Under the right conditions, dermoscopy allows us to observe the vascular features of many different types of skin lesions. The visualization and identification of vessels with a characteristic morphology can be the key to diagnosis, especially in hypopigmented lesions in which the typical pigmented structures are not visible.
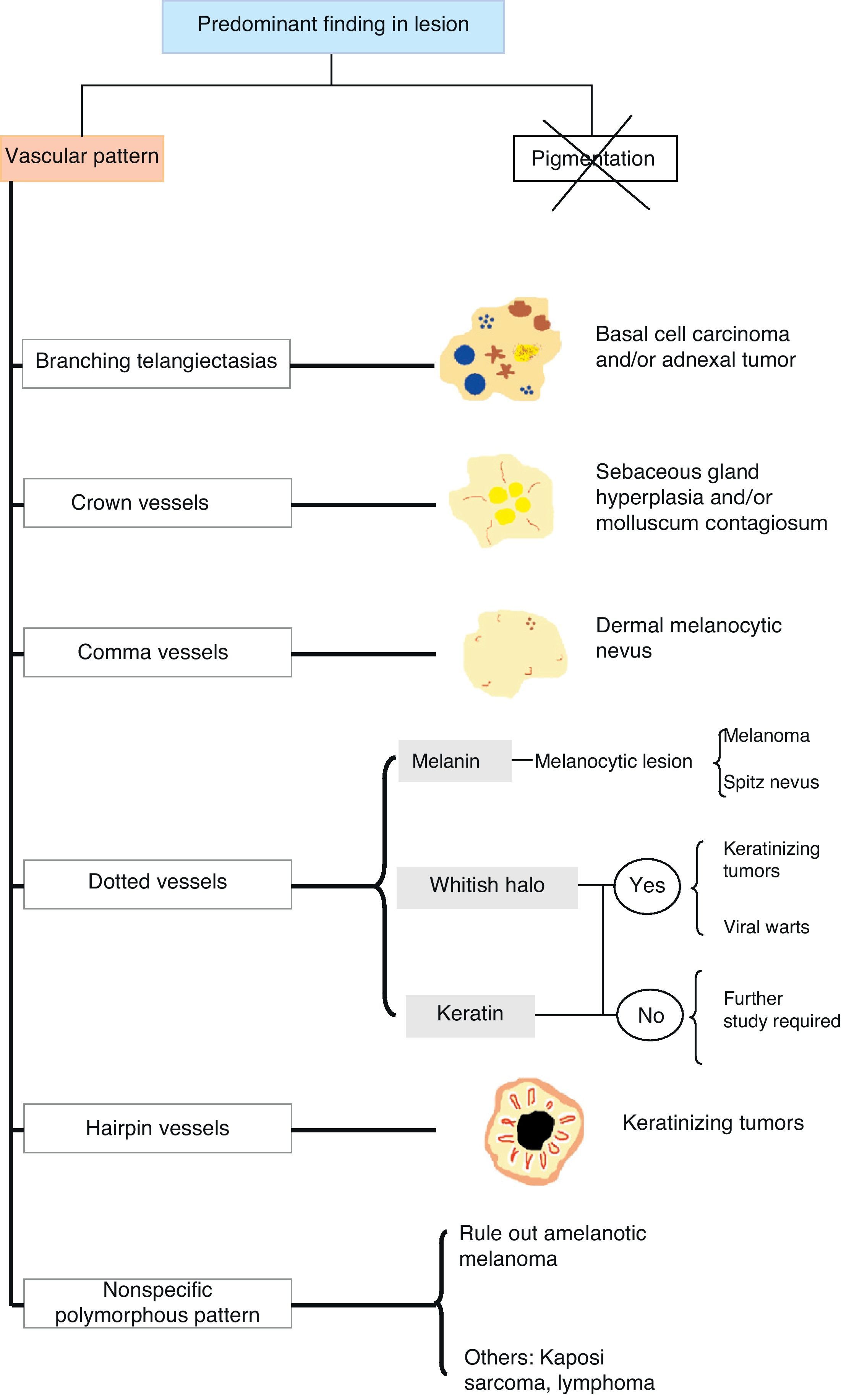
Some of the more characteristic associations are the presence of crown vessels in sebaceous hyperplasia, arborizing telangiectasias in basal cell carcinoma, comma-shaped vessels in intradermal and compound nevi, dotted vessels in Spitz nevi and melanoma, and hairpin vessels in seborrheic keratoses.
The recognition of distinctive vascular features can be of great help in the diagnosis of many types of skin lesions, and very often such patterns are the only key to the diagnosis of melanoma.
La dermatoscopia permite observar, en las condiciones adecuadas, la vascularización presente en las lesiones cutáneas de muy diversa índole. La visualización e identificación de vasos de morfología característica puede ser la clave en el diagnóstico de determinadas lesiones, especialmente en el caso de aquellas hipopigmentadas, en las que no es posible identificar las estructuras pigmentadas clásicas.
Así pues, algunas de las asociaciones más características son la presencia de vasos «en corona» en la hiperplasia sebácea, las «telangiectasias arboriformes» en el carcinoma basocelular, los vasos «en coma» en los nevos intradérmicos y compuestos, los vasos «en cabeza de alfiler» en los nevos de Spitz o melanoma, o los vasos «en horquilla» en las queratosis seborreicas.
El reconocimiento de estructuras vasculares distintivas puede ser de gran ayuda para el diagnóstico de numerosas lesiones. A menudo, constituyen la única clave para el diagnóstico del melanoma.
Dermoscopy is a noninvasive diagnostic technique that allows us to visualize morphologic features invisible to the naked eye; it combines a method that renders the corneal layer of the skin translucent with an optical system that magnifies the image projected onto the retina.1–4
Dermoscopy has become a routine technique in dermatology practice in the last decade and has contributed to our improved knowledge of the morphology of numerous cutaneous lesions.5 Additionally, it permits closer follow-up of patients with multiple melanocytic nevi and has substantially improved the accuracy of the clinical diagnosis of pigmented and nonpigmented cutaneous lesions.
