 | −22 | |||||||
| Variable | N | % | N | % | p | N | % | N | % | p |
| Sex | ||||||||||
| Male | 30 | 51.7% | 7 | 53.8% | 0.89 | 33 | 62.3% | 12 | 54.5% | 0.534 |
| Female | 28 | 48.3% | 6 | 46.2% | 20 | 37.7% | 10 | 45.5% | ||
| Sí | 1 | 1.7% | 1 | 7.7% | 2 | 3.8% | 0 | 0% | ||
| Location | ||||||||||
| Trunk | 23 | 39.7% | 3 | 23.1% | 0.725 | 25 | 47.2% | 8 | 36.4% | 0.102 |
| Head and neck | 6 | 10.3% | 2 | 15.4% | 8 | 15.1% | 0 | 0% | ||
| Upper limb | 15 | 25.9% | 4 | 30.8% | 7 | 13.2% | 4 | 18.2% | ||
| Lower limb | 14 | 24.1% | 4 | 30.8% | 13 | 24.5% | 10 | 45.5% | ||
| Histologic subtype | ||||||||||
| Lentigo maligno | 32 | 59.3% | 5 | 41.7% | 0.642 | 5 | 9.6% | 0 | 0% | 0.002 |
| Superficial spreading melanoma | 4 | 7.4% | 1 | 8.3% | 31 | 59.6% | 5 | 25.00% | ||
| Nodular melanoma | 5 | 9.3% | 1 | 8.3% | 2 | 3.8% | 0 | 0% | ||
| Acral lentiginous melanoma | 13 | 24.1% | 5 | 41.7% | 14 | 26.9% | 15 | 75.00% | ||
| Lymphovascular invasion | ||||||||||
| Absent | 57 | 98.3% | 13 | 100.00% | 1 | 52 | 98.1% | 21 | 100.00% | 1 |
| Present | 1 | 1.7% | 0 | 0% | 1 | 1.9% | 0 | 0% | ||
| Ulceration | ||||||||||
| Absent | 45 | 77.6% | 9 | 69.2% | 0.496 | 49 | 92.5% | 13 | 59.1% | 0.001 |
| Present | 13 | 22.4% | 4 | 30.8% | 4 | 7.5% | 9 | 40.9% | ||
| Regression | ||||||||||
| Absent | 55 | 94.8% | 13 | 100.00% | 1 | 51 | 96.2% | 21 | 95.5% | 1 |
| Present | 3 | 5.2% | 0 | 0% | 2 | 3.8% | 1 | 4.5% | ||
| SLNB location | ||||||||||
| Axillar | 30 | 53.6% | 6 | 46.2% | 0.574 | 28 | 52.8% | 9 | 40.9% | 0.056 |
| Cervical | 4 | 7.1% | 2 | 15.4% | 7 | 13.2% | 0 | 0% | ||
| Inguinal | 18 | 32.1% | 5 | 38.5% | 16 | 30.2% | 13 | 59.1% | ||
| Other | 4 | 7.1% | 0 | 0% | 2 | 3.8% | 0 | 0% | ||
| Lymphadenectomy result | ||||||||||
| Negative | 3 | 60.00% | 7 | 77.8% | 0.58 | 0 | 0% | 11 | 57.9% | – |
| Positive | 2 | 40.00% | 2 | 22.2% | 0 | 0% | 8 | 42.1% | ||
| Exitus due to melanoma | ||||||||||
| Si | 4 | 100% | 1 | 100% | – | 6 | 100% | 6 | 100% | – |
| SNLB complication | ||||||||||
| No | 53 | 91.4% | 11 | 84.6% | 0.553 | 44 | 83.00% | 21 | 95.5% | 0.333 |
| Seroma | 4 | 6.9% | 2 | 15.4% | 7 | 13.2% | 1 | 4.5% | ||
| Paraesthesia | 1 | 1.7% | 0 | 0% | 2 | 3.8% | 0 | 0% | ||
| Complication outcome | ||||||||||
| Complete remission | 5 | 100% | 2 | 100% | – | 8 | 88.9% | 1 | 100.00% | 1 |
| −SNLB | +SNLB | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | N | Media | DT | N | Media | DT | t | p |
| Age | 53 | 81.55 | 4.66 | 22 | 85.86 | 6.61 | −2.788 | 0.009 |
| Breslow index | 52 | 2.25 | 1.98 | 20 | 3.37 | 1.98 | −2.148 | 0.035 |
| Mitosis | 53 | 3.19 | 5.74 | 22 | 4.50 | 7.89 | −0.804 | 0.424 |
SLNB: sentinel node biopsy. Inferior: Bivariate studies between study category and outcome using independent samples T test for quantitative variables in the ≥75 year old group. The p values<0.1 were considered statistically significant and become part of the logistic regression model.
Logistic regression model the behaviour of the variables is studied using a backward stepwise selection model. The variables selected to perform the study are chosen among the ones that showed statistical significance on the regression model.
| Variable forced introduction | Backward stepwise selection | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI95% | OR | CI95% | OR | |||||||||
| B | ET(B) | Sig. | OR | Inferior | Superior | B | ET(B) | Sig. | OR | Inferior | Superior | |
| Ulceration | 1.046 | 1.139 | 0.359 | 2.846 | 0.305 | 26.556 | 1.945 | 0.869 | 0.025 | 6.997 | 1.275 | 38.404 |
| Age≥82 | .277 | 0.092 | 0.003 | 1.32 | 1.101 | 1.581 | 1.418 | 0.801 | 0.077 | 4.127 | 0.859 | 1.834 |
Includes all variables that p<0.1 on bivariate analysis. χ2=24.41; gl=3; p≤0.001; R2L=0.287; R2Cox&Snell=0.288; R2Nagalkerke=0.415.
Many reports have found age-dependent prognostic factors on SLNB status but most randomized trials and major studies have excluded patients older than 70–75 years.5,6 Consequently, data on the feasibility and diagnostic accuracy of the method in this patient group are so far lacking. Also, there are some existing models to predict SN status in melanoma patients that combine clinicopathologic factors depending on the primary tumour, but they do not take age into account or are not specific for the elderly.7 It has been postulated that SLNB is a poor predictor of prognosis in older patients because its positivity declines with increasing age.8 In a large study (n=858), the frequency of sentinel node (SN) metastases decreased with increasing age from 18 to 70 years, despite an increase in other poor prognostic factors. Whether this represents a lower sensitivity of the procedure or a different biological behaviour of melanomas in older patients remains unanswered.9 In our cohort, positivity was 11 points higher in the ≥75-year-old group (29.3% vs 18.3%).
Patients in the ≥75-year-old group, with ulcerated melanoma, older than 82 years-old have the higher likelihood of positivity in SLNB in our cohort. The present study focuses on elderly patients aged 75 years or older. As it is retrospective, bias may exist. The study confirms that SLNB might be recommended in the elder safely. Although SLNB remains controversial due to its lack of impact on survival, it is still the best staging system for micrometastases. It is the authors opinion that it should be offered in elderly patients.
Conflict of interestThe authors declare they have no conflict of interest.
The authors are grateful to AM for the statistics and MGG for the correction of English language.