Oral ivermectin is an alternative therapy for human scabies infection due to its ease of administration and good safety profile. However, there is no definitive consensus on the optimal dosing regimen.
ObjectiveTo describe the treatment of human scabies with different dosages of oral ivermectin and the possible adverse events.
Methods23 patients with human scabies were treated with oral ivermectin: 10 patients received a single oral dose of 200μg/kg and 13 a dose of 400μg/kg. A second, or even a third dose, was administered in cases of treatment failure.
ResultsA complete clinical response was achieved by all of the patients. The first ten patients required at least two (80%) or three (20%) doses of ivermectin for complete resolution of the infection. The remaining cases resolved with a single 400μg/kg oral dose. Within the first 72h after the administration of oral ivermectin, new cutaneous lesions were observed in eleven patients (47.8%). Cutaneous biopsies showed signs of subacute eczema. The eruption was treated with topical corticosteroids and emollient therapy. There was no other new drug administration or a history of irritants. There was no history of atopic diathesis except for one patient.
ConclusionsOral ivermectin is an effective therapy for the treatment of human scabies. A single 400μg/kg oral dose demonstrated high efficacy and good tolerance. However, the appearance of eczematous cutaneous lesions induced by oral ivermectin has not previously been reported in the literature. Dermatologists should be aware of this possible adverse event.
La ivermectina oral es una alternativa terapeutica en el tratamiento de la escabiosis humana debido a su fácil administración y buen perfil de seguridad. Sin embargo, no existe un consenso definido sobre un esquema adecuado de dosificación.
ObjetivoDescribir el tratamiento de escabiosis en humanos con diferentes dosis de ivermectina oral y sus posibles efectos adversos. Métodos: 23 pacientes con escabiosis fueron tratados con ivermectina oral; 10 pacientes recibieron una única dosis de 200 μg/kg y 13 pacientes, una dosis de 400 μg/kg. Una segunda, e incluso, una tercera dosis fueron administradas en casos de fallo terapeútico.
ResultadosTodos los pacientes tuvieron respuesta clinica al tratamiento. Los primeros 10 pacientes necesitaron, al menos, 2 dosis (80%) o 3 dosis (20%) para conseguir una remisión completa de la infección. En el resto de pacientes se resolvió con una única dosis oral de 400 μg/kg. En las primeras 72 horas tras la administración de ivermectina oral se observaron nuevas lesiones cutáneas en 11 pacientes (47,8%). Las biopsias cutáneas mostraron signos de eccema subagudo. Se realizó tratamiento con corticoterapia tópica y emolientes. No había antecedentes de toma de otros fármacos, contacto con agentes irritantes ni historia de dermatitis atópica salvo en 1 paciente.
ConclusionesIvermectina oral es una terapia eficaz en el tratamiento de escabiosis humana. Una dosis única de 400 μg/kg demostró una alta eficacia y buena tolerancia. Sin embargo, la aparición de lesiones cutaneas eccematosas inducidas por ivermectina oral no había sido descrito previamente en la literatura y por tanto, consideramos que los dermatólogos deberían conocer este posible efecto adverso.
Traditionally, ivermectin has been extensively used to control and treat onchocerciasis, a disease caused by the filarial worm Onchocerca volvulus. However, it has also been demonstrated to act strongly against a wide variety of insects, nematodes, and acarine parasites, including lice and scabies.1,2
Oral ivermectin has been recommended as a systemic alternative to topical scabicides due to its ease of administration, convenience, safety and favourable side effect profile. It has the additional advantage of alleviating the problems of noncompliance, misuse, and inadequate application associated with topical therapy. Therapy may be effective after a single 200μg/kg dose for uncomplicated scabies, but multiple doses are usually required to achieve complete resolution.1 Ivermectin has proven to be remarkably safe for different indications in adults, and it is well tolerated without serious adverse events. The majority of side effects reported in onchocerciasis and other filarial diseases are minor and rare. These side effects include mild gastrointestinal upset, abdominal pain, asthenia, somnolence, dizziness, pruritus and rare biochemical abnormalities such as hypertransaminasaemia and leukopenia.2 Only rarely, cutaneous side effects following scabies treatment have been reported.3
We describe 23 patients with human scabies treated with oral ivermectin. We emphasize the development of an eczematous eruption in 11 of these patients, a finding not previously reported in the literature.
Materials and methodsThis is a retrospective, observational, case-series study that included 23 otherwise healthy outpatients diagnosed with a human scabies infection who received oral ivermectin (200μg/kg or 400μg/kg). With the corresponding authorization, and after the signed informed consent by the patients, the medication was supplied, out of indication, by the Madrid Community Health Department. The diagnosis of scabies was based on typical clinical features and was confirmed microscopically by the demonstration of mites, eggs or faecal pellets (scybala) in skin scrapings. The severity of the disease was recorded as mild (less than 11 lesions), moderate (11–49 lesions), severe (50 or more lesions) or crusted (Norwegian). The intensity of pruritus was determined with a visual analogue scale (0–10). At baseline, clinical data were recorded, and a complete physical and dermatologic examination was performed. Baseline complementary tests included haematological and biochemical tests and serologic testing for hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV).