The color seen through a dermoscope depends on various factors. The 2 main chromophores detected are melanin, which appears as a black, brown, bluish, or grayish color depending on its depth in the skin, and hemoglobin, which can exhibit red, bluish, or even purple tones, depending on its depth, degree of oxidation, and the presence or absence of thrombosis.6
In histological examination, it is difficult to fully appreciate the morphologic features of vessels as histology provides a vertical view of sections of lesions; dermoscopy, by contrast, provides a horizontal view of the lesion, allowing the identification of a wide variety of vascular structures, each with characteristic morphologic and architectural features, that can be of enormous diagnostic value as the technique provides additional information to that obtained in the traditional 2-step approach (melanocytic vs nonmelanocytic lesions and benign vs malignant lesions). The examination of vessels is of particular interest in the diagnosis of nonpigmented lesions, where vascular features are often the only clues that suggest the presence of a melanoma.7
Technical ConsiderationsThe identification and evaluation of vascular structures on dermoscopy depends largely on the optical system and examination technique used. There are several important aspects to consider: the method (contact dermoscopy vs polarized light dermoscopy), the resolution of the dermoscope, and the choice of immersion fluid.6
In contact dermoscopy, the choice of an appropriate immersion fluid (the fluid placed between the skin and the glass plate of the dermoscope) and the correct use of the optical system are key to obtaining a clear image of tumor vessels as the closer these are to the surface, the more likely it is that the pressure on the skin will compress the vessels. When a contact dermoscope is used, it is thus essential to apply minimal pressure to prevent the collapse of vessels.
A range of immersion fluids can be used, including water, alcohol, immersion oil, and ultrasound gel. When examining vessels without polarized light, ultrasound gel is the best option thanks to its high viscosity. Less viscous fluids tend to flow off and they also absorb less pressure.
Immersion or interface fluids are not required in polarized light dermoscopy, although they can be useful in lesions where hyperkeratosis or crusting result in considerable refraction that makes it difficult to visualize the structures clearly.8,9 In such cases, the use of an immersion fluid helps to reduce the refraction of light.
Another important aspect is the magnification power of the dermoscope. A magnification of at least 30x is recommended for visualizing vascular structures as very small capillaries are difficult to analyze at lower magnifications.
Unfortunately, most of the hand-held dermoscopes available produce magnifications of between 10x and 20x, making it very complicated to visualize vessels correctly.
In digital systems, video camera resolution is also a limitation when it comes to evaluating vascular structures, and in our experience, handheld devices offer a much sharper image than some of the video cameras used in digital dermoscopy, except at the highest magnifications.
There are also digital photography dermoscopic systems that can be coupled to cameras to produce high-quality images. Examples are the Heine Delta 20 dermatoscope and the DermLite PhotoSystem.
General ConsiderationsVascular structures show up better in hypopigmented or nonpigmented lesions, or in lighter areas of pigmented tumors.
The most important chromophore in nonpigmented cutaneous tumors is hemoglobin, a pigment found in the erythrocytes of the vascular lumen.7
Because dermoscopy provides a horizontal view of the skin, vessels that run parallel to the skin surface are visualized as lines, while those that run perpendicularly are generally viewed as dots, or even loops.
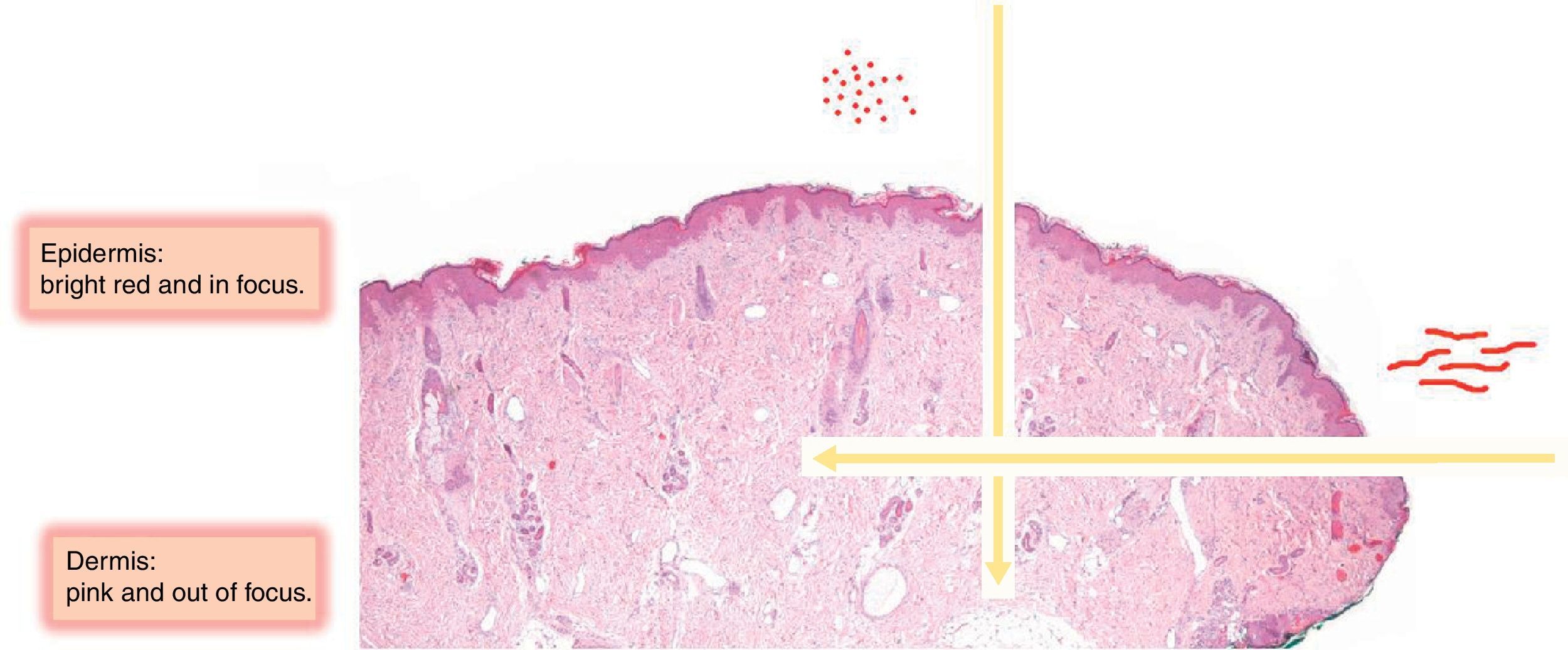
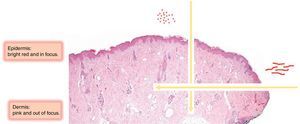
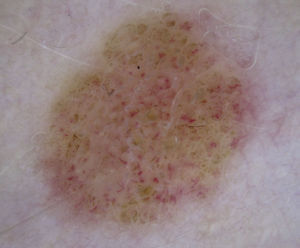
Vessels located in the dermis are generally pink and appear out of focus due to the effect of the dispersion of light through the dermal connective tissue; those found closer to the surface (immediately under the epidermis), by contrast, are bright red and in focus7 (Fig. 1).
The predominant vascular pattern also depends on the volume of the tumor and its proliferation pattern.1 The flat part of a tumor, for example, may have vessels that differ from those in the nodular component, which typically develop through neovascularization phenomena within the tumor that give rise to blood vessels with varying degrees of aberrant morphology.
