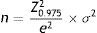To date, no formal study has been published regarding how Colombian patients with skin disorders could be affected according to their perception of disease.
ObjectiveTo determine the impact in quality of life of skin diseases in a Colombian population.
MethodsThis multicenter study included patients with skin disease from almost the whole country. Individuals >18 years old; of any gender; with any skin disease and who signed informed consent, were included. We applied the Colombian validated version of the Skindex-29 instrument.
ResultsA total of 1896 questionnaires had sufficient information for the analyses. No significant differences in sociodemographic characteristics of patients who returned the questionnaire incomplete vs. complete, were found. Participants mean age was 41.5 years. There were no statistical differences in men vs. women regarding the global (p=0.37), symptoms (p=0.71) and emotions (p=0.32) domains, whereas statistical differences were found in the function domain (p=0.04; Mann–Whitney U test). Psoriasis, contact dermatitis, atopic dermatitis, urticaria, hair disorders, Hansen's disease, scars, hyperhidrosis and genital human papillomavirus disease scored the highest.
LimitationsSkindex-29 score variability as a result of differences in the location of the skin lesions, their inflammatory or non-inflammatory nature, and the start of therapy.
ConclusionsEven the most localized or asymptomatic skin lesion in our population leads to a disruption at some level of patient's wellness. This study adds well supported scientific data of the burden of skin diseases worldwide.
En Colombia se carece de estudios que hayan evaluado formalmente el impacto de las enfermedades dermatológicas en la calidad de vida de los pacientes que las padecen.
ObjetivoDeterminar el impacto en la calidad de vida de las enfermedades cutáneas en una población colombiana.
MétodosEstudio multicéntrico que incluyó a individuos>18 años de edad; de cualquier sexo, con cualquier trastorno cutáneo y que firmaron el consentimiento informado. Se aplicó la versión validada en Colombia del instrumento Skindex-29.
ResultadosUn total de 1.896 cuestionarios se incluyeron en el análisis. No se observaron diferencias significativas en las características sociodemográficas entre los que devolvieron el cuestionario incompleto vs. completo. La edad promedio fue de 41,5 años. No hubo diferencias significativas entre hombres y mujeres con respecto al puntaje global del instrumento, ni de los dominios sintomático o emocional, mientras que sí las hubo en el dominio funcional. Entre las enfermedades que más afectaron la calidad de vida se incluyen: psoriasis, dermatitis de contacto, dermatitis atópica, urticaria, trastornos capilares, lepra, cicatrices, hiperhidrosis y las verrugas genitales.
LimitacionesLas puntuaciones del Skindex-29 mostraron una gran variabilidad explicable por diferencias en la localización de las lesiones de la piel, su naturaleza inflamatoria/no inflamatoria, y la iniciación o no del tratamiento.
ConclusionesCualquier lesión dermatológica por más localizada o asintomática que sea, condujo a una alteración en algún grado de la calidad de vida dermatológica. Este estudio añade soporte científico a la carga de enfermedad que generan los trastornos cutáneos en el mundo.
The importance of skin disease is often underestimated by its chronic nature and low mortality, but its frequency is so high that it has been determined that between 21% and 87% of the population may suffer from some type of skin disorder.1 Indeed, they constitute a quarter of consultations in primary health care due to its related significant physical and psychological impairment,1,2 as self-image is an essential element in the personality structure which not only affects mental health, but also the attitude toward the environment.3,4 In addition, it has been described that self-image is predictive of overall satisfaction and, therefore, has a major effect on human's quality of life (QOL)5 to a level in which a favorable self-image leads to positive emotions, while a unfavorable self-image leads to anxiety, fear, anger, depression and social maladjustment.6 Moreover, skin appearance is so important for the human being that previous work has shown that up to 50% of patients with severe eczema or 49% of psoriasis patients would be willing to employ two or more hours each day in their treatment if this would allow them to have a normal skin during the rest of the day.7,8
In the dermatology field, the search for a better QOL expressed in terms of a better skin perception or in the improvement of the image that we project to others has become more apparent in recent years in both women and men.9 Additionally, skin diseases can alter very relevant aspects such as sexuality, the performance of certain tasks or they can even lead to work inability.10
Among the skin diseases that mostly affect self-image and QOL (including those related to the visible areas of the skin), are acne vulgaris, vitiligo and hair loss,11–13 and also those that can affect the whole body and are also accompanied by itching or burning symptoms such as psoriasis or atopic dermatitis.7,14
Several instruments have been developed to measure QOL in dermatology.15–18 The Skindex-29 instrument was developed in the United States by Chren et al.18 It has been validated in the American population in whom it demonstrated good measurement properties, as it was found to be internally consistent (Cronbach's α=0.87–0.96) and replicable.19 The original version and the translated and adapted version in Spain of this instrument have both demonstrated construct and content validity as higher scores have been reported in patients with eczema and psoriasis than in those with isolated skin lesions.18,20,21 In addition, the Skindex-29 version from Spain and its Colombian version have also demonstrated construct validity and a high test–retest reliability.20–22
As skin disease perception and impact among patients could vary from one country to another, the aim of this study was to quantify such patient-reported effects in Colombian patients.
