Polymorphic light eruption (PLE) is a common idiopathic photodermatosis that typically presents with pruritic papular or papulovesicular lesions on sun-exposed skin between spring and autumn. In many subjects PLE is mild, and can usually be prevented by the use of broad-spectrum topical sunscreens and a gradual increase in sunlight exposure. However, in some individuals, sunlight exposure results in florid PLE and they often benefit from prophylactic desensitization treatment using phototherapy in early spring, an artificial method that induces a “hardening” phenomenon.
ObjectiveTo describe and evaluate the efficacy of a short desensitization protocol, based on a one-month-treatment, administered twice a week with narrow band UVB in subjects with severe polymorphic light eruption (PLE).
MethodsA retrospective, open planned and non-randomized study to assess the efficacy of UVB phototherapy in prevention of polymorphic light eruption.
ResultsFifteen subjects diagnosed with severe PLE were treated with the standard protocol in our Photobiology Unit between 2014 and 2015. The effect of hardening was sustained during follow up in 87.5% of desensitization treatments. A statistically significant association (p<0.05) between the years of duration of the PLE and the response to treatment was found.
ConclusionsThe effect of hardening was maintained in the vast majority of subjects, obtaining a good benefit with no PLE episodes during all the summer. We demonstrate that our standard protocol is effective, and produces a successful outcome for the majority of PLE subjects. Our protocol is shorter than those currently applied, being favourable both for the patient and the physician.
La erupción polimorfa lumínica (EPL) es una fotodermatosis idiopática que se presenta típicamente en forma de lesiones papulares o pápulo-vesiculosas pruriginosas en áreas fotoexpuestas, típicamente entre primavera y otoño. En la mayoría de pacientes la EPL es leve, y se previene mediante el uso de fotoprotectores y una exposición gradual a la luz solar. En algunos casos la EPL es muy florida, y requiere una desensibilización profiláctica en primavera, que induce fenómeno de hardening.
ObjetivoDescribir y evaluar la eficacia de un protocolo de desensibilización que se basa en la administración de UVB de banda estrecha, 2 veces a la semana, durante un mes.
ResultadosSe trataron un total de 15 sujetos con el protocolo de desensibilización entre los años 2014 y 2015. Se realizaron un total de 24 tratamientos. El efecto hardening se mantuvo en el 87,5% de los casos tratados. Se encontró una asociación estadísticamente significativa (p<0,05) entre los años de progresión de la enfermedad y la respuesta al tratamiento.
ConclusionesLos efectos del hardening se mantuvieron en la mayoría de los sujetos, los cuales presentaron un buen control de la EPL y ausencia de brotes durante el verano. Se demuestra la efectividad del protocolo de desensibilización en los sujetos con EPL, el cual tiene una duración más corta que los previamente descritos en la literatura.
Polymorphic light eruption (PLE) is a common idiopathic photodermatosis that typically presents with pruritic papular or papulovesicular lesions on sun-exposed skin between spring and autumn. The prevalence is higher in females (ratio 2:1) and usually onsets in the first 3 decades of life.1–4 The cause of PLE is not yet well understood. It is thought to be due to an imbalance between the immunosuppressive and stimulative effects of UV radiation in favour of the latter, that could give rise to delayed-type hypersensitivity reaction to photoinduced endogenous neoallergens. Some cases of PLE caused by UVC have been recently described.5 Diagnosis of PLE is based on patient history, morphology of the lesions and results of phototesting.6
In many subjects PLE is mild, and can usually be prevented by the use of broad-spectrum topical sunscreens and a gradual increase in sunlight exposure. However, in some individuals, sunlight exposure results in florid PLE and they often benefit from prophylactic desensitization treatment using phototherapy in early spring, an artificial method that induces a “hardening” phenomenon.1,7,8 The mechanism of action of phototherapy in the desensitization process is not fully understood but it seems to be the result of hyperpigmentation, epidermal hyperplasia, thickening of stratum corneum, immunosuppression, an increase in the number of regulatory T cells,9 and a gradual exposure to the main trigger of PLE.10–12
Previous studies have demonstrated that the use of narrow band ultraviolet B phototherapy (NB-UVB, TL-01, 311±2nm bandwidth) is as effective as psoralen plus ultraviolet (UV) A (PUVA) photochemotherapy in the induction of skin tolerance to sunlight in PLE subjects.7,13–15 In some centres desensitization with narrowband UVB (NB-UVB) has gradually replaced PUVA, becoming the treatment of choice.1,10,15 Advantages of UVB include absence of psoralen and its associated gastrointestinal upset, avoidance of wearing photoprotective glasses in the post-treatment period, the ability to be used in children and pregnancy, and the supposed lower carcinogenic potential of UVB irradiation.10,15,16 Nevertheless treatment regimens vary greatly between centres,17 and a standardized therapy has still not been implemented.10
In this study we describe a short desensitization protocol based on a four-week treatment with narrow band UVB (NB-UVB, TL-01, 311±2nm bandwidth) that may guide other physicians intending to use these forms of therapy.