Other important factors are patient phototype and age and lesion location.10 In individuals with fair skin, it is important to compare melanocytic nevi to nevi in other locations to check for similarities in pattern, color, and vascular structure. While vessels are easier to identify in fair-skinned individuals, who generally have less pigmented lesions, they need to be examined very closely as they are sometimes the only clues that can lead to a diagnosis or aid long-term follow-up.11
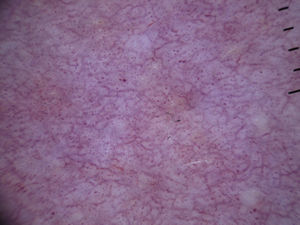
In certain anatomic areas, above all on the breasts and the buttocks, the normal vascular plexus, which is formed by papillary dermal vessels (seen as small red dots) and the upper dermal plexus (seen as a coarse, somewhat blurred network of vessels) are very clear and may be mistaken for tumor vessels6 (Fig. 2). A patient's age can also influence the appearance of blood vessels with a dermoscope. Vessels may be easier to visualize in areas with marked elastosis, common in elderly patients, but this, again, can result in normal vessels being confused with tumor vessels.
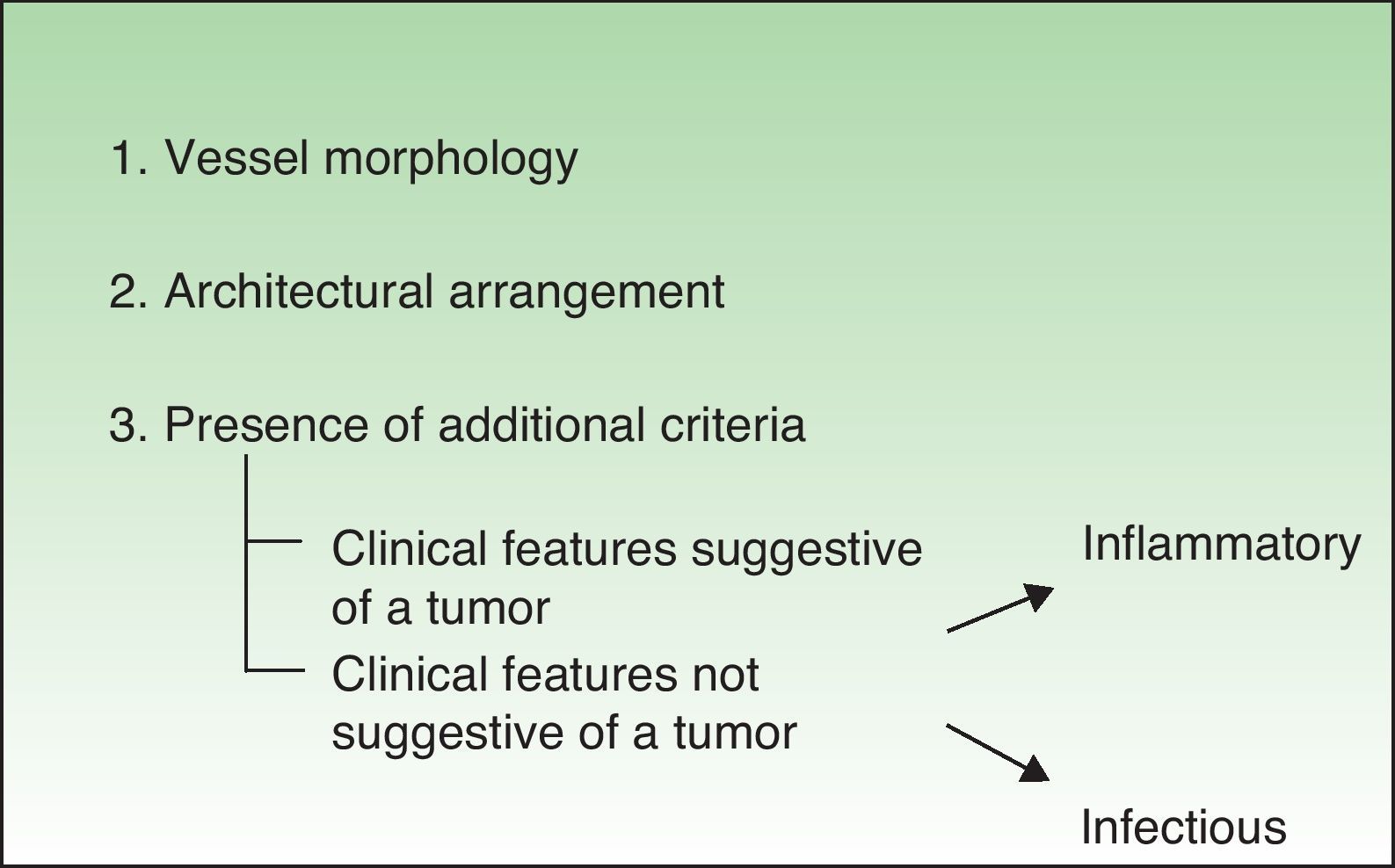
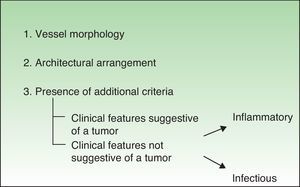
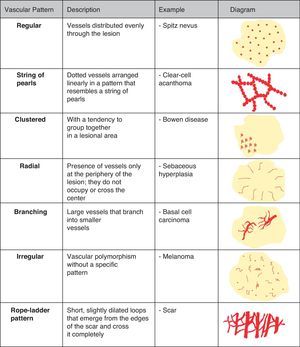
Diagnostic ProcedureThere are 3 essential features that should be analyzed when evaluating the vascular structure of a lesion with a dermoscope: vessel morphology, architectural arrangement, and finally, additional features that might help to narrow a diagnosis.
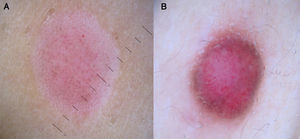
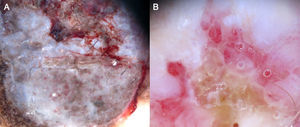
It is also essential to determine if the clinical appearance of the lesion is that of a tumoral or a nontumoral (inflammatory or infectious) lesion because the vascular patterns may be similar7 (Fig. 3).