MethodsA cross-sectional, non-interventional study was performed at 7 main and 34 smaller cities in Colombia. Due to the descriptive nature of the study, data of the percentage of patients’ refusal to participate was not considered. Inclusion criteria corresponded to individuals older than 18 years, of any gender, and with any skin disease. Biopsy confirmation of skin disease was not mandatory. Eligible patients also had to express their willing to participate in the study and had to fill out an informed consent.
Exclusion criteria corresponded to patients with any mental and/or physical, psychological, or psychiatric disability that could interfere with skin disease perception or the self-reporting of the questionnaire.
In this study, no control group was planned for comparison, as the burden of skin diseases in our culture was previously demonstrated in the studies of cross cultural adaptation and validation of the instrument22,23 where such type of group was included.
Sociodemographic characteristics such as age; gender; education level; living place; time-lapse of skin disease (in months or years); socioeconomic classification; social security type, and personal history of comorbidities were included in the analyses.
In respect to socioeconomic classification, the study classified patients according to the National Administrative Department of Statistics (DANE),23 a Colombian stratification which has been based on public services payment related to housing type and its urban or rural environment. Such variables have been statistically proved by DANE to be a good surrogated marker for individual's or family income. Therefore, classification has been stratified as follows: Stratum 1: Low–Low; Stratum 2: Low; Stratum 3: Medium–Low; Stratum 4: Medium; Stratum 5: Medium–High; Stratum 6: High.23
Moreover, the study based patients’ social security classification according to the Colombian general social security health system,24 which operates in two main affiliation regimes: the contributory scheme (employees, independent workers) and the subsidized regime (poorest people). There is also a special regime which includes the military force, the police, all school teachers and the Colombian national petroleum company (Ecopetrol). Lastly, according to people's will and economic capacity, individuals can choose to pay for the provision of any health service (private patients).
InstrumentThe validated Colombian version of the Skindex-29©25 was applied to consecutive dermatology patients attending any outpatient clinic, any private practice, or any health institution in the country. Both the informed consent and the questionnaire were handled to each patient for self-response before consultation. Sociodemographic variables and diagnosis of skin condition were completed by the attending physician after the consultation.
The Colombian skindex-29 version contains 29 consecutive items, and as the original instrument, it covers 3 domains: symptoms, emotions and functioning. Each item is rated on a 5-point Likert scale (never, rarely, sometimes, often, at all times), and higher scores reflect greater QOL impairment.
The instrument was monitored in each health institution, clinic or office by a certified dermatologist whom was in charge of verifying data completeness.
Data analysesSample size was calculated according to the following equation based on the mean estimate:
where “e” was the maximum expected statistical error for this study (value: 3), a confidence interval of 95% and a standard deviation σ of 20.77 extracted from the overall score obtained in the validation process of the Colombian version of Skindex-29©.22 These calculations resulted in a sample size of 217 patients per institution or dermatologist, with an adjustment for a 15% patient's loss.To ensure the quality of the data, all the information collected on printed questionnaires were double-typed by a research assistant. For that purpose, the Microsoft Access® 2010 (Microsoft Corporation, Redmond, WA) software was used. Questionnaires with any missing value in the Skindex-29 scale were excluded.
All responses of the entire instrument were transformed to a linear scale ranging from 0 to 100. Normality of data was assessed using the Kolmogorov–Smirnov test. A univariate analysis was performed, and for qualitative variables absolute and relative frequencies were used, whereas quantitative variables were analyzed utilizing central tendency and dispersion measurements. Differences between proportions were assessed using the Chi2 test or Fisher exact test, as required.
The Kruskal–Wallis or Mann–Whitney U tests were used to determine whether there were QOL scores differences for groups by sociodemographic variables. Data analysis was performed with the SPSS software, version 19.0.
ResultsA total of 4340 questionnaires were sent to 29 Colombian dermatologists who agreed to participate as investigators and data monitors, and 2102 were returned. Of these, 206 were returned with incomplete data leaving a total of 1896 valid questionnaires for the analyses.
There were no significant differences in sociodemographic characteristics such as socioeconomic classification, level of education and gender when the group of individuals who returned the questionnaire incomplete when compared to the ones who completed the survey and returned it (p>0.13; Chi2 Test).
More women than men responded the questionnaire (65.9% vs. 34.1%, respectively) (Table 1), and the mean age of participants was 41.5 years (standard deviation: 16.8, range: 18–91 years). There were no statistical differences in the global (median score: 21.5 vs. 19.8; p=0.37), symptoms (median score: 24.5 vs. 24.9; p=0.71) and emotions (median score: 30 vs. 30; p=0.32) Skindex-29 domains when men and women were compared respectively, whereas statistical differences were found in the function domain (median score: 10.4 vs. 8.3; p=0.04; Kruskal–Wallis test) (Table 2).