Materials and methodsSubjectsInclusion criteria were consecutive subjects 18 years or older diagnosed of PLE between 2014 and 2015 which reported benefit with natural hardening and who accepted the 2 days per week regime during one month period.
The diagnosis of PLE had been previously established by clinical history, phototesting and laboratory tests (including antinuclear antibodies (ANAs) and porphyrins to rule out lupus erythematous (LE), erythropoietic protoporphyria (EPP) and other sun induced skin diseases). None of the subjects were taking photosensitizing drugs at the time of diagnosis. All subjects presented frequent episodes of PLE and had not benefited from the use of broad-spectrum sunscreens, oral beta-carotene or antihistaminic drugs. Sex, age, clinical presentation, time since the onset of the disease, serum antinuclear antibodies and vitamin D levels, and the result of phototest were registered in our database. The result of the phototest was classified between normal or pathologic, defined as a decreased Minimal Erythema Dose (MED) to UVB or an abnormal UVA reaction.18
Study designThis is a retrospective, open planned and non-randomized study to assess the efficacy of UVB phototherapy in prevention of polymorphic light eruption.
PhototestPhototesting was performed using a template on uninvolved skin on the patient's back, and subsequent exposure to different doses of UVA and UVB (Waldmann® UVA 700L and UV 801 BL). Each patient was evaluated 24h later for the development of erythema. The minimal erythema dose (MED) was defined as the lowest dose of UVB, or UVA, that produced perceptible erythema, covering the entire irradiated area.18 Photoprovocation was not performed.
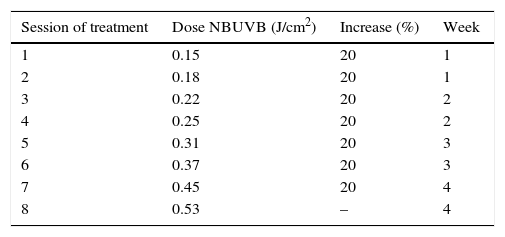
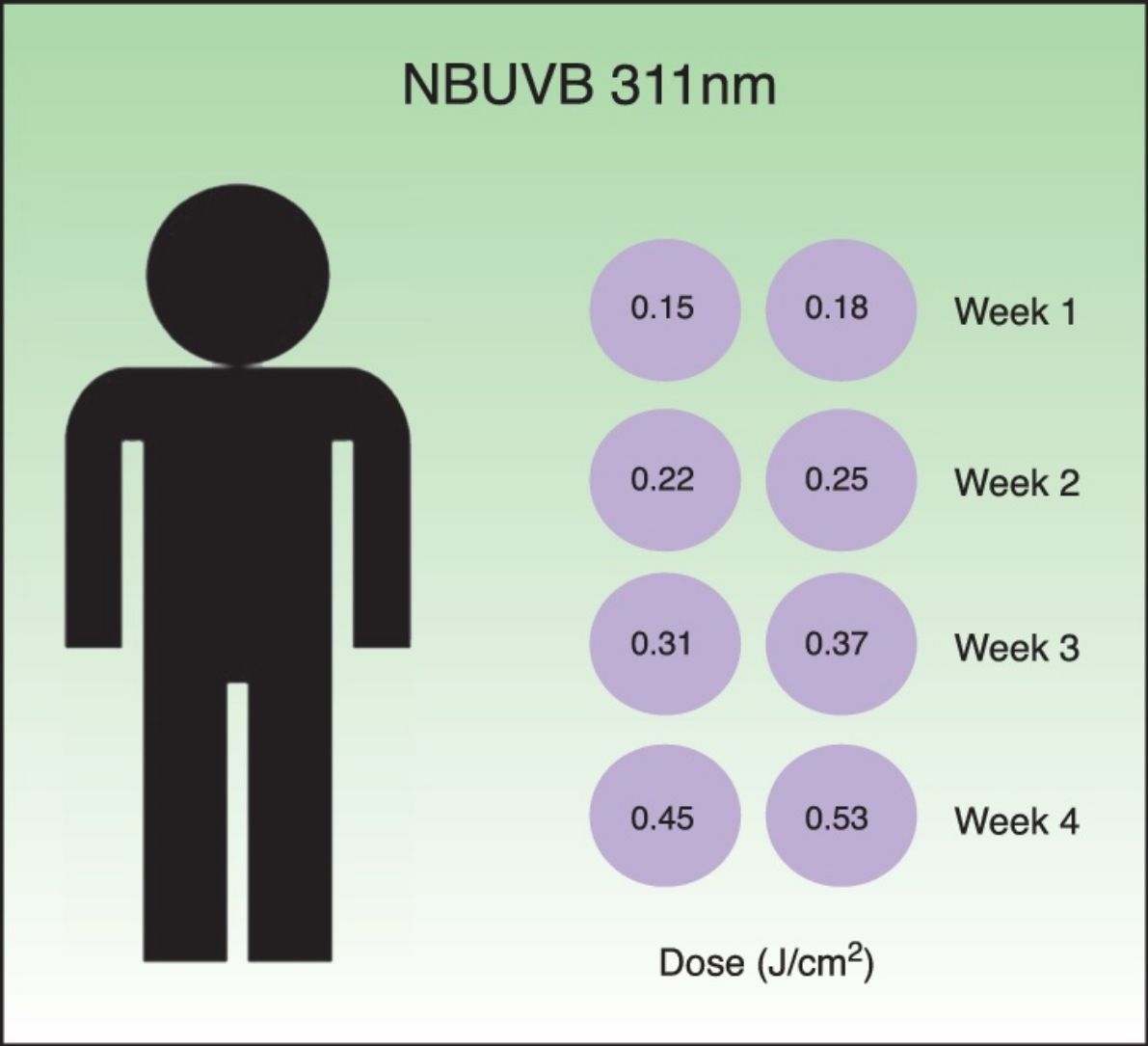
Desensitization treatmentDesensitization treatment courses were offered to each subject once a year in spring in 2014 and in 2015. All subjects were whole body irradiated with Waldmann® UV 7001K light stand calibrated annually. The standard treatment protocol for narrowband UVB (TL-01) is shown in Table 1. The starting