Patients were evaluated each 2 weeks after the first dose of ivermectin was administered and a physical examination including the collection of skin scrapings were repeated. The first 10 patients received a single 200μg/kg oral dose of ivermectin. A second 200μg/kg dose of ivermectin was administered 2 weeks later, in the case of treatment failure, which was defined as the presence of clinical signs of active scabies, new lesions and/or microscopic evidence of scabies. A third dose of ivermectin was administered at the end of the fourth week to the second dose non-responders. Complete resolution of the infection was considered as the absence of clinical evidence of scabies and a lack of positive signs of scabies in skin scrapings two weeks after ivermectin administration. No topical or any other systemic scabicide treatment was provided within thirty days of treatment initiation or during the study. Ivermectin was administered orally as 6-mg tablets. The total dose ranged from 12 to 30mg, depending on patient body weight.
Because the first 10 patients demonstrated a high proportion of treatment failure and eczematous dermatitis after a single oral ivermectin administration, the subsequent 13 patients with a diagnosis of scabies were treated with a single 400μg/kg oral dose of ivermectin and daily topical emollients.
Patients in both groups were evaluated clinically and microbiologically every two weeks until the scabies infection completely resolved. The last visit was made three months after the last ivermectin dose. The severity of the lesions, the intensity of pruritus and the possible appearance of side effects were recorded at each visit. Haematological and biochemical analyses were performed two weeks after each ivermectin dose.
ResultsThe study included 23 patients (15 males and 8 females; mean age of 36.1 years, range 14–77) (Table 1). Mild scabies was present in one patient, moderate in twelve, severe in nine, and the remaining patient presented with crusted scabies. The mean intensity of pruritus was 7.9 (5–10). None of the patients had a history of scabies infection.
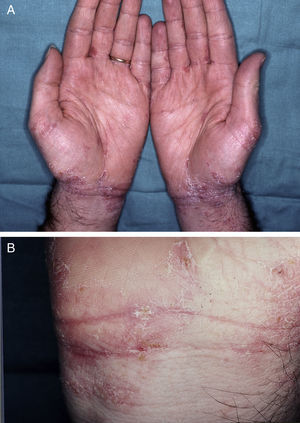
Summary of distribution, treatment and evolution of ivermectin-induced subacute eczema.
| Patient no./Sex/Age | Severity of scabies | Oral dose of ivermectin (μg/kg) | No. of doses | Doses (mg) | Weight (kg) | Xerodermia | Ezcema | Ezcema distribution | Treatment of eczematous eruption | Preventive emollient Therapy | Outcome of eczematous eruption |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/M/40 | Moderate | 200 | 2 | 18 | 82 | Yes | Yes | Trunk, UE, LE | TC | Resolution | |
| 2/M/25 | Severe | 200 | 2 | 18 | 92 | Yes | Yes | Trunk, UE | TC | Resolution | |
| 3/M/37 | Moderate | 200 | 2 | 18 | 87 | No | Yes | Trunk, UE, LE | TC | Resolution | |
| 4/M/34 | Severe | 200 | 3 | 18 | 80 | No | Yes | Trunk | TC | LF-U | |
| 5/M/50 | Severe | 200 | 2 | 12 | 60 | No | Yes | Trunk, UE, LE | TC | Resolution | |
| 6/M/27 | Severe | 200 | 2 | 15 | 70 | Yes | Yes | Trunk, UE | TC | Resolution | |
| 7/F/35 | Moderate | 200 | 2 | 12 | 62 | Yes | No | ||||
| 8/F/27 | Moderate | 200 | 3 | 15 | 70 | No | No | ||||
| 9/M/60 | Severe | 200 | 2 | 18 | 99 | Yes | No | ||||
| 10/F/14 | Severe | 200 | 2 | 12 | 58 | No | No | ||||
| 11/F/70 | Crust | 400 | 1 | 24 | 60 | No | Yes | Trunk, UE, LE | TC | Yes | Resolution |
| 12/F/77 | Severe | 400 | 1 | 24 | 55 | No | No | Yes | |||
| 13/M/46 | Severe | 400 | 1 | 28.5 | 72 | No | No | Yes | |||
| 14/M/24 | Moderate | 400 | 1 | 24 | 67 | No | No | Yes | |||
| 15/M/26 | Moderate | 400 | 1 | 30 | 75 | No | Yes | Thighs, hands | TC | Yes | Resolution |
| 16/F/21 | Severe | 400 | 1 | 18 | 52 | No | No | Yes | |||
| 17/F/16 | Mild | 400 | 1 | 18 | 45 | No | No | Yes | |||
| 18/M/19 | Moderate | 400 | 1 | 24 | 65 | NK | No | Yes | |||
| 19/M/50 | Moderate | 400 | 1 | 30 | 78 | No | No | Yes | |||
| 20/M/58 | Moderate | 400 | 1 | 24 | 60 | No | Yes | UE, LE | TC | Yes | Resolution |
| 21/F/26 | Moderate | 400 | 1 | 24 | 52 | No | No | Yes | |||
| 22/M/28 | Moderate | 400 | 1 | 30 | 80 | No | Yes | Hands, LE | TC | Yes | NK |
| 23/M/21 | Moderate | 400 | 1 | 30 | 80 | No | Yes | UE, LE | TC | Yes | NK |
UE: upper extremities, LE: lower extremities, TC: topical corticosteroids, NK: not known, LF-U: lost follow-up.