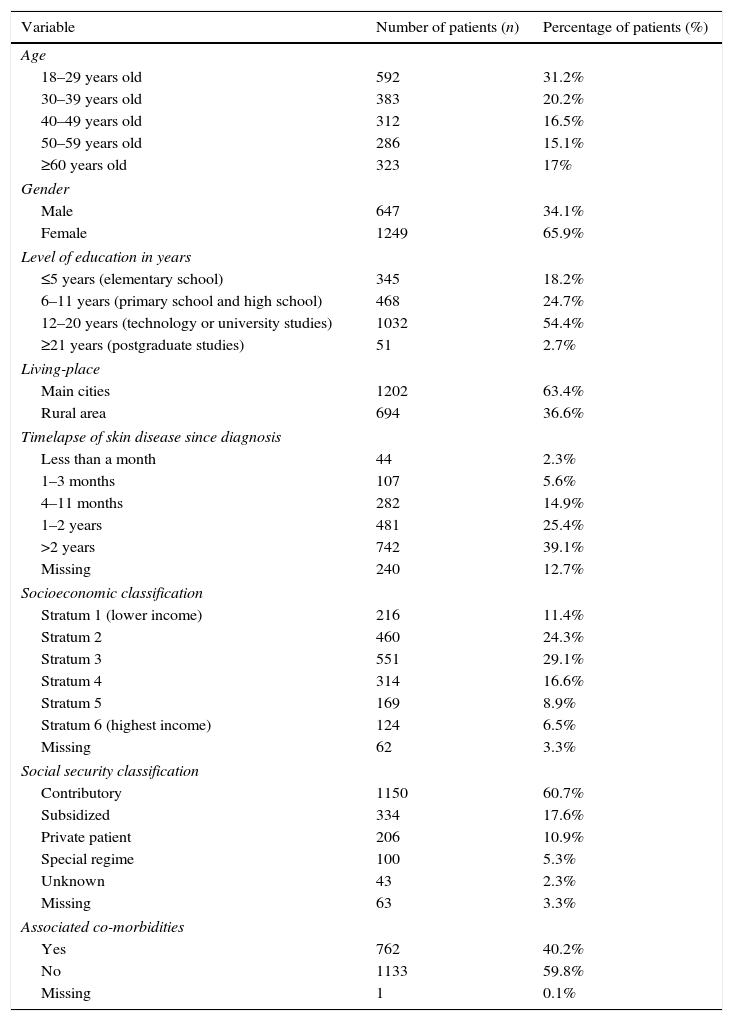
Sociodemographic characteristics of included patients.
| Variable | Number of patients (n) | Percentage of patients (%) |
|---|---|---|
| Age | ||
| 18–29 years old | 592 | 31.2% |
| 30–39 years old | 383 | 20.2% |
| 40–49 years old | 312 | 16.5% |
| 50–59 years old | 286 | 15.1% |
| ≥60 years old | 323 | 17% |
| Gender | ||
| Male | 647 | 34.1% |
| Female | 1249 | 65.9% |
| Level of education in years | ||
| ≤5 years (elementary school) | 345 | 18.2% |
| 6–11 years (primary school and high school) | 468 | 24.7% |
| 12–20 years (technology or university studies) | 1032 | 54.4% |
| ≥21 years (postgraduate studies) | 51 | 2.7% |
| Living-place | ||
| Main cities | 1202 | 63.4% |
| Rural area | 694 | 36.6% |
| Timelapse of skin disease since diagnosis | ||
| Less than a month | 44 | 2.3% |
| 1–3 months | 107 | 5.6% |
| 4–11 months | 282 | 14.9% |
| 1–2 years | 481 | 25.4% |