dose was 0.15J/cm2. This was followed by a 20% incremental dosage, twice a week, during four weeks. The total number of sessions was eight and the final dose achieved was 0.53J/cm2. Each course of treatment was carefully documented including the total number of treatment sessions administered and the frequency of PLE flares. If PLE was provoked, oral corticosteroids were administered without discontinuation of the treatment.
Standard treatment protocol for NBUVB (311±2nm). The starting dose was 0.15J/cm2 followed by a 20% incremental dosage. The total number of courses of treatment was eight and the final dose was 0.53J/cm2. The treatment was administered twice a week, during four weeks.
| Session of treatment | Dose NBUVB (J/cm2) | Increase (%) | Week |
|---|---|---|---|
| 1 | 0.15 | 20 | 1 |
| 2 | 0.18 | 20 | 1 |
| 3 | 0.22 | 20 | 2 |
| 4 | 0.25 | 20 | 2 |
| 5 | 0.31 | 20 | 3 |
| 6 | 0.37 | 20 | 3 |
| 7 | 0.45 | 20 | 4 |
| 8 | 0.53 | – | 4 |
Subjects were told to keep exposing themselves to sunlight through over the summer after the desensitization protocol. As the photo-protective effects of desensitization treatments are temporary with a complete loss of the hardening phenomenon once subjects are no longer exposed to sunlight or the intensity of sunlight decreases (e.g. winter); we considered each treatment course as an independent one, and did not perform a separate analysis of first and second course. Treatment courses in different years were considered not cumulative and were analyzed separately.
Follow upSubjects were followed up both after the end of the desensitization treatment and at the beginning of autumn. Each subject's response to treatment during the subsequent months was tabulated and analyzed. Subjects were asked whether they have had flares during the summer, considering complete responders those who reported from 0 to 1 flare and partial responders those who reported 2 flares. Subjects who had more than 2 flares were considered non-responders. Taking extra treatment during the summer such as oral beta-carotene or antihistaminic drugs was also registered.