The first 10 patients required at least two (8/10, 80%) or three (2/10, 20%) 200μg/kg doses of oral ivermectin to completely resolve the infection. Three months after the last dose of ivermectin, there was no evidence of disease in nine patients. One patient was lost to regular follow-up.
The remaining 13 patients were cured with a single 400μg/kg oral dose. No patient required a second ivermectin oral dose. The patients fulfilled the criteria for complete resolution after four (12 patients) or six weeks (a 70-year-old immunocompetent woman with psychiatric disturbances who was diagnosed with crusted scabies). Three months after ivermectin treatment, clinical and/or microbiological evidence of disease was not evident in eleven patients. Two patients did not return for the three-months’ follow-up visit.
All of the patients tested negative for HBV, HCV and HIV. Throughout the treatment, the complementary tests showed no changes in relation to baseline findings. Ivermectin was well-tolerated, and no severe side effects were reported at any of the follow-up visits.
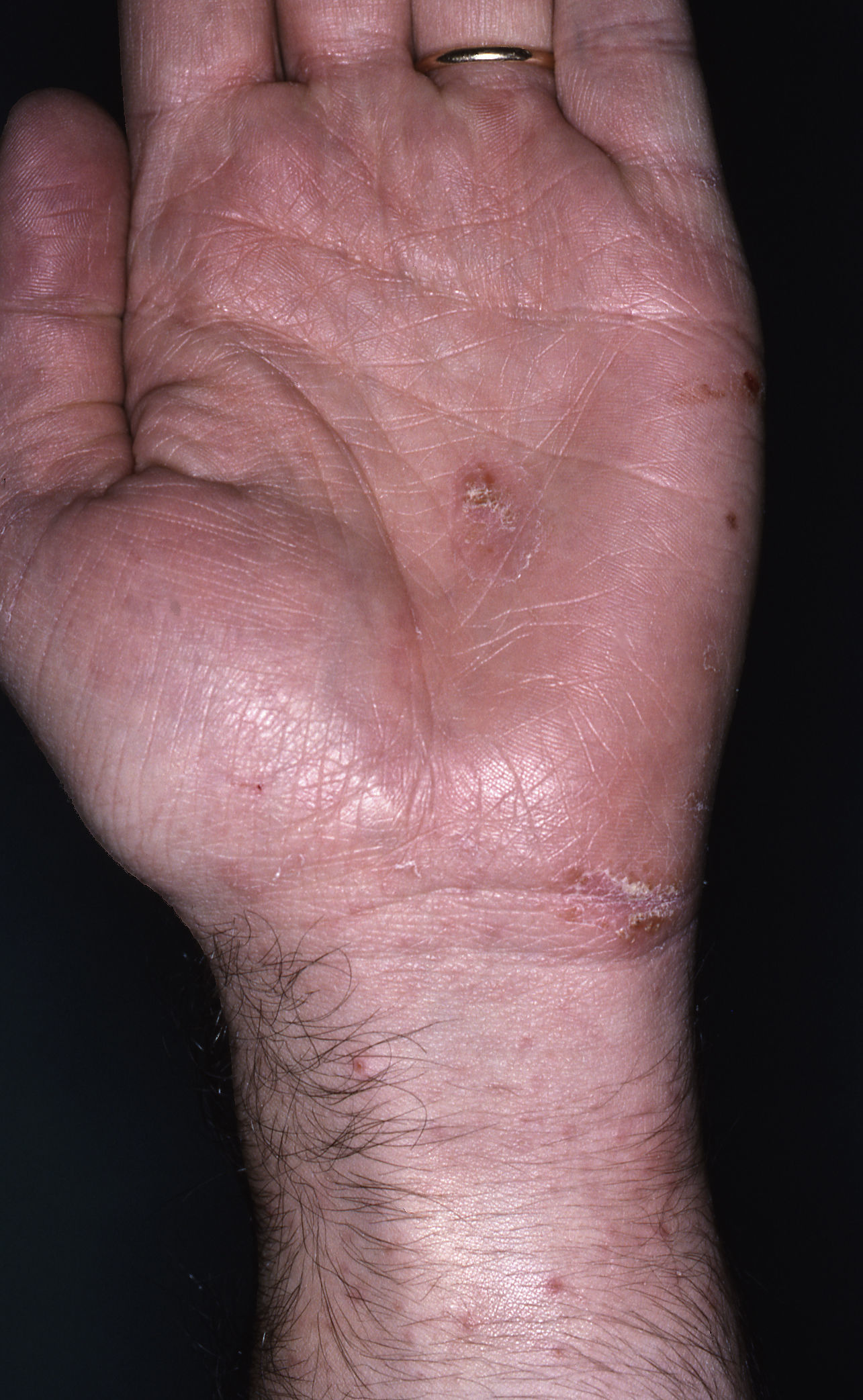
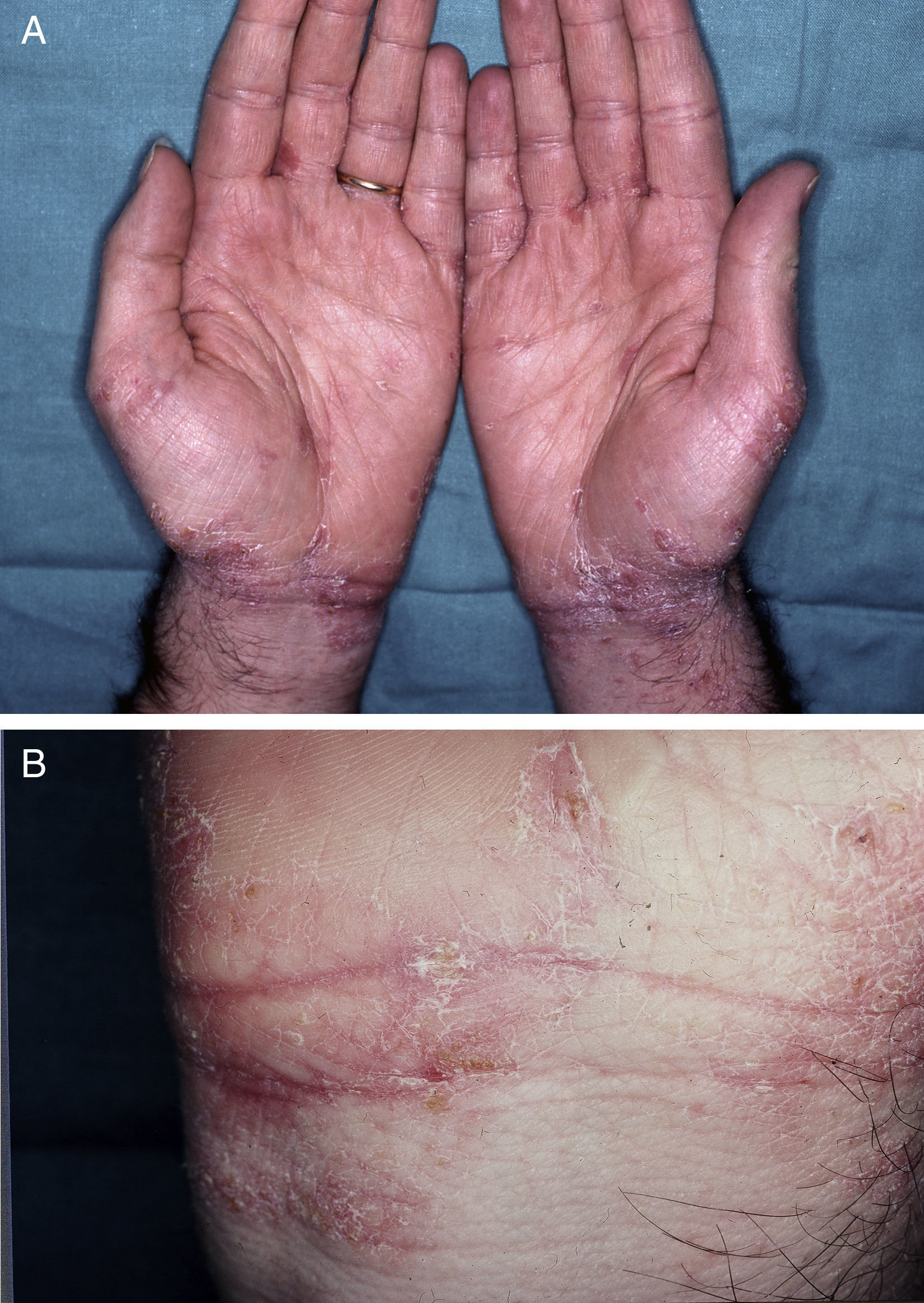