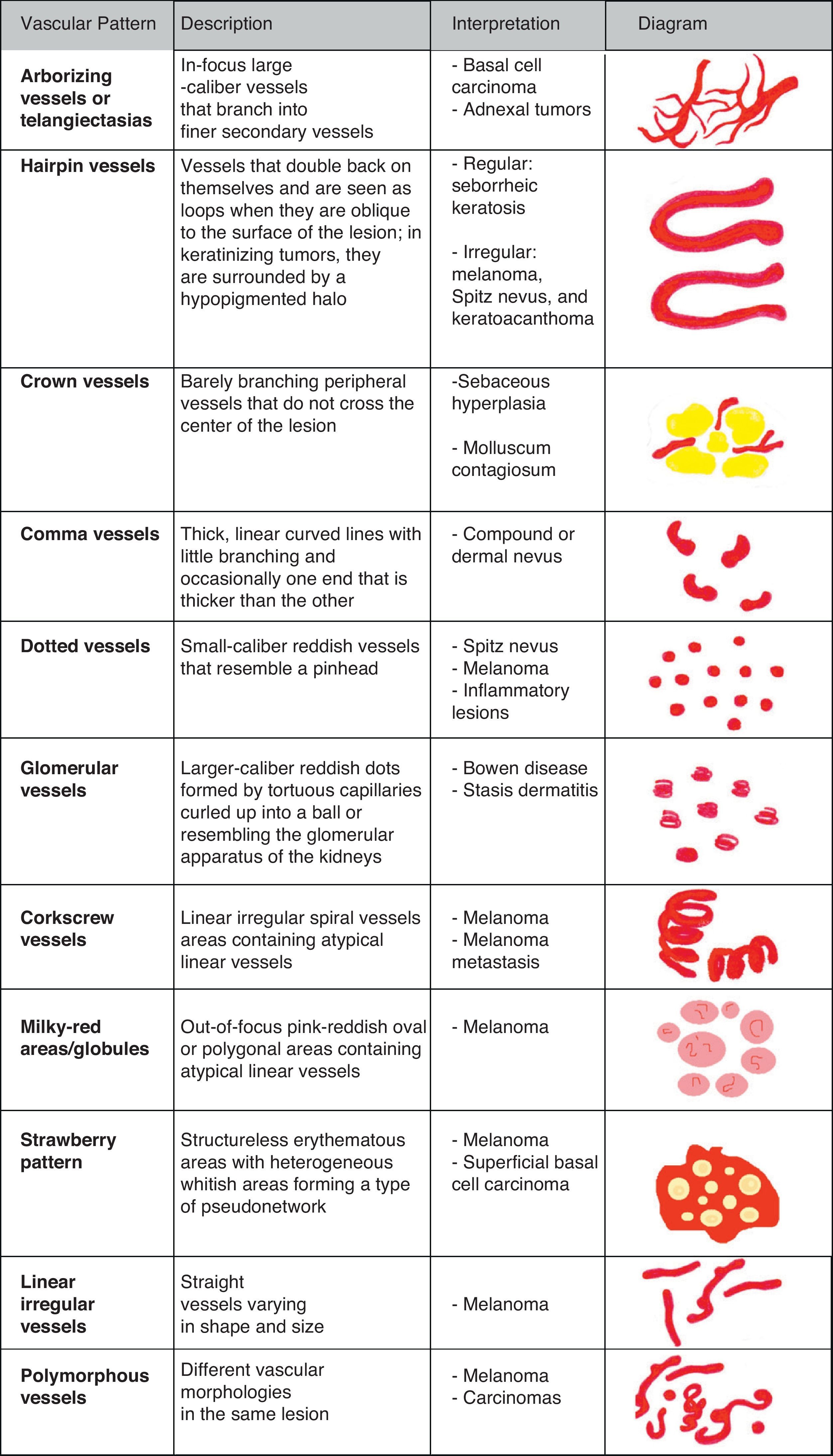
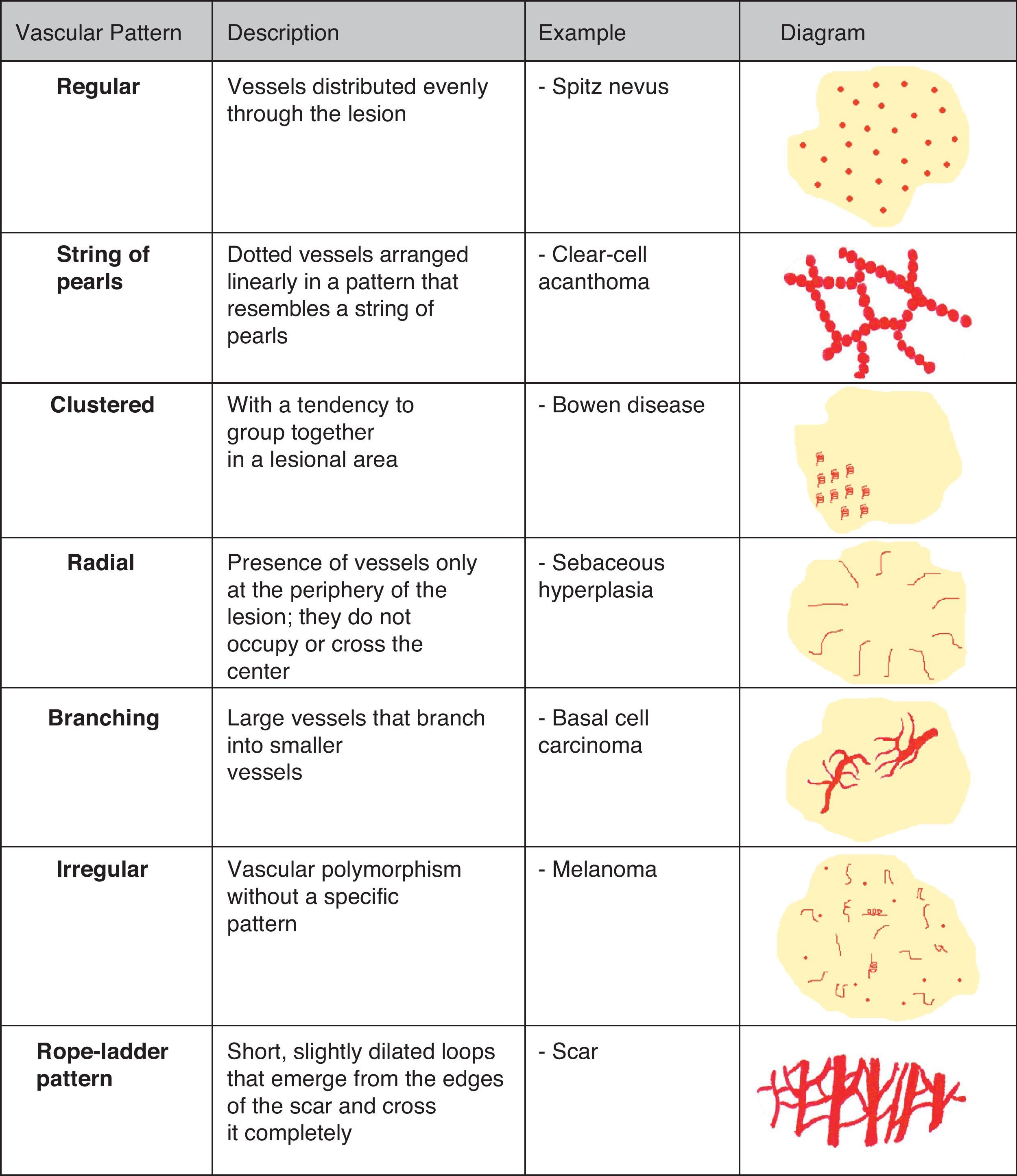
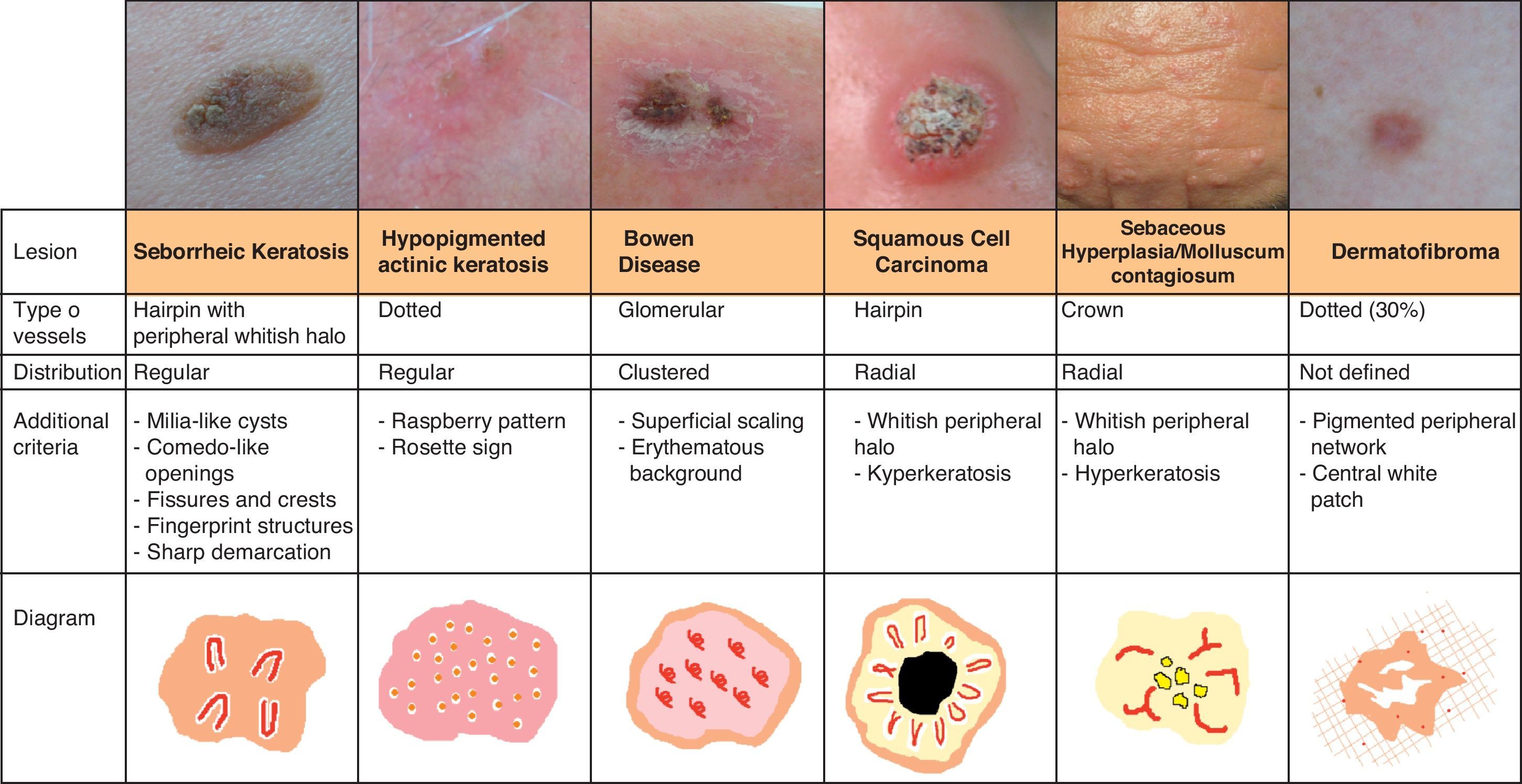
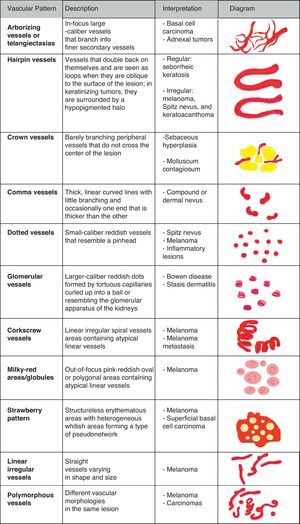
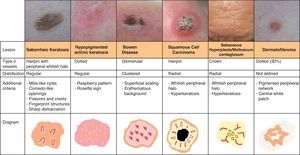
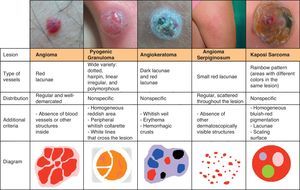
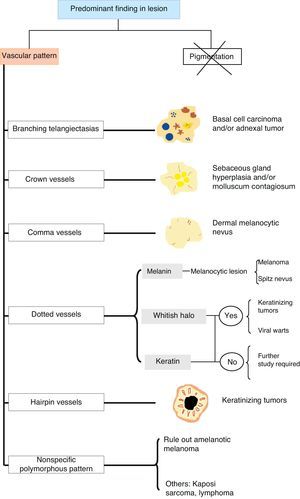
Vascular StructuresDifferent vascular patterns are identified on dermoscopy (Fig. 4). The most important ones are comma vessels, dotted vessels, linear irregular vessels, hairpin vessels, glomerular vessels, arborizing vessels, crown vessels, the strawberry pattern, milky-red areas or globules, and lacunae.6,7,12–14
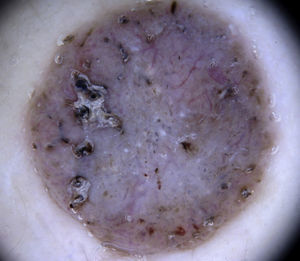
Architectural ArrangementOnce the morphologic features of the vessels have been analyzed, it is essential to examine the architectural features, as these provide essential clues for diagnosing nonpigmented cutaneous tumors, many of which have the same vessels but different architectural arrangements6,7 (Fig. 5).