Statistical analysesStatistical analyses were performed with SPSS (version 20.0; SPSS Inc., Chicago, IL). Descriptive analysis of the sample was performed, including percentages for categorical variables, and mean, minimum, maximum and standard deviation values for continuous variables. Comparisons of continuous variable means were performed using Student's exact t-test when variables followed a normal distribution. Comparisons of discrete variable means were performed using the Mann–Whitney non-parametric test. Comparisons between category variables were performed with tests and Fisher corrections were performed when required.
ResultsSubjectsBetween 2014 and 2015, 16 subjects with the diagnosis of polymorphic light eruption (PLE) were seen at our Photobiology Unit fulfilled the inclusion criteria for desensitization and agreed to complete the treatment protocol. Desensitization treatment with narrow band UVB (NBUVB) was offered to 15 subjects. The remaining one was not finally included because of personal history of melanoma.
Regarding sex distribution, there were 4 men (26.7%) and 11 women (73.3%). The mean age was 42.4 years (range 16–62). All of them had decreased serum levels of 25-hydroxyvitamin-D3 (<25ng/ml) in blood tests.
Sixty-six per cent of subjects were found to be ANA positive, but only 3 of them had levels >1:160. However, none of them met the diagnostic criteria for lupus erythematous (LE) of the American College of Rheumatology.19
The mean duration of PLE at presentation was 6.13 years (range, 1–15 years). There were no differences between the duration of the disease and the age and sex of the subjects (p>0.05).
Phototesting revealed that the minimal erythema dose for UVB was decreased in 64.8% of subjects tested while the remaining 35.7% fell within normal limits.18 The result of phototesting did not show differences (p>0.05) between age and sex of subjects.
Treatment coursesNine subjects were treated both in 2014 and in 2015 and 6 started phototherapy in 2015. Therefore, a total of 24 desensitization treatment courses were performed between 2014 and 2015. All treatment courses were administered in spring: 8.3% started in March, 50% in April, 33.3% in May and 8.3% in June. All subjects completed their desensitization courses.
Polymorphous light eruption (PLE) was provoked in 8 treatment courses (33.3%). All of the induced PLE were mild, and only 3 of them (12.5%) required the administration of 20mg of oral prednisone in decreasing doses during 10 days. The induced flares resolved in all subjects, and the interruption of the desensitization treatment course was not required in any case.