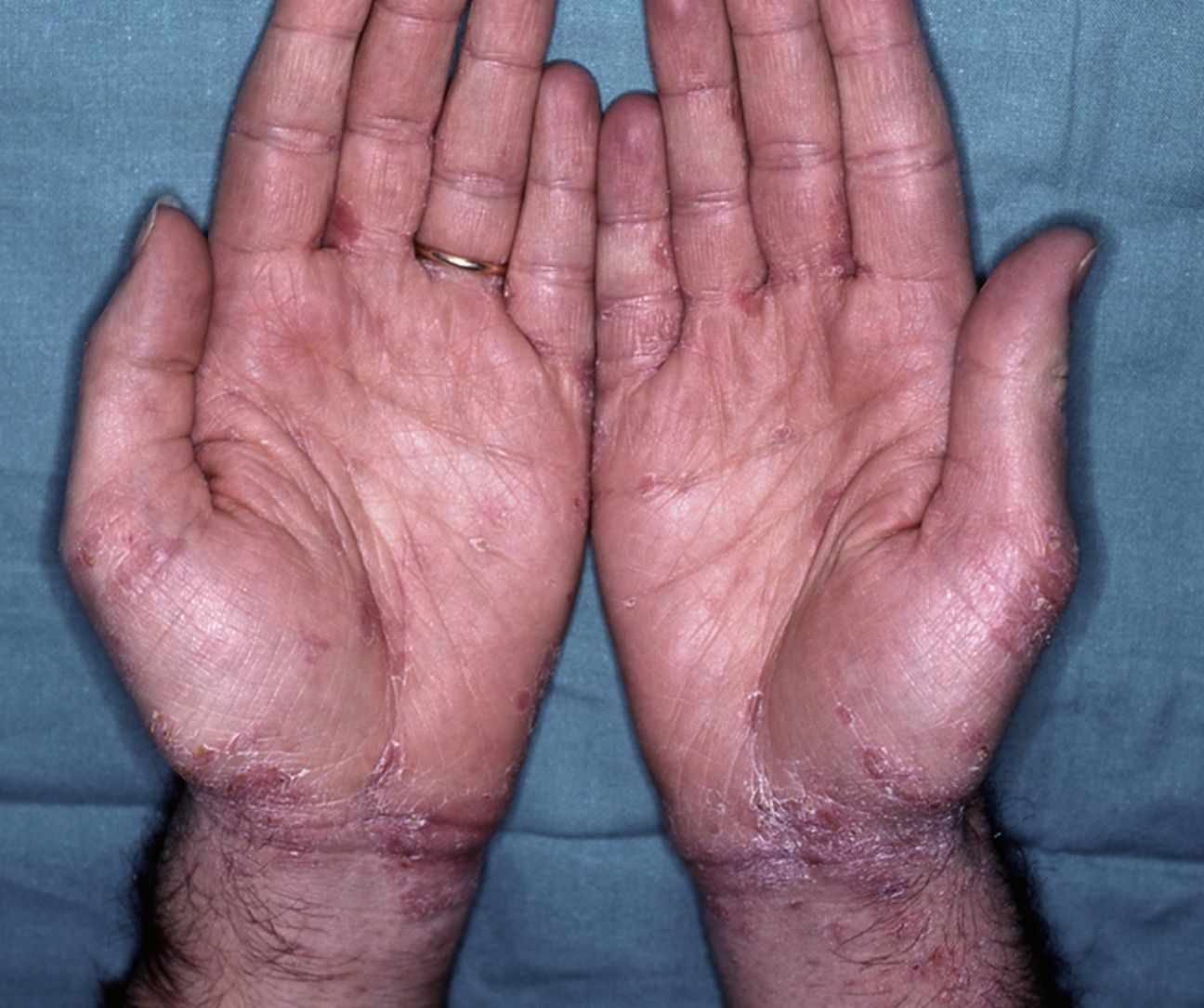
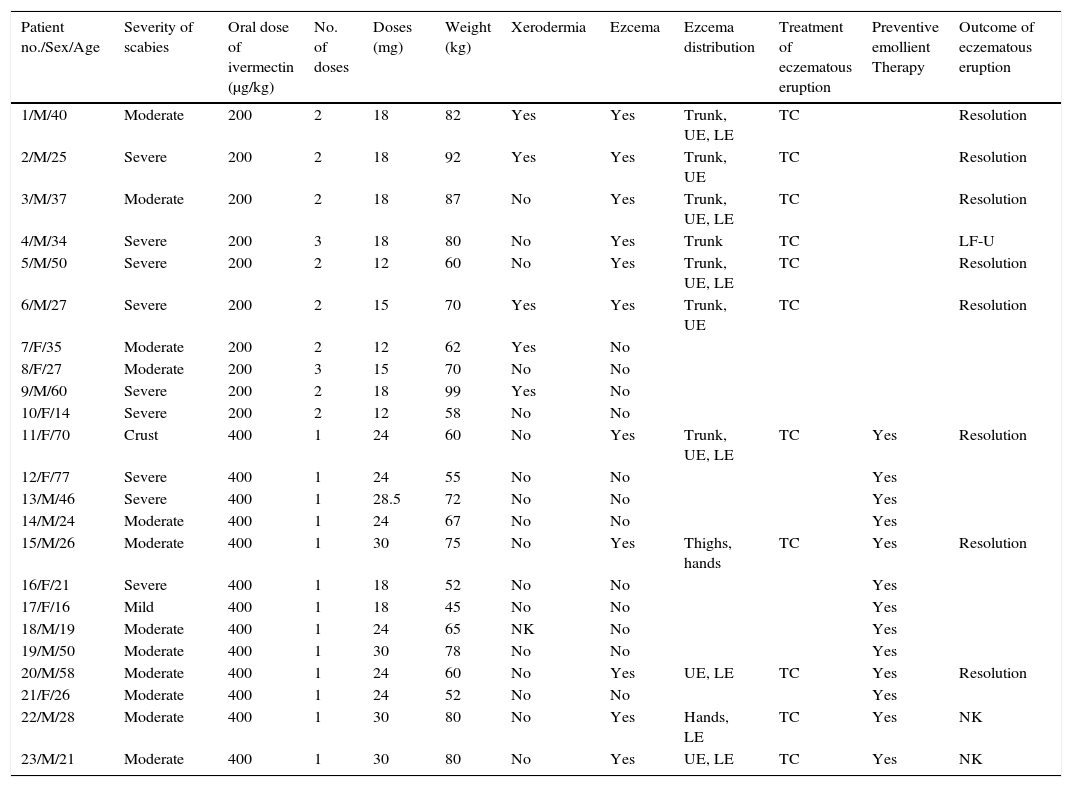
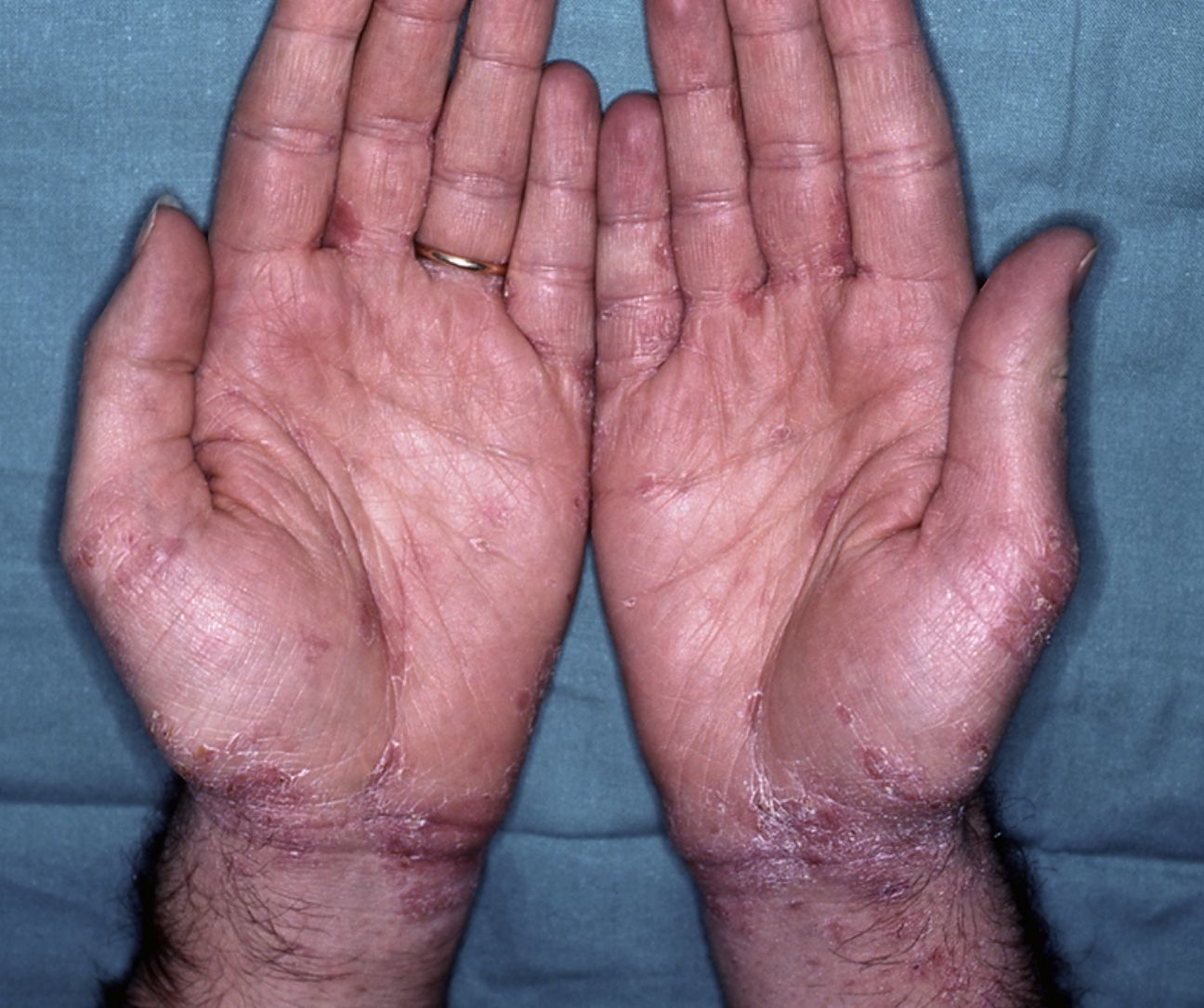
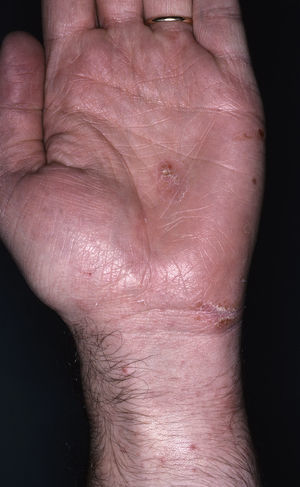
Within twenty-four to seventy-two hours after the administration of oral ivermectin, eleven patients (47.8%) presented new cutaneous lesions that were clinically distinct from those characteristic of scabies. The skin eruptions appeared after receiving the first oral ivermectin dose in 9 patients, after receiving the second dose in 1 patient, and after both the first and second dose in 1 patient. In all of the cases, the eruptions were characterized by itchy, scaly erythematous eczematous plaques located on the trunk and extremities at the time of onset, but they were much more prominent in areas previously infected with scabies, particularly the interdigital folds. Although the eruptions simulated an exacerbation of the disease they were clinically distinct (Figs. 1, 2a and 2b). The patients clearly distinguished the quality of pruritus as distinct from scabies. Some patients also presented an accompanying xerosis.
(a) and (b) Eczematous cutaneous lesions induced by oral ivermectin. Same patient as Figure 1.