| >2 years | 742 | 39.1% |
| Missing | 240 | 12.7% |
| Socioeconomic classification | ||
| Stratum 1 (lower income) | 216 | 11.4% |
| Stratum 2 | 460 | 24.3% |
| Stratum 3 | 551 | 29.1% |
| Stratum 4 | 314 | 16.6% |
| Stratum 5 | 169 | 8.9% |
| Stratum 6 (highest income) | 124 | 6.5% |
| Missing | 62 | 3.3% |
| Social security classification | ||
| Contributory | 1150 | 60.7% |
| Subsidized | 334 | 17.6% |
| Private patient | 206 | 10.9% |
| Special regime | 100 | 5.3% |
| Unknown | 43 | 2.3% |
| Missing | 63 | 3.3% |
| Associated co-morbidities | ||
| Yes | 762 | 40.2% |
| No | 1133 | 59.8% |
| Missing | 1 | 0.1% |
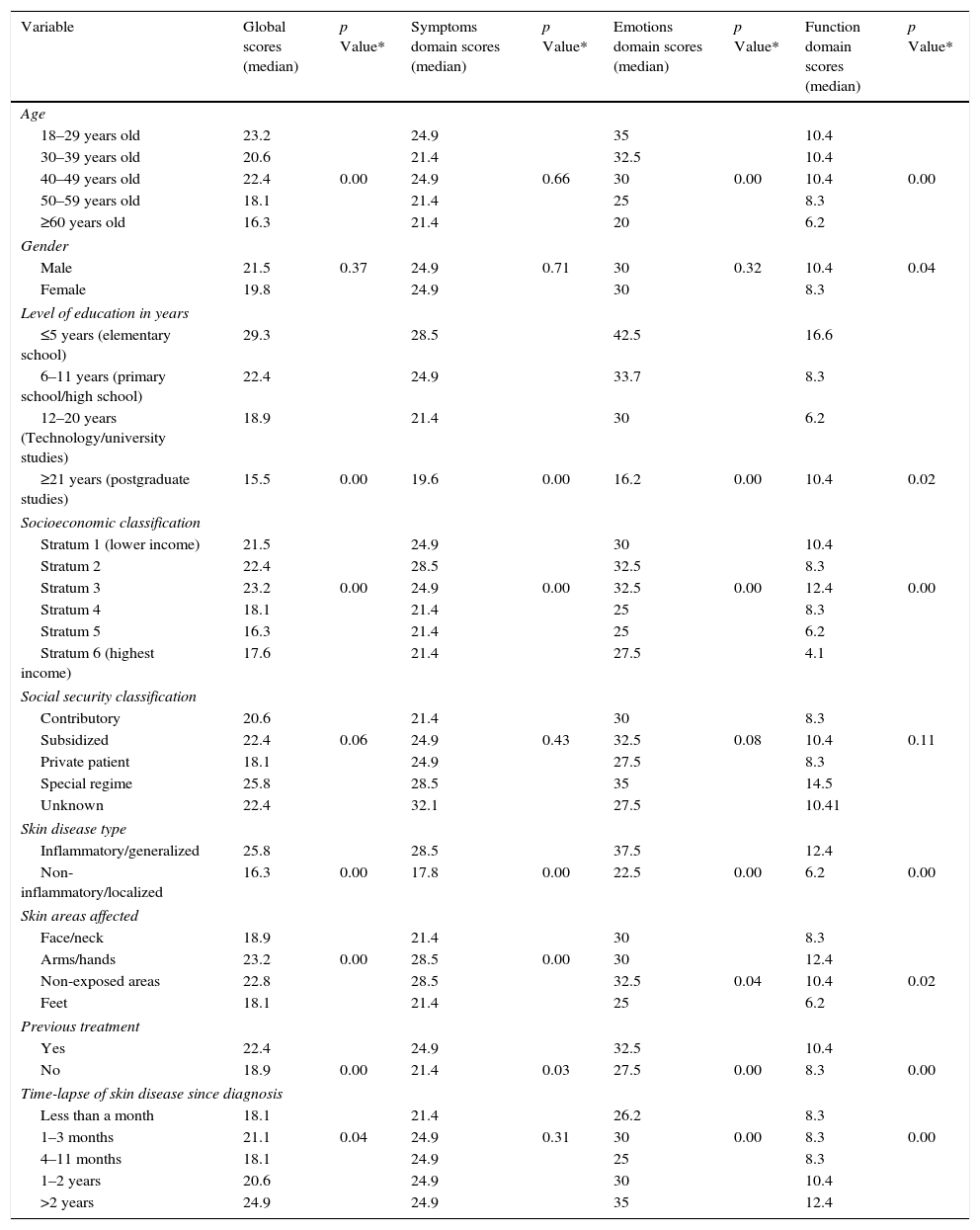
Median Skindex-29 scores according to gender, level of education, socioeconomic classification, social security classification, skin disease type, affected skin areas, previous treatment and timelapse of skin disease since diagnosis.