Response/Follow upThe evaluation of response was performed both after finishing the desensitization treatment and at the beginning of autumn. The effect of hardening was sustained during the follow up after 21 desensitization treatment courses, as subjects reported a complete remission of symptoms during all the sunny Spanish summer. The remaining 3 desensitization treatment courses were ineffective and subjects continued having disease outbreaks. The nine subjects, who received 2 desensitization treatment courses, were complete responders both in 2014 and 2015.
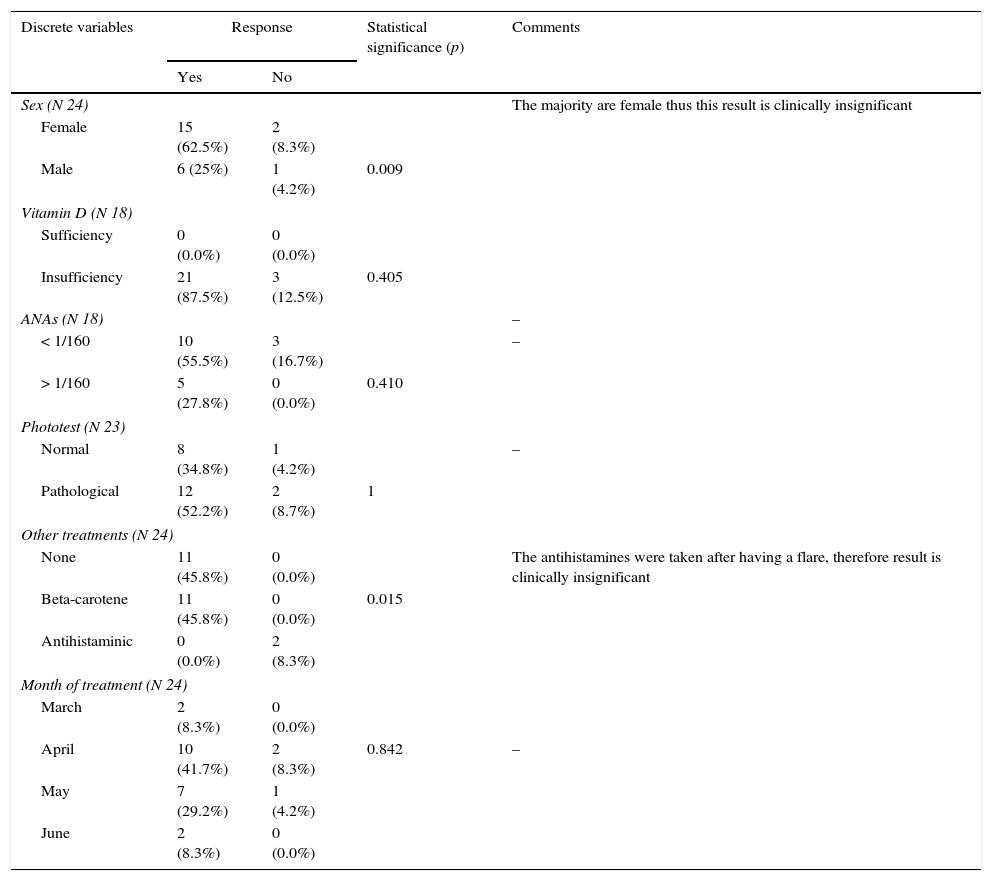
There was no association between the maintained response to the desensitization treatment and demographic characteristics of the subjects. No association was found either between response and serum levels of 25-hydroxyvitamin-D3, positive antinuclear antibodies (ANAs) in laboratory tests or the result of previous subjects’ phototest (Table 2). Female sex and low vitamin D levels were associated with a better response although this could not be considered statistically significant due to the high percentage of female subjects and the suboptimal vitamin D levels in all the cases.
Response to treatment regarding discrete variables: sex, levels of vitamin D, ANAs, result of phototest, month of treatment and medication taken after desensitization treatment.
| Discrete variables | Response | Statistical significance (p) | Comments | |
|---|---|---|---|---|
| Yes | No | |||
| Sex (N 24) | The majority are female thus this result is clinically insignificant | |||
| Female | 15 (62.5%) | 2 (8.3%) | ||
| Male | 6 (25%) | 1 (4.2%) | 0.009 | |
| Vitamin D (N 18) | ||||
| Sufficiency | 0 (0.0%) | 0 (0.0%) | ||
| Insufficiency | 21 (87.5%) | 3 (12.5%) | 0.405 | |
| ANAs (N 18) | – | |||
| < 1/160 | 10 (55.5%) | 3 (16.7%) | – | |
| > 1/160 | 5 (27.8%) | 0 (0.0%) | 0.410 | |
| Phototest (N 23) | ||||
| Normal | 8 (34.8%) | 1 (4.2%) | – | |
| Pathological | 12 (52.2%) | 2 (8.7%) | 1 | |
| Other treatments (N 24) | ||||
| None | 11 (45.8%) | 0 (0.0%) | The antihistamines were taken after having a flare, therefore result is clinically insignificant | |
| Beta-carotene | 11 (45.8%) | 0 (0.0%) | 0.015 | |
| Antihistaminic | 0 (0.0%) | 2 (8.3%) | ||
| Month of treatment (N 24) | ||||
| March | 2 (8.3%) | 0 (0.0%) | ||
| April | 10 (41.7%) | 2 (8.3%) | 0.842 | – |
| May | 7 (29.2%) | 1 (4.2%) | ||
| June | 2 (8.3%) | 0 (0.0%) | ||
Pearson Chi square. Fisher's exact test was performed when required.
We found a statistically significant association (p<0.05) between the years of progression of the polymorphous light eruption (PLE) at presentation and the complete remission of symptoms after the desensitization treatment (Table 3). The mean time from when the PLE began was 5.9 years (std. deviation 4.318) in the 87.5% of subjects that presented a sustained response during the summer, while the mean time was 8 years (std. deviation 6.245) in those who did not respond.
