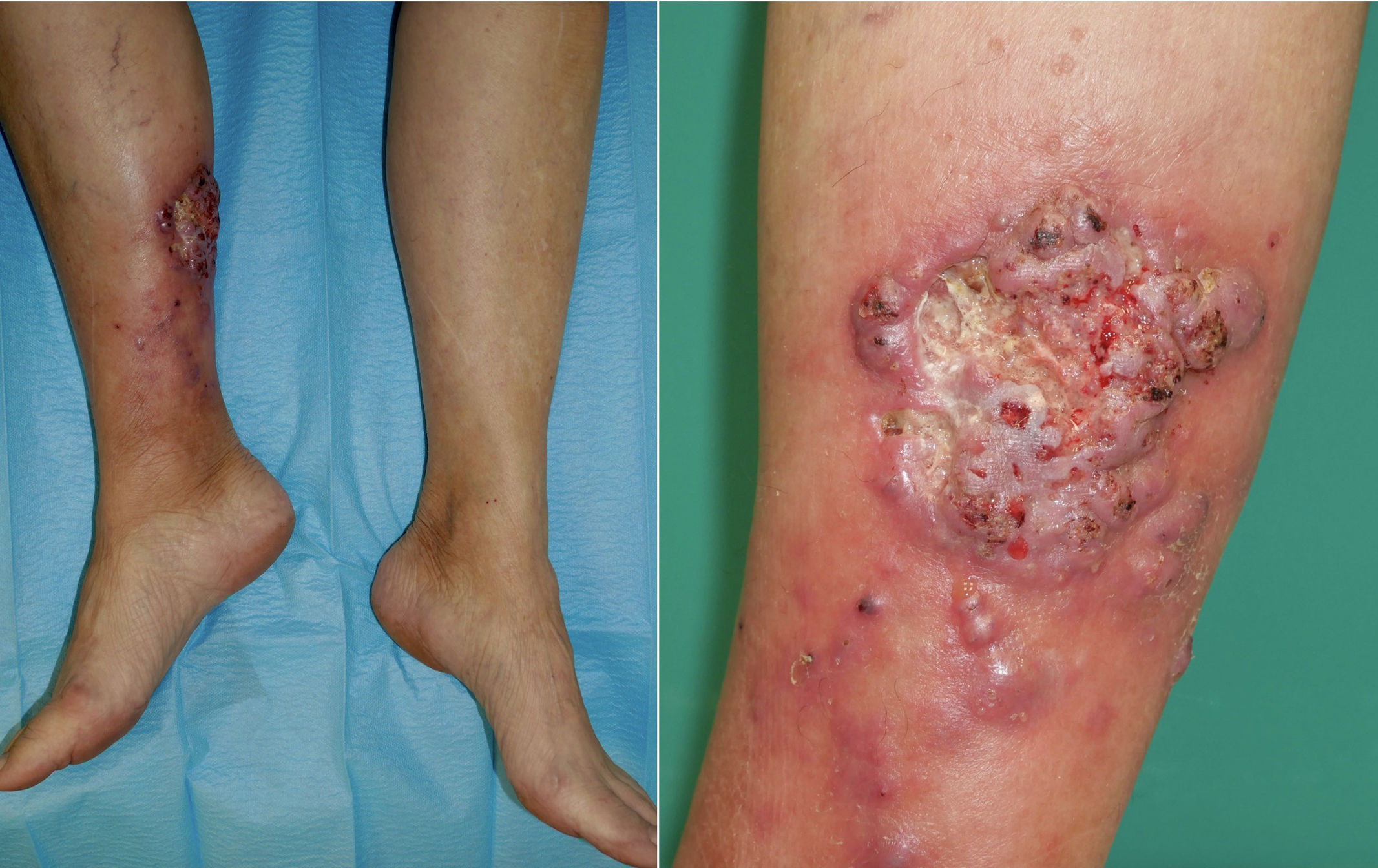
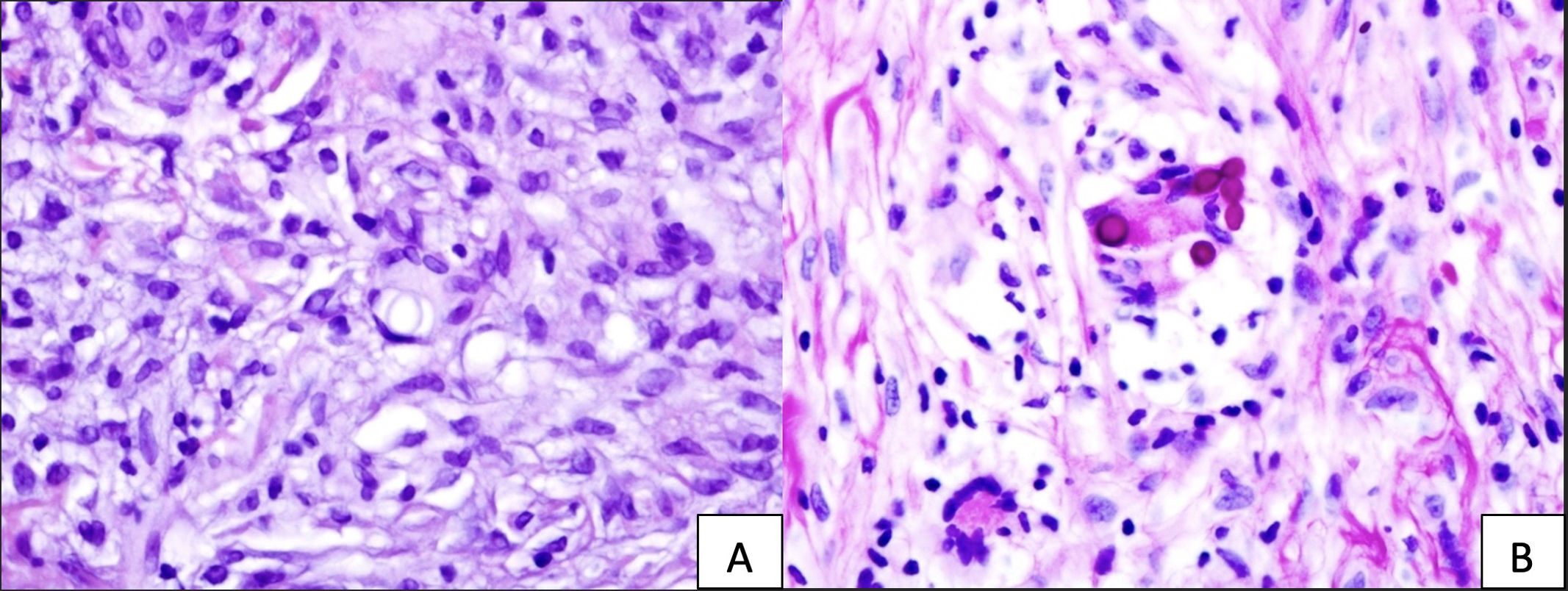
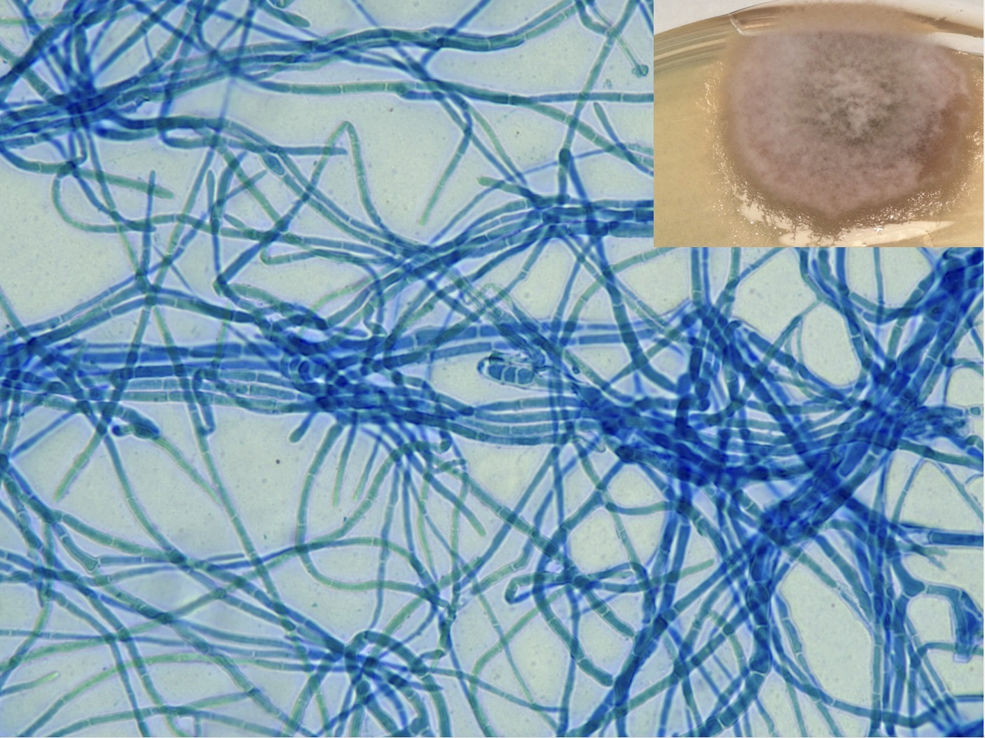
A 69-year-old woman who underwent kidney transplantation in 2014 and on triple immunosuppressive therapy [prednisone (5mg/day), mycophenolic acid (180mg b.i.d), and tacrolimus (8mg/day)] sought medical attention with a 3-month history of a progressively evolving cutaneous lesion on her right lower extremity. Physical examination revealed the presence of a 10cm×7cm exophytic skin plaque characterized by poorly defined and mamillated borders, violaceus nodules in the surrounding region following a sporotrichoid lymphocutaneous pattern, and ulceration with a fibrinous base at the center. The lesion exhibited induration and adherence to deeper margins, with concomitant serous and serosanguinous suppuration (Fig. 1). The patient denied pain or systemic symptoms, and no inguinal lymphadenopathy was detected. She acknowledged occasional insulin injections in the affected area and no epidemiological context was identified. Over the course of 3 months, the lesion underwent therapeutic interventions with topical and systemic antibiotics and corticosteroids, yielding no improvement. Histopathologic examination revealed pseudoepitheliomatous hyperplasia and granulomas within the upper and medium dermis. Transparent oval structures with a double membrane were detected and Periodic acid-Schiff diastase (PAS-D) was performed (Fig. 2). A culture was performed on Sabouraud-Chloramphenicol medium, followed by microscopic evaluation using lactophenol blue staining (Fig. 3).