| Variable | Global scores (median) | p Value* | Symptoms domain scores (median) | p Value* | Emotions domain scores (median) | p Value* | Function domain scores (median) | p Value* |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| 18–29 years old | 23.2 | 24.9 | 35 | 10.4 | ||||
| 30–39 years old | 20.6 | 21.4 | 32.5 | 10.4 | ||||
| 40–49 years old | 22.4 | 0.00 | 24.9 | 0.66 | 30 | 0.00 | 10.4 | 0.00 |
| 50–59 years old | 18.1 | 21.4 | 25 | 8.3 | ||||
| ≥60 years old | 16.3 | 21.4 | 20 | 6.2 | ||||
| Gender | ||||||||
| Male | 21.5 | 0.37 | 24.9 | 0.71 | 30 | 0.32 | 10.4 | 0.04 |
| Female | 19.8 | 24.9 | 30 | 8.3 | ||||
| Level of education in years | ||||||||
| ≤5 years (elementary school) | 29.3 | 28.5 | 42.5 | 16.6 | ||||
| 6–11 years (primary school/high school) | 22.4 | 24.9 | 33.7 | 8.3 | ||||
| 12–20 years (Technology/university studies) | 18.9 | 21.4 | 30 | 6.2 | ||||
| ≥21 years (postgraduate studies) | 15.5 | 0.00 | 19.6 | 0.00 | 16.2 | 0.00 | 10.4 | 0.02 |
| Socioeconomic classification | ||||||||
| Stratum 1 (lower income) | 21.5 | 24.9 | 30 | 10.4 | ||||
| Stratum 2 | 22.4 | 28.5 | 32.5 | 8.3 | ||||
| Stratum 3 | 23.2 | 0.00 | 24.9 | 0.00 | 32.5 | 0.00 | 12.4 | 0.00 |
| Stratum 4 | 18.1 | 21.4 | 25 | 8.3 | ||||
| Stratum 5 | 16.3 | 21.4 | 25 | 6.2 | ||||
| Stratum 6 (highest income) | 17.6 | 21.4 | 27.5 | 4.1 | ||||
| Social security classification | ||||||||
| Contributory | 20.6 | 21.4 | 30 | 8.3 | ||||
| Subsidized | 22.4 | 0.06 | 24.9 | 0.43 | 32.5 | 0.08 | 10.4 | 0.11 |
| Private patient | 18.1 | 24.9 | 27.5 | 8.3 | ||||
| Special regime | 25.8 | 28.5 | 35 | 14.5 | ||||
| Unknown | 22.4 | 32.1 | 27.5 | 10.41 | ||||
| Skin disease type | ||||||||
| Inflammatory/generalized | 25.8 | 28.5 | 37.5 | 12.4 | ||||
| Non-inflammatory/localized | 16.3 | 0.00 | 17.8 | 0.00 | 22.5 | 0.00 | 6.2 | 0.00 |
| Skin areas affected | ||||||||
| Face/neck | 18.9 | 21.4 | 30 | 8.3 | ||||
| Arms/hands | 23.2 | 0.00 | 28.5 | 0.00 | 30 | 12.4 | ||
| Non-exposed areas | 22.8 | 28.5 | 32.5 | 0.04 | 10.4 | 0.02 | ||
| Feet | 18.1 | 21.4 | 25 | 6.2 | ||||
| Previous treatment | ||||||||
| Yes | 22.4 | 24.9 | 32.5 | 10.4 | ||||
| No | 18.9 | 0.00 | 21.4 | 0.03 | 27.5 | 0.00 | 8.3 | 0.00 |
| Time-lapse of skin disease since diagnosis | ||||||||
| Less than a month | 18.1 | 21.4 | 26.2 | 8.3 | ||||
| 1–3 months | 21.1 | 0.04 | 24.9 | 0.31 | 30 | 0.00 | 8.3 | 0.00 |
| 4–11 months | 18.1 | 24.9 | 25 | 8.3 | ||||
| 1–2 years | 20.6 | 24.9 | 30 | 10.4 | ||||
| >2 years | 24.9 | 24.9 | 35 | 12.4 | ||||
Furthermore, and regarding the age of participants, statistical differences were found in the global (p=0.00), function (p=0.001) and emotions (p=0.00) Skindex-29 domains, whereas non statistical differences were found only in the symptoms domain (p=0.66; Kruskal–Wallis test) (Table 2).
As described in Table 1, more than half of the population was under 39 years old (51.4%) and with some level of education (81.8%), although with a lower socioeconomic (SE) classification (64.8%). Statistical differences of QOL scores in all domains were found in SE classification (p=0.00) and the level of education (p=0.00), whereas no differences were found for social security classification (p=0.06; Kruskal–Wallis test) (Table 2).