Response to treatment regarding time of evolution. An association between the time since the onset of PLE and the response to the desensitization treatment was found, being better responders those who had a short lasting disease.
| Continuous variables | Response | Statistical significance (p) | |
|---|---|---|---|
| Yes | No | ||
| Time of evolution (years) | 5.9 std. deviation 4.318 | 8.0 std. deviation 6.245 | <0.001 |
T-test.
With respect to the year's period of treatment, there were no differences (p>0.05) between the sustained response to the desensitization and the month in which photo hardening was performed.
Nearly half of the cases (45.8%) spent the summer without need for any other treatment. In 11 treatment courses (45.8%) subjects attempted to strengthen the response with oral beta-carotene, and only in 2 cases (8.4%) antihistaminic drugs were taken. However, there were no differences (p>0.05) between the maintained response to photohardening and the use of extra treatments after the desensitization course.
DiscussionIn accordance with what has been reported in the literature, in our study PLE was predominantly affecting females. The mean age of onset was 42.4 years, slightly higher than previously described.2,20,21
Consistent with what Gruber-Wackernagel et al. defined,22 our PLE subjects had low 25(OH) vitamin D serum levels, probably related to the avoidance of sun exposure. It has been reported that 311nm UVB phototherapy increases those levels, and that boosting levels of vitamin D may be important in ameliorating PLE.22,23
None of the ANA-positive subjects had or developed systemic lupus erythematous during follow-up. Previous studies have shown elevated levels of antinuclear antibodies (ANA) in subjects with polymorphic light eruption; however, PLE is a benign disease without tendency to progress to LE.24,25
In regard to phototest results in PLE subjects, data are diverse in the literature. Phototesting is useful to determine the responsible ultraviolet action spectrum and to exclude differential diagnoses like photosensitive eczema, lupus erythematous or chronic actinic dermatitis. It is reported that the majority of subjects with PLE have results that fell within normal limits when it refers to UVA and UVB MED determination.1,6,18,21,23,26–28 However, in our series 64.8% of the subjects had decreased UVB MED, a higher percentage compared to what has been previously described. In accordance with our results, phototest and photoprovocation showed no significant relationship with clinical disease severity and response in other series.28
When discussing a desensitization course in a PLE patient, it is important to highlight that each patient will respond differently, and treatment is almost always individualized.17 However, we are of the opinion that a standard treatment course should be implemented, in order to facilitate the management of PLE subjects.
Despite its widespread use, treatment regimes vary greatly between health clinics, and in most centres, narrowband UVB has gradually replaced broadband UVB and PUVA and is now considered the treatment of choice.
In this paper we report our experience in PLE subjects being treated with our standard protocol, based on narrowband UVB (TL-01) phototherapy, starting with 0.15J/cm2 followed by a 20% incremental dosage, until 0.53J/cm2 achieving a total number of 8 sessions administered twice a week, during four weeks.
The effect of hardening was sustained in the vast majority of our subjects, obtaining a good benefit with no PLE episodes during all the summer. With these results, we demonstrate that this standard protocol is effective, and produces a successful outcome for the majority of PLE subjects.
Moreover, we found a direct association between the time from the onset of PLE and the response to the desensitization treatment, being better responders those who had a short lasting disease. Therefore, it seems that treatment should be administrated as soon as the broad-spectrum topical sunscreens and the gradual increase in sunlight exposure fail to control the PLE symptoms in order to achieve better results.
Although PLE may be provoked during desensitization and subjects must be forewarned, it should not prevent them from continuing treatment, as acute flares can be treated with oral corticosteroids, and it is not predictive for poor outcome.
Contrary to what was believed,18,29,30 we found no significant results regarding the maintained response to photohardening and the use of beta-carotene or oral antihistaminic after the desensitization course. Nearly half of our subjects could spend the whole summer free of symptoms without any extra treatment, which implies an increase in their quality of life.
The protocol that we propose is shorter than those currently applied, being favourable both for the patient and the physician. Thereby, treatment adherence will probably increase and the cost of the treatment might be reduced.
Treatment needs to be repeated yearly in early spring, as the photoprotective effects of phototherapy are temporary. There appears to be no loss of benefit with subsequent courses.
LimitationsImportant limitations of studies like this include the small numbers of subjects due to the low prevalence of the disease, and a study design that is not double-blinded and placebo controlled.
Conflict of interestThe authors declare that they have no conflicts of interest.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
We would like to thank the nursing staff for their contribution in the management of the subjects in this study.