A 10cm×7cm plaque, exophytic with ill-defined borders, along with violaceous nodules in the surrounding area. The center of the plaque reveals the presence of an ulcer with a fibrin background, surrounded by vegetative borders, mamillated edges and friable tissue. The lesion exhibited induration and adherence to deeper layers, with serohematic suppuration.
(A) Hematoxylin and eosin staining displays conspicuous oval, transparent structures with a distinct double membrane at the center. (B) PAS-D staining highlights the affirmative response of these structures within a multinucleated cell, situated at the image focal point and enclosed by a well-demarcated membrane.
Sabouraud-Chloramphenicol medium petri dish, an ash-colored fluffy colony featuring a faint perimeter and a black undersurface. The microscopic evaluation in lactophenol blue staining revealed dark septate hyphae. The image exhibits conidia with transverse septa positioned at the core of the image.
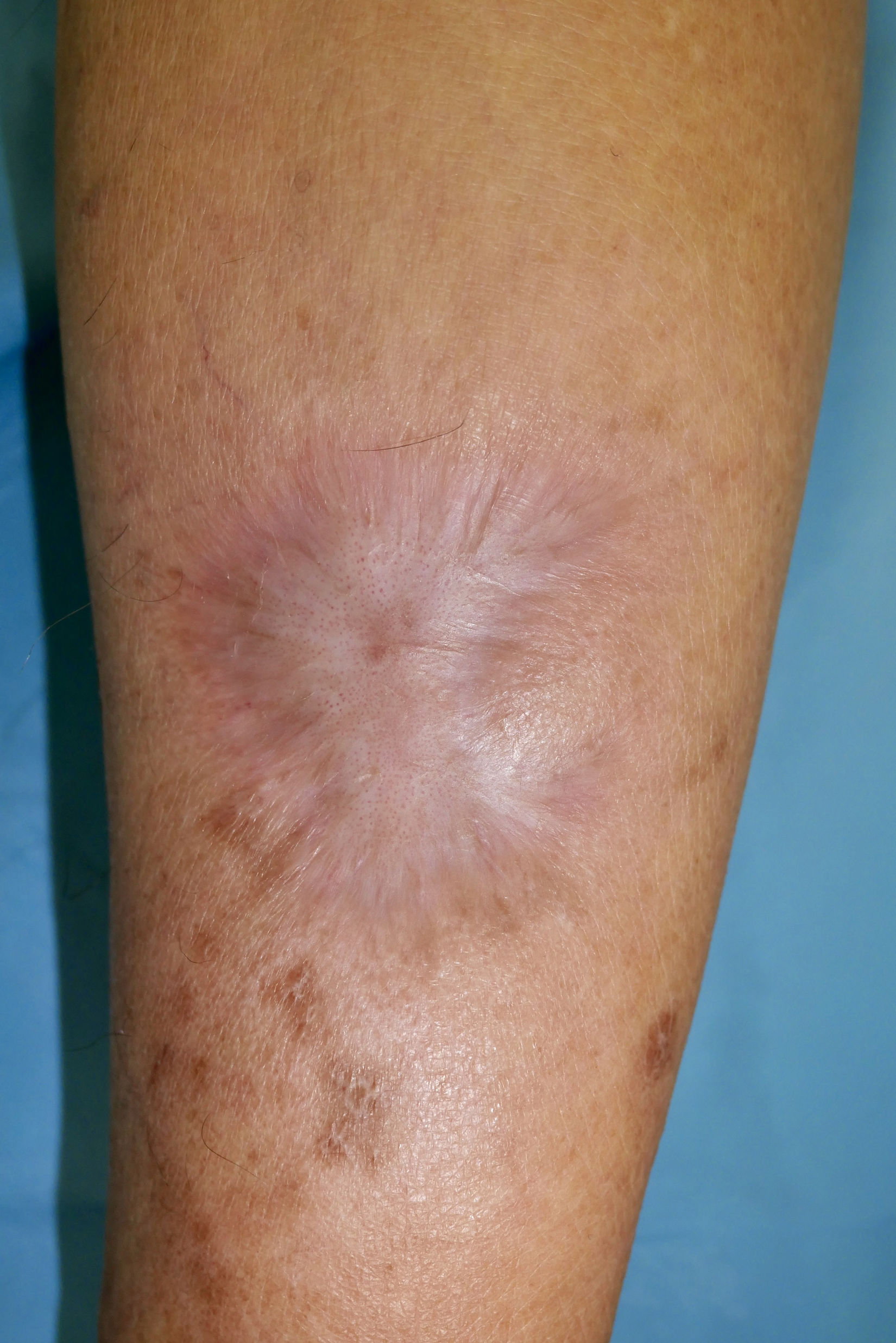
The fungal culture isolated a dematiaceous fungus consistent with Alternaria species (Fig. 3). DNA sequencing confirmed Alternaria infectoria as the causative agent and a whole-body PET-SCAN excluded invasive fungal dissemination. Treatment included aggressive surgical resection followed by a 2-month regimen of amphotericin B followed by oral voriconazole. Transient renal impairment and slightly higher transaminase levels were detected during amphotericin therapy, both of which resolved upon treatment completion. The patient remains asymptomatic after 6 months of diligent follow-up (Fig. 4).
Phaeohyphomycosis (PHM), a term introduced by Ajello et al. in 1974 refers to fungal infections distinguished by darkly pigmented hyphal structures.1 A limited number of clinical forms of PHM have been documented in the literature, with Alternaria species being rare causative agents.2 This widespread dematiaceous mold has increasingly been identified as an opportunistic pathogen, causing cutaneous and subcutaneous infections particularly in immunocompromised individuals.2–4 Such infections typically manifest as isolated lesions on extremities, often following a traumatic event.2,3,5A. infectoria infections are commonly described in solid organ recipients, especially those with kidney transplantation.3 However, a sporotrichoid lymphocutaneous pattern is a rare finding,6 leading to a wide range of differential diagnosis. Prior to the biopsy, immunocompromised individuals with this rare pattern usually encounter misdiagnoses, often linked to