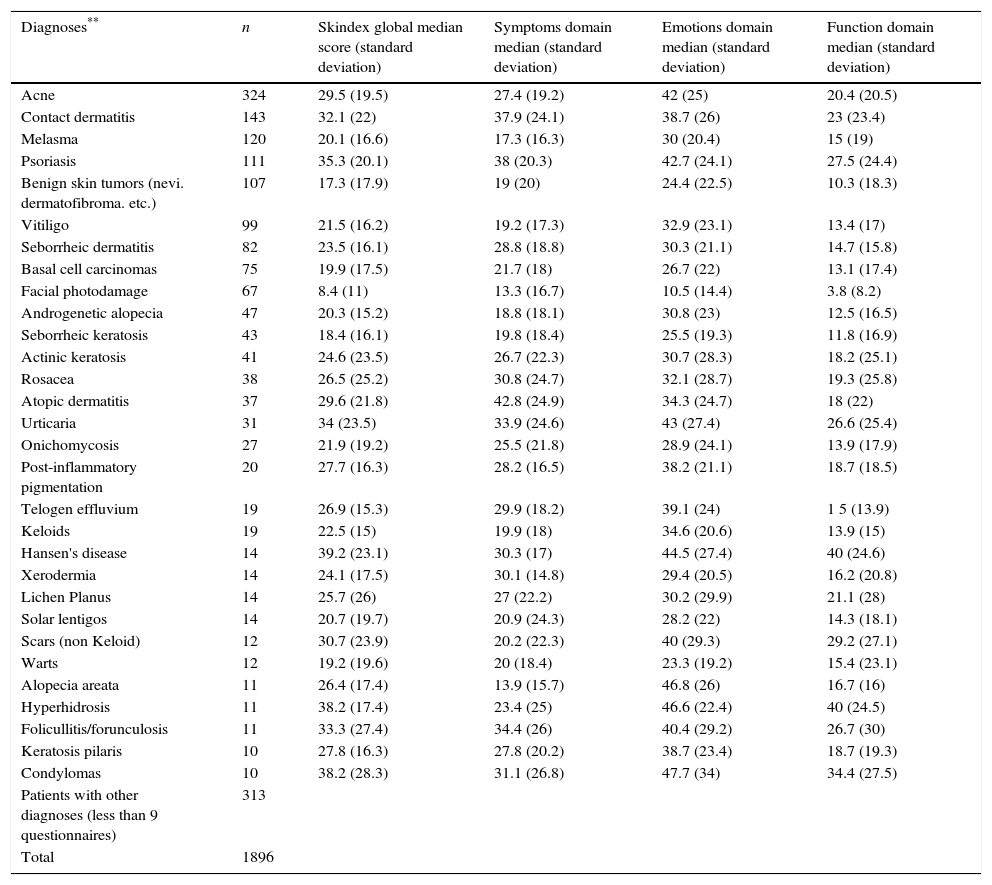
The highest Skindex-29 scores were found in psoriasis (global median score: 35.3 symptoms: 38; emotions: 42.7 and function: 27.5); contact dermatitis (global median score: 32.1 symptoms: 37.9; emotions: 38.7 and function: 23); atopic dermatitis (global median score: 29.6 symptoms: 42.8; emotions: 34.3 and function: 18); urticaria (global median score: 34 symptoms: 33.9; emotions: 43 and function: 26.6); and condylomas or anogenital warts (global median score: 38.2 symptoms: 31.1; emotions: 47.7 and function:34.4) (Table 3).
Global, symptoms, emotions and function Skindex scores according to skin disease.
| Diagnoses** | n | Skindex global median score (standard deviation) | Symptoms domain median (standard deviation) | Emotions domain median (standard deviation) | Function domain median (standard deviation) |
|---|---|---|---|---|---|
| Acne | 324 | 29.5 (19.5) | 27.4 (19.2) | 42 (25) | 20.4 (20.5) |
| Contact dermatitis | 143 | 32.1 (22) | 37.9 (24.1) | 38.7 (26) | 23 (23.4) |
| Melasma | 120 | 20.1 (16.6) | 17.3 (16.3) | 30 (20.4) | 15 (19) |
| Psoriasis | 111 | 35.3 (20.1) | 38 (20.3) | 42.7 (24.1) | 27.5 (24.4) |
| Benign skin tumors (nevi. dermatofibroma. etc.) | 107 | 17.3 (17.9) | 19 (20) | 24.4 (22.5) | 10.3 (18.3) |
| Vitiligo | 99 | 21.5 (16.2) | 19.2 (17.3) | 32.9 (23.1) | 13.4 (17) |
| Seborrheic dermatitis | 82 | 23.5 (16.1) | 28.8 (18.8) | 30.3 (21.1) | 14.7 (15.8) |
| Basal cell carcinomas | 75 | 19.9 (17.5) | 21.7 (18) | 26.7 (22) | 13.1 (17.4) |
| Facial photodamage | 67 | 8.4 (11) | 13.3 (16.7) | 10.5 (14.4) | 3.8 (8.2) |
| Androgenetic alopecia | 47 | 20.3 (15.2) | 18.8 (18.1) | 30.8 (23) | 12.5 (16.5) |
| Seborrheic keratosis | 43 | 18.4 (16.1) | 19.8 (18.4) | 25.5 (19.3) | 11.8 (16.9) |
| Actinic keratosis | 41 | 24.6 (23.5) | 26.7 (22.3) | 30.7 (28.3) | 18.2 (25.1) |
| Rosacea | 38 | 26.5 (25.2) | 30.8 (24.7) | 32.1 (28.7) | 19.3 (25.8) |
| Atopic dermatitis | 37 | 29.6 (21.8) | 42.8 (24.9) | 34.3 (24.7) | 18 (22) |
| Urticaria | 31 | 34 (23.5) | 33.9 (24.6) | 43 (27.4) | 26.6 (25.4) |
| Onichomycosis | 27 | 21.9 (19.2) | 25.5 (21.8) | 28.9 (24.1) | 13.9 (17.9) |
| Post-inflammatory pigmentation | 20 | 27.7 (16.3) | 28.2 (16.5) | 38.2 (21.1) | 18.7 (18.5) |
| Telogen effluvium | 19 | 26.9 (15.3) | 29.9 (18.2) | 39.1 (24) | 1 5 (13.9) |
| Keloids | 19 | 22.5 (15) | 19.9 (18) | 34.6 (20.6) | 13.9 (15) |
| Hansen's disease | 14 | 39.2 (23.1) | 30.3 (17) | 44.5 (27.4) | 40 (24.6) |
| Xerodermia | 14 | 24.1 (17.5) | 30.1 (14.8) | 29.4 (20.5) | 16.2 (20.8) |
| Lichen Planus | 14 | 25.7 (26) | 27 (22.2) | 30.2 (29.9) | 21.1 (28) |
| Solar lentigos | 14 | 20.7 (19.7) | 20.9 (24.3) | 28.2 (22) | 14.3 (18.1) |
| Scars (non Keloid) | 12 | 30.7 (23.9) | 20.2 (22.3) | 40 (29.3) | 29.2 (27.1) |
| Warts | 12 | 19.2 (19.6) | 20 (18.4) | 23.3 (19.2) | 15.4 (23.1) |
| Alopecia areata | 11 | 26.4 (17.4) | 13.9 (15.7) | 46.8 (26) | 16.7 (16) |
| Hyperhidrosis | 11 | 38.2 (17.4) | 23.4 (25) | 46.6 (22.4) | 40 (24.5) |
| Folicullitis/forunculosis | 11 | 33.3 (27.4) | 34.4 (26) | 40.4 (29.2) | 26.7 (30) |
| Keratosis pilaris | 10 | 27.8 (16.3) | 27.8 (20.2) | 38.7 (23.4) | 18.7 (19.3) |
| Condylomas | 10 | 38.2 (28.3) | 31.1 (26.8) | 47.7 (34) | 34.4 (27.5) |
| Patients with other diagnoses (less than 9 questionnaires) | 313 | ||||
| Total | 1896 |