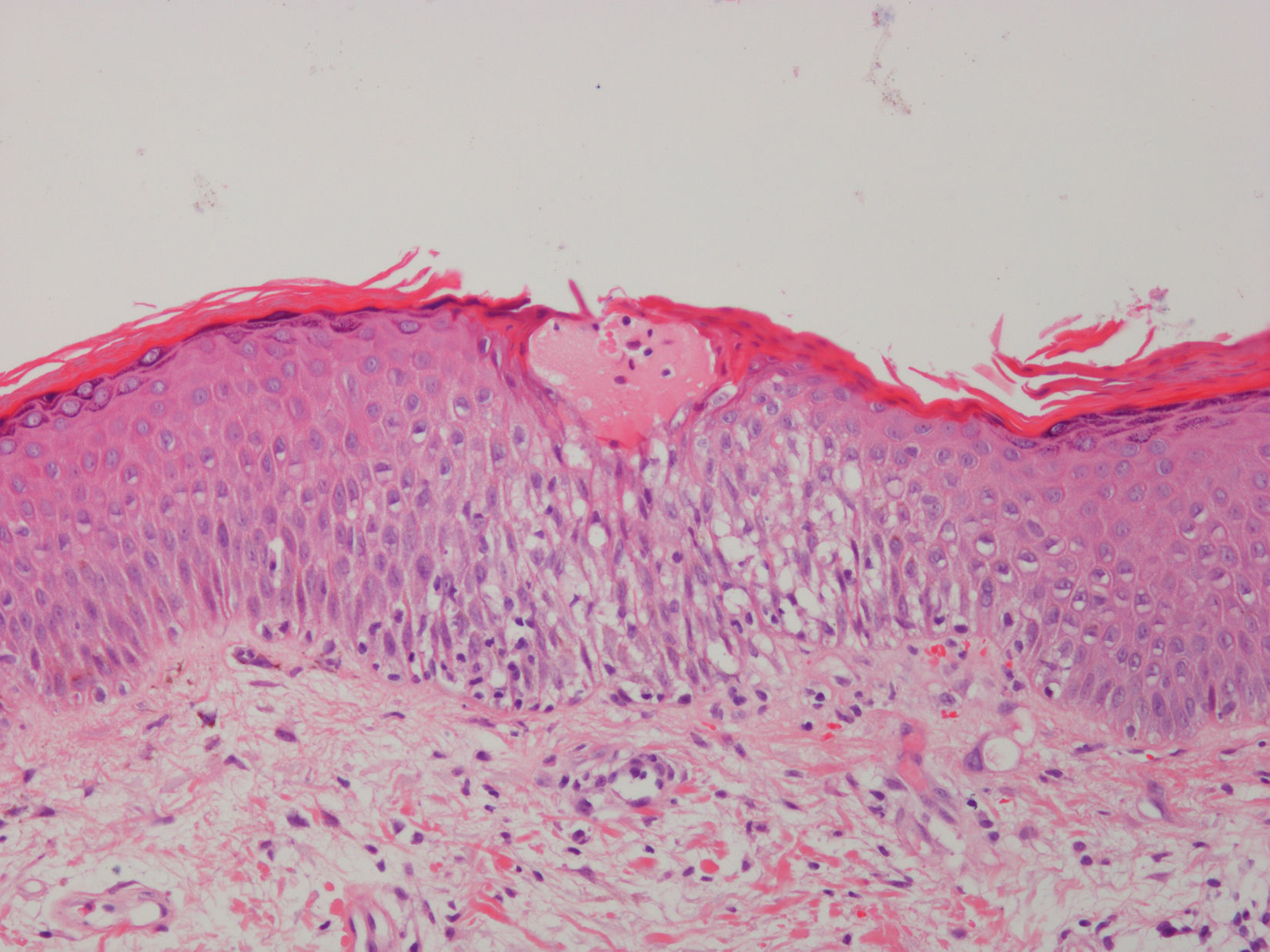
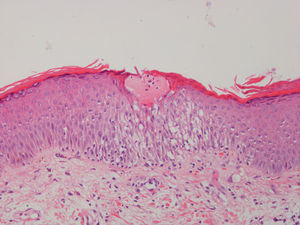
We performed a cutaneous biopsy in three patients showing similar histopathological findings characterized by parakeratosis, small droplets of plasma limited to the stratum of Malpighi and epidermal spongiosis. An inflammatory cellular infiltrate, mainly composed of lymphocytes and eosinophils, was present within the papillary and upper reticular dermis. The spongiotic dermatitis findings were characteristic of subacute eczema (Fig. 3).
No patient required an interruption of treatment with ivermectin. The skin eruptions were treated with topical diflucortolone valerate and emollient therapy and cleared within one week. The eczematous eruptions developed in 6 of the 10 patients (60%) treated orally with 200μg/kg ivermectin, while only 5 of the 13 patients (38.4%) who received daily emollient therapy and 400μg/kg oral ivermectin presented the same eruption, suggesting that the emollients helped to prevent the development of xerosis and cutaneous lesions. Specifically asked, only one patient with this peculiar side effect had a history of other skin diseases (atopic dermatitis). None referred signs or symptoms of atopic diathesis (asthma, pollen sensitivity, spring rhinoconjunctivitis, etc.). None of the patients reported the use of irritants or changes in the use of soaps or fragrances. Haematological tests in the patients with the eczematous eruption did not show eosinophilia at baseline. IgE determination in 5 of them was within normal limits. No other systemic drugs were administered to the patients prior to the eczematous changes that could be chronologically related.