infectious origins such as atypical mycobacteria or neoproliferative processes involving in-transit metastases or lymphoproliferative conditions such as type B lymphomas. These misdiagnoses can subsequently lead to delayed implementation of appropriate infection management strategies. Histopathological findings vary depending on the stage of the lesion; early lesions (<3-month history) usually show epidermal changes, dermal mixed inflammatory infiltrate, and fungal structures within histiocytes, appearing as round-to-oval, thick refractile-walled hyphae.2,4 In contrast, advanced lesions (>3-month history) typically demonstrate prominent granulomatous inflammation in deeper skin layers.2,4 Biopsy usually shows the presence of fungal structures characterized by round-to-oval shapes and thick refractile walls.2 Microbiological diagnosis in Alternaria infections has gained importance due to the emergence of novel species being A. infectoria the most common species identified in recent studies.3,4 Accurate diagnosis is pivotal to guide antifungal treatment, optimize patient outcomes avoiding ineffective interventions, and predict the best drug for treatment purposes.3,4,7,8 Management involves immunosuppression reduction, excisional surgery, and a 3–6-month regimen of extended antifungal therapy, primarily involving azoles.8 As the number of cases continues to rise, additional studies are needed to establish the most effective management approach for this susceptible, immunosuppressed population.3
Although, in transplant recipients, A. infectoria infections are well-documented, the sporotrichoid lymphocutaneous pattern is still a rare finding. Early identification of these fungal infections, particularly in atypical presentations such as sporotrichoid lymphocutaneous is of paramount importance for optimal management in immunosuppressed individuals. This case highlights the crucial contribution of dermatologists in conducting meticulous differential diagnoses and fostering early clinical suspicion. Moreover, it underscores the fundamental role of a rigorous diagnostic process, as well as the imperative of individualized antifungal treatments to ensure both appropriateness and efficacy in management.
Conflict of interestThe authors declare that they have no conflict of interest.
We sincerely wish to thank Alba Romero Caballero, Esther Sanchis Vidal, Anabel Fernández Navarro, Marc Blasi Brugue, Ariadna Quer Pi-Sunyer, and Inés Perezpayá Alonso for their invaluable contributions in image acquisition and the development of this article.