When skin diseases were grouped according to the localization of lesions (face, hands, feet or non-exposed areas), or according to the inflammatory or non-inflammatory nature of the disease, there were statistical differences of Skindex-29 scores in all domains (p=0.00; Kruskal–Wallis test/Mann–Whitney U Test) (Table 2).
Similarly, Skindex-29 scores in all domains were statistically different when non-treated patients vs. treated patients were compared respectively: (global median score: 22.4 vs. 18.9; p=0.00), symptoms (median score: 24.9 vs. 21.4; p=0.03), emotions (median score: 32.5 vs. 27.5; p=0.00) and function (median score: 10.4 vs. 8.3; p=0.00; Mann–Whitney U Test) (Table 2).
DiscussionPatient-reported outcomes have become very relevant in clinical research in the last decades. Among these, quality of life emerges as one of the most important endpoints to be evaluated, as it has been required by regulatory agencies to be included not only as a primary endpoint in clinical trials, but also as a “must” outcome to be evaluated in systematic reviews and metanalysis.26,27
To date there is no standard approach for the Interpretation of Skindex-29 scores. Anchor-based and distribution-based methods have been used, with some remarkable differences in the categorization of scores when both methods are compared.28–32 However, if we round-off the cutoffs for mild (≥20), moderate (≥30) and severe (≥40) impairment as suggested by Prinsen et al., according to obtained results, psoriasis, contact dermatitis, atopic dermatitis, urticaria, hair disorders, Hansen's disease, scars, hyperhidrosis and genital human papillomavirus disease are among the skin illnesses that have a major impact in QOL in Colombia. Furthermore, even the most localized or asymptomatic skin lesion may lead to a disruption at some level of patient's wellness. Such findings are in agreement with previous reports21,33 and with others in which some of these skin disorders (namely psoriasis, atopic dermatitis) are perceived by patients as equally concerning as having hypertension, diabetes, asthma, cystic fibrosis or depression.34,35
The majority of interviewed patients were of ages between 18 and 49 (68%). Such finding not only reinforces exhaustive previous work2,36,37 but is also very relevant in this study, as this is the age at which individual's productivity and labor could be more affected by an impaired QOL.
Regarding other sociodemographic characteristics, more women participated in the study, a finding that is not unusual, as several studies have shown that women seek more frequently a dermatologist than men.38–40 But interestingly, statistical differences were only found in the function domain when both groups were compared. This result could be explained by differences in the kind of job performed either by men or women, as it could be hypothesized that this domain could be perceived more impaired by men, particularly when physical work is an important task in their jobs. Importantly, the majority of participants did not have a co-morbidity, a characteristic that if presented, might have influenced final QOL scores. Another interesting finding was that participants’ age did affect Skindex-29 scores, except for the symptoms domain. Such result has been reported before in other studies41,42 and could be explained by the fact that a symptom (i.e. itch) is decoded by the human brain in the same way either in a young person or in an old individual, whereas emotions and function could be more related to patient's context and job-type in accordance with age.