DiscussionIvermectin is derived from a class of broad-spectrum antiparasitic agents called avermectins, which were isolated from products of the bacteria Streptomyces avermitilis. It is a macrocyclic lactone that is structurally similar to macrolide antibiotics but devoid of antibacterial activity. Ivermectin has been used to treat a variety of infestations and infections in animals.2 In humans, this drug was introduced against Onchocerca volvulus infection by Aziz et al. in 1982, but it is also highly effective for the treatment of other nematodes (e.g., Strongyloides stercolaris, filariasis) and arthropods.4
Ivermectin has been used by several investigators to treat scabies infection, and excellent results have been obtained with the 200μg/kg dosage. In 1995, Meinking et al.1 showed success treating normal scabies and Norwegian scabies in HIV-infected individuals who were cleared with a single dose. Moreover, they suggested that a single oral dose of 200μg/kg ivermectin cured most cases of uncomplicated scabies.1 Some authors have shown similar results even with crusted scabies,5 although in these cases, additional treatment could be required.2,6
In our study, all of the patients with scabies were successfully cured with this drug. However, 0 of 10 patients were cured with a single oral dose of 200μg/kg; all 10 patients required a second (80%) or third 200μg/kg dose (20%). Recent studies have shown a higher rate of treatment failure with a single dose of ivermectin (200μg/kg) than with topical benzyl benzoate7 or permethrin.8 However, the cure rate increased if a second dose of ivermectin was administered to patients who did not response to the first dose. It has been postulated that the lower efficacy of a single dose of ivermectin compared to two doses may reflect the lack of ovicidal action of the drug.2,9 Moreover, the eggs hatch every 6–7 days, so it is recommended that the treatment be repeated two or three times separated by 1–2-week intervals because it acts only at certain stages of the parasite life cycle. This drug might not be effective against the younger stages because the parasite nervous system has not yet developed.6,9 At present, the parasiticidal effect and the optimal dosage of ivermectin in the various stages of the mite have not yet been determined. In our study, the 13 patients who received a single initial 400μg/kg oral dose of ivermectin (including one patient with crusted scabies) demonstrated complete resolution of the scabies infection.
Ivermectin acts by selectively binding to glutamate-gated chloride ion channels, which are found in invertebrate nerve and muscle cells. The selective binding leads to a change in the permeability of the cell membrane, causing hyperpolarisation of the cells, paralysis and the death of the mite. Most mammals do not have glutamate-gated chloride channels, and the drug appears almost devoid of side effects, with most studies reporting none.2,10 This result is also true for humans in which ivermectin has been proven to be remarkably safe. The adverse reactions described include fever, headache, chills, arthalgia, eosinophilia and anorexia. In most cases, the reactions are transient and mild. A Mazzotti-type reaction occasionally occurs when ivermectin is used in the treatment of onchocerciasis and other filarial diseases due to the death of numerous microfilariae and the release of their toxic products. More severe complications, such as neurological reactions, have been reported following ivermectin administration to patients heavily infected with Loa loa.11
The use of ivermectin to treat scabies infection has not been conclusively associated with any serious adverse effects, although some articles mention transitory and mild events. Some studies have reported pruritus within a few hours of taking 200μg/kg of oral ivermectin that spontaneously disappeared after two to three days.1,8,12 Dourmishev et al. described an enhancement of pruritus accompanied with a vesicle-pustular rash in some patients (3 out of 19) between the second and fourth day after the oral ivermectin administration.3
In our study, we observed that a high percentage of patients developed new cutaneous eczematous lesions within the first week after oral ivermectin administration. We believe that these lesions were induced by ivermectin based on the strong temporal relationship with a similar time interval (3–4 days) between the administration of the drug and the appearance of the condition. The distribution and severity of the eruption was more pronounced in the areas previously infected with scabies, which could be explained by a local release of parasite antigens following the death of the mites after ivermectin treatment, resulting in a mild skin Mazzotti-like reaction. Although cutaneous symptoms, such as pruritus, have been associated with the use of ivermectin, to our knowledge “ivermectin-induced eczema” has not been previously reported in the literature. We have not found any factor that could predict this reaction. The triggering of the eczematous lesions by irritants (soaps, fragrances, etc.) was reasonably ruled out by asking the patients. Only one of the patients that developed the eczematous changes had a history of atopic dermatitis. The remaining 10 patients did not have a history of atopic diathesis despite specifically asked for it, nor did they present a baseline eosinophilia or an increased IgE in those in which this test had been performed, also reasonably ruling out atopy as an underlying disorder. Patients clearly distinguished the difference between the pruritus by the scabies from the symptoms of the eczematous eruptions and, in fact, while original scabiotic lesions (burrows, papules, etc.) cleared, the new eczematous lesions persisted until corticosteroid therapy was started.
We administered emollient therapy before and after oral ivermectin to the last 13 patients to prevent xeroderma and the eczematous eruptions that had been observed in the first 10 patients. Only five of these patients (38.4%) experienced similar skin reactions within the first week, a markedly lower proportion than the first group of patients who had not received pre-treatment with emollients, suggesting that this therapy may prevent a number of cases of secondary skin disorders following ivermectin administration.
Limitations were the sample size, the retrospective nature of the study, the lack of a randomized clinical trial with a control arm, the variability of doses and the influence of emollients on the results.
In conclusion, ivermectin is a useful drug for the treatment of scabies infection. It has a high therapeutic effectiveness when administered as a single 400μg/kg oral dose, good tolerance and a low incidence of serious side effects. We emphasize the appearance of xeroderma and eczematous eruptions induced by ivermectin, a reaction not previously reported in the literature, which can be prevented with the use of emollients in some cases. Dermatologists should be aware of this side effect to avoid confusion with a recurrence or exacerbation of the scabies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNil.
Conflicts of interestThe authors have no conflicts of interest to declare.