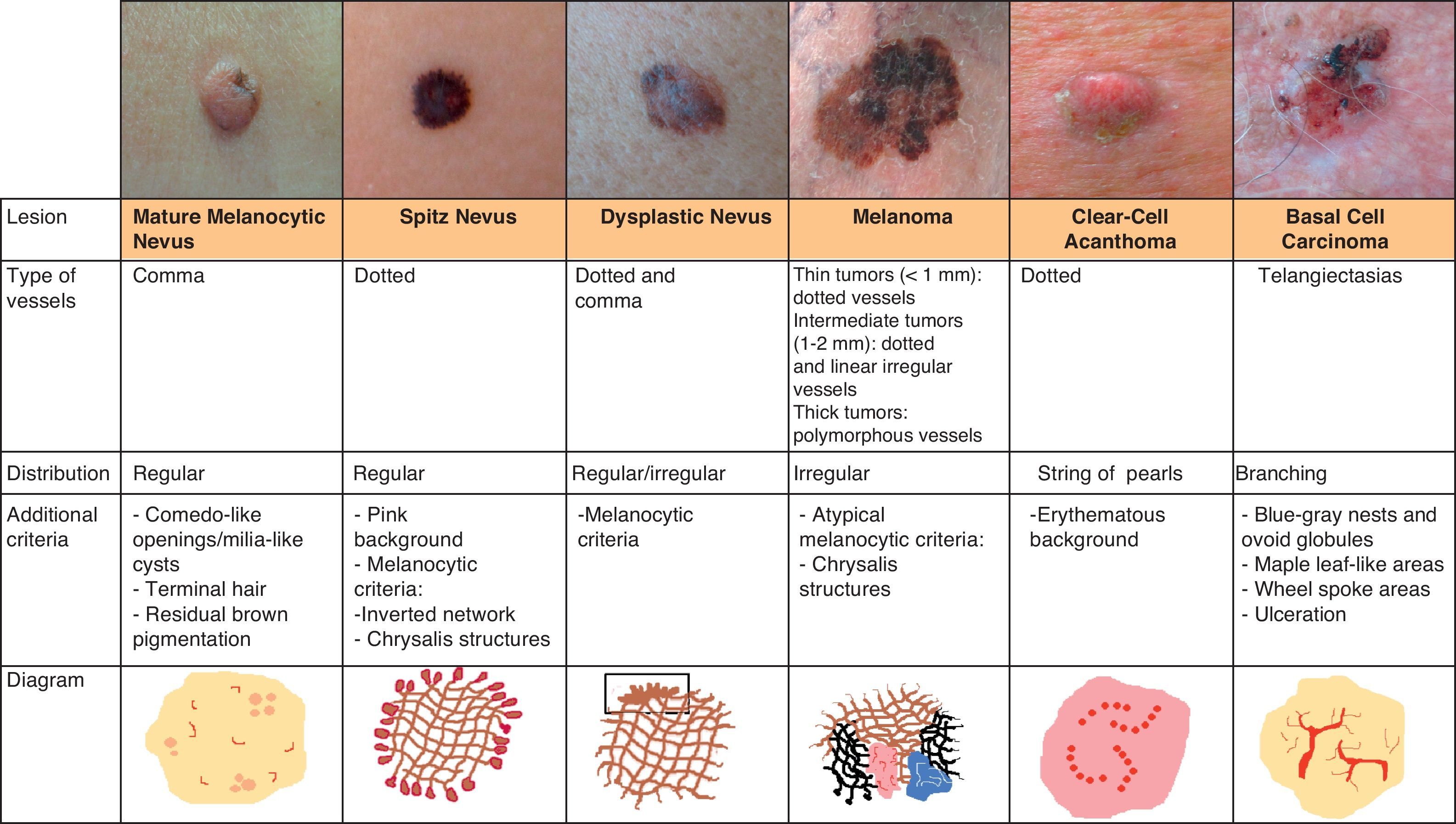
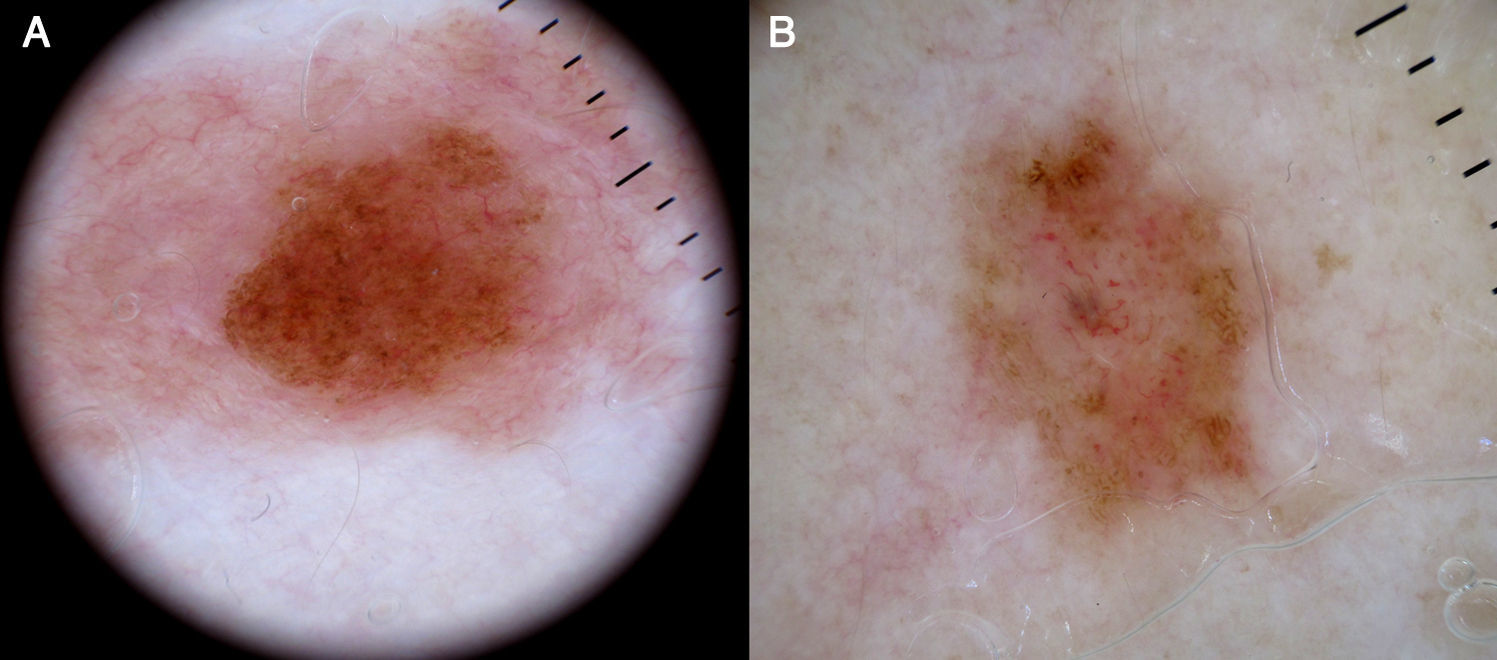
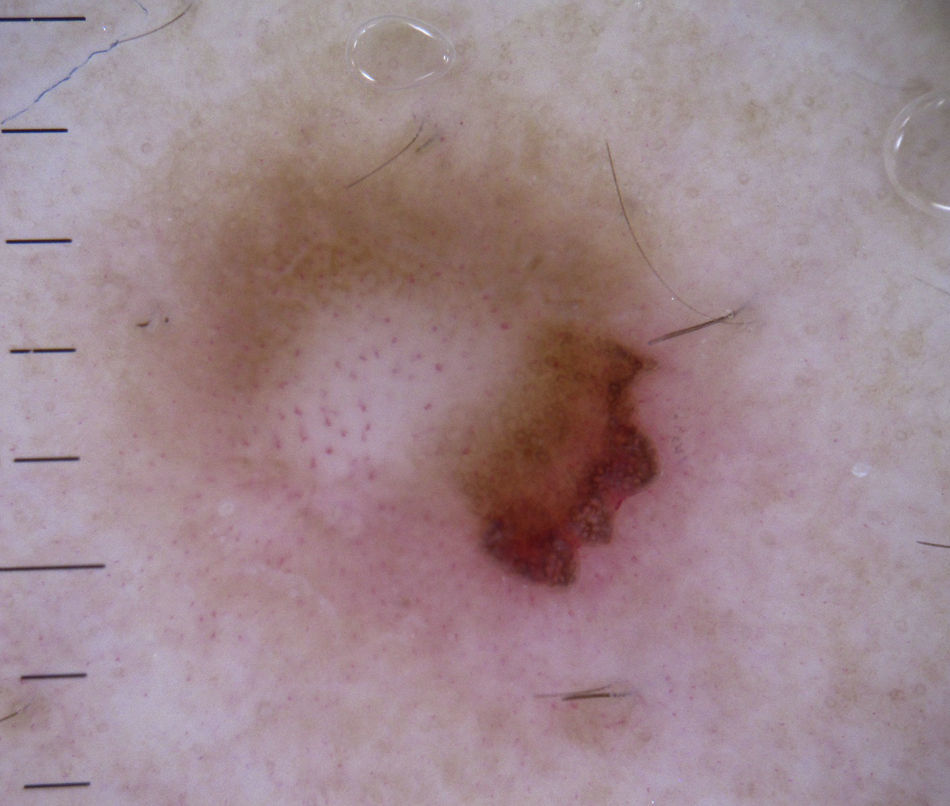
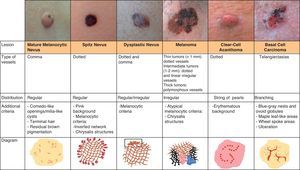
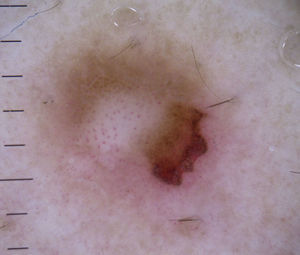
Melanocytic TumorsMature Melanocytic NeviThe key dermoscopic finding for the diagnosis of intradermal melanocytic nevus is the presence of comma vessels. When present, the probability of the lesion being an intradermal melanocytic nevus is 94% (positive predictive value).12,15
In the case of melanoma, however, comma vessels are a significant negative predictor.16
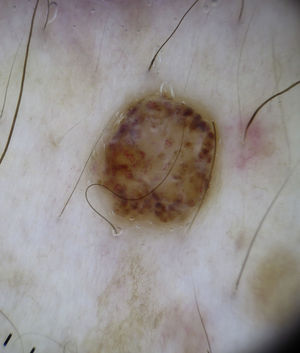
Comma vessels are thick, slightly curved, barely branching vessels that vary enormously in size and caliber and are generally evenly distributed throughout the tumor. Other characteristic dermoscopic findings in mature melanocytic nevi are terminal hair, milia-like cysts, and lightly pigmented globules (Figs. 6 and 7).
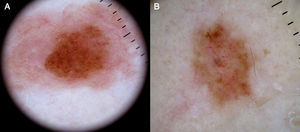
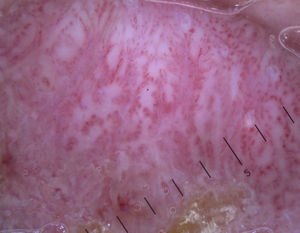
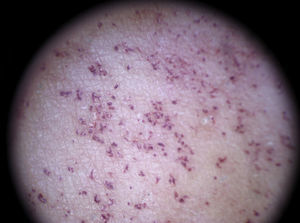
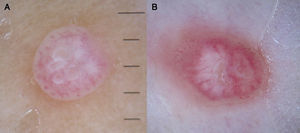
Dotted vessels are highly predictive (90%) for melanocytic lesions in general and for Spitz nevi in particular (they are seen in 77.8% of these lesions).12,17 They correspond to small capillary vessels in the papillary dermis and are arranged close together in a vertical pattern. Dotted vessels are common findings in the hypopigmented center of Spitz nevi, where they are highly monomorphous, regularly distributed, tightly packed, and appear against a milky pink background (Fig. 8). While chrysalis structures are a common feature of melanoma,14 they may occasionally be seen in Spitz nevi.15 They are shiny white streaks that correspond to collagen alterations in the papillary dermis and can only be seen with polarized light dermoscopy.13 In lightly pigmented Spitz nevi, the identification of a residual starburst pattern can also aid diagnosis.16
As is widely known, it can be impossible to distinguish between spitzoid lesions and melanoma on dermoscopy, particularly in nodular and atypical variants as these have atypical vascular structures impossible to differentiate from those seen in thick hypomelanotic melanoma (linear irregular and glomerular vessels and a pink-white veil). Furthermore, the dotted lesions typically seen in flat spitzoid lesions are also a common finding in hypomelanotic or amelanotic melanoma. In adult patients, it is advisable to excise all lesions with dermoscopic features of spitzoid lesions, although the identification of dotted vessels alone may not be sufficient to justify excision; in such cases, there should be at least one other dermoscopic or clinical sign (Fig. 7).