According to our results, skin disease duration in the majority of participants was >1 year, a fact that could explain higher Skindex-29 scores particularly in patients with chronic cutaneous disorders. This result is consistent with previous reports which have demonstrated that QOL is more impaired in long-standing disease.43–45
Regarding the level of education, most of the included individuals finished either high school, college or had a university degree. Although the level of education of the Colombian population tends to be lower, this could be explained by the inclusion in the study of an important number of patients with contributive social security which in our country usually corresponds to people having a formal job, therefore it represents a better income. However, the distribution of participants according to the socioeconomic status was more in agreement with Colombian current statistics and stratification.23 Interestingly, significant differences of QOL scores were shown for socioeconomic and education level. However, such finding is not surprising as schooling level in Colombia is highly dependent on income.
Importantly, the inflammatory nature of skin disease and its localization (e.g. exposed areas) impacted QOL scores of participants, a finding that has been reported in other countries21,46,47 and that is also in agreement with clinical practice. Similarly, having a treatment or not leads to significant differences in Skindex-29 scores, a result that also agrees with dermatology practice.
Although questionnaire return rate was low (only 52%),sociodemographic characteristics of the non-returning vs. the returning group were similar and overall missing information rate was low. Therefore, in our opinion, this result was more due to Dermatologists scarce time to interview patients during their regular practice with busy waiting rooms rather than to methodological issues, as most health institutions in Colombia (with some exceptions) do not have time or trained personnel for performing research during outpatient consultation.
Limitations of our study include some variability in the scores of the studied population, a result that could be more related to differences in the localization of skin lesions, their inflammatory or non-inflammatory nature and the initiation of therapy, and not in the disease itself (e.g. a sudden loss of hair in the frontal area in a woman has a higher impact in QOL when compared to the gradual appearance of a small vitiligo lesion in a hidden area).
The strength of this study relies on a large sample that included patients with different socioeconomic status, level of education and social security as well as individuals not only from the majority of Colombian main cities, but also from rural areas. This research is also strengthened by the fact that it is the first Colombian study that applied a formal validated dermatological QOL scale, becoming a valuable tool for global clinical practice, for the performance of local clinical trials, and for decision makers.
In conclusion, this study reinforces previous research regarding the burden of skin diseases in quality of life20,21,46 and adds well-supported scientific data to health authorities.
Such contribution gains importance in countries where the health system has misinterpreted cutaneous disorders (unfortunately with few exceptions) as having a “cosmetic” origin instead of them being an organic disorder which could affect the individual's socialization, mental and physical health, and human wellness and satisfaction.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis study has been sponsored by the Asociación Colombiana de Dermatología y Cirugía Dermatológica (Asocolderma). The administration and logistics of the study were both performed by Fundacion Dermabase.
Conflict of interestThe authors declare no conflict of interest.
María-del-Pilar Villegas, MD (Private practice office, Pereira, Colombia)
Martha-Susana Ramírez, MD (Somes, Bucaramanga, Colombia)
Rosario Betancourt, MD (Private Practice office, Sincelejo, Colombia)
Liliana Tamayo, MD (Universidad Pontificia Bolivariana, Medellín, Colombia)
Mónica Gaviria M, MD (Universidad Pontificia Bolivariana, Medellín, Colombia)
Joaquín Berrio J, MD (Facultad de Medicina, Universidad del Quindío, Armenia, Colombia)
Roberto Díaz, MD (Private Practice office, Sincelejo, Colombia)
Mufith Salaiman, MD (Private Practice office, Sincelejo, Colombia)
María-Victoria Hoyos, MD (Private Practice office, Sincelejo, Colombia)
Gabriel Matamoros, MD (Private Practice office, Cúcuta, Colombia)
Celmira Vargas, MD (Centro Dermatológico Federico Lleras Acosta, Bogotá, Colombia)
Edwin Bendek, MD, PhD (Centro Dermatológico Federico Lleras Acosta, Bogotá, Colombia)
Raquel Eraso, MD (Centro Dermatológico Federico Lleras Acosta, Bogotá, Colombia)
María-Victoria Franco, MD (Centro Dermatológico Federico Lleras Acosta, Bogotá, Colombia)
Maria-Isabel Barona, MD (Centro Medico Imbanaco,Cali, Colombia)
Lucia Montes, MD (Private Practice office, Sincelejo, Colombia)
Gener-Alejandro Mancilla, MD (Grupo de Investigación Dermatológica (GRID), Medellín; Centros
Especializados San Vicente Fundación, Rionegro, Colombia)
Eucaris Meza, MD (Private Practice office, Sincelejo, Colombia).