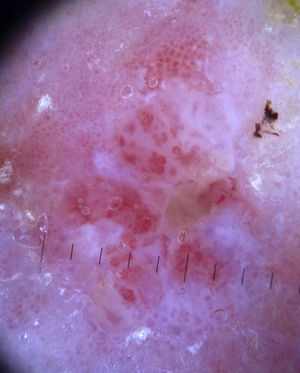
Atypical Melanocytic NeviA common finding in lightly pigmented atypical nevi, which are found in fair-skinned individuals, is the presence of dotted or comma vessels on a slightly raised center surrounded by a flat peripheral area with a predominantly light brown reticular or homogeneous pattern (Fig. 9).
Dotted vessels are not as tightly packed in atypical nevi as they are in Spitz nevi, and there are only few comma vessels. Another difference is that in Spitz nevi the background is clearly pink, while in atypical nevi it is darker7,18 (Fig. 7).
Hypomelanotic or Amelanotic MelanomaThe predominant morphology and arrangement of vessels in amelanotic melanomas depend largely on the thickness of the tumor.7 Melanoma vessels are new, tumor-induced blood vessels located in the deep layers of the skin, explaining why they may appear out of focus when viewed under a dermoscope (Fig. 10).The main finding in flat lesions is the presence of dotted vessels with a regular shape and distribution, similar to those seen in hypopigmented Spitz nevi. In raised lesions, the vessels have an irregular distribution and are longer, thicker, and more polymorphous.16,19,20 Common dermoscopic features of hypomelanotic melanomas of intermediate thickness are dotted vessels combined with hairpin or linear irregular vessels forming a polymorphous pattern against a pink-reddish background. Dotted vessels, however, may sometimes be the only vessels seen; while their distribution tends to be somewhat more irregular than in Spitz nevi, they are not always discernible on dermoscopy.21–23Other structures that are suggestive of melanoma, such as the presence of reticular depigmentation or chrysalis structures, can also be seen in Spitz nevi, hence the recommendation to excise all lesions with Spitzoid features in adult patients.24–26
Thick melanomas have a different vascular supply system and may have arborizing vessels and/or milky-red globules in addition to linear irregular, hairpin, and corkscrew vessels.7
The serpentine branching of arborizing vessels is more regular than that seen in basal cell carcinoma. While seen less frequently, red-pink globules are highly suspicious for hypomelanotic melanoma. In fact they are the feature with the highest positive predictive value (77.8%) for melanoma. A small proportion of hypomelanotic melanomas have a milky-red background. This dermoscopic feature is also found in basal cell carcinoma and pyogenic granuloma,27 but is rare in benign tumors.
Dotted vessels are rare in thick melanomas and are normally seen in the flat portion of the tumor.
Different clinical variants of melanoma can exhibit specific dermoscopic features. Nodular melanoma, for example, may lack a clear vascular pattern, and in such cases, the identification of polychromatism is the only clue to diagnosis.28–30
Eczema-like melanoma exhibits marked vascular polymorphism (dotted, glomerular, or atypical linear vessels) in association with a residual brown-grayish color.31
Melanomas that have undergone complete regression can exhibit scar-like depigmentation, a pink background, linear irregular vessels, dotted vessels, residual pigment, and whitish transverse bands.
Desmoplastic melanoma, in turn, has features indicative of regression such as areas of scarring, pepper-like granules, multiple colors, and melanoma-associated vascular patterns such as linear irregular vessels and milky-red globules.32,33
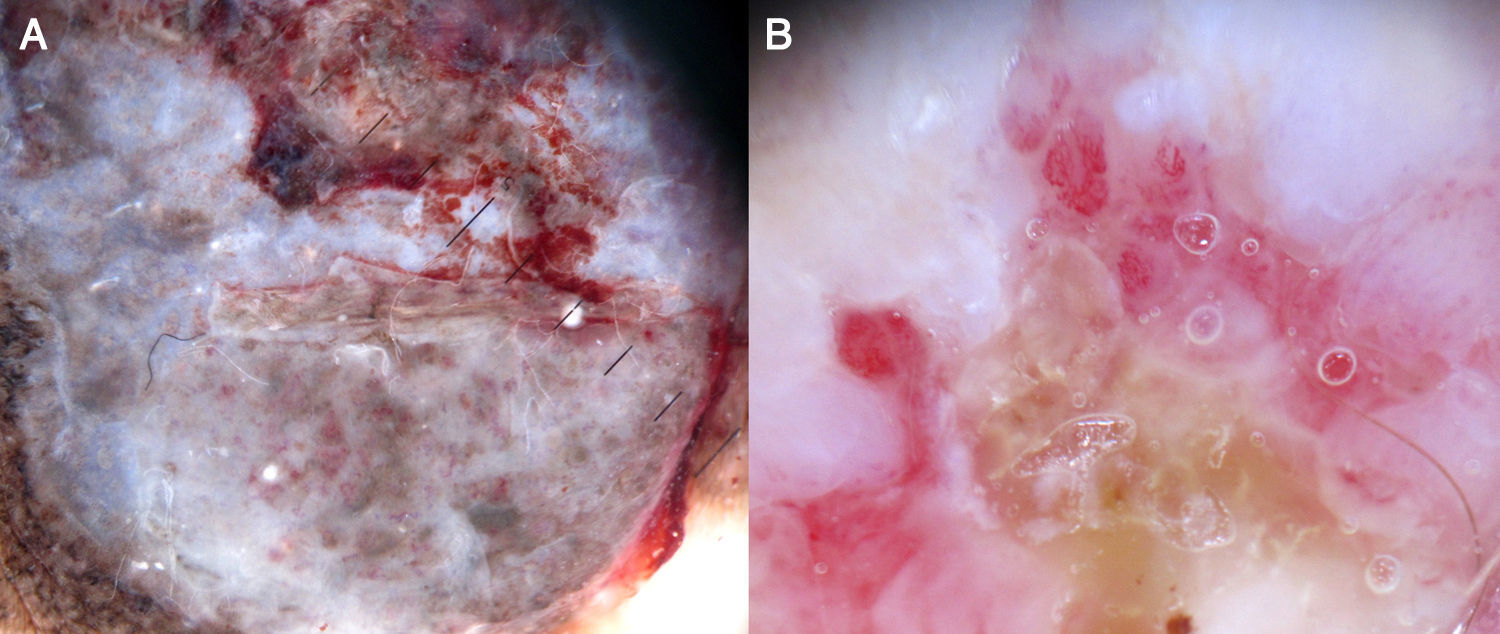
Another finding that can help in the diagnosis of melanoma is poppyfield bleeding, which refers to the appearance of droplets of blood when the dermoscope is pressed firmly against the lesion, causing an increase in blood pressure on the vessel wall and subsequent rupture. The histological correlates of these droplets are dilated, fragile tumor vessels. They are seen in thick melanomas that generally have a Breslow depth of over 2.5mm and histologic ulceration.34
Vascular polymorphism is not always indicative of malignancy and can be found, with more or less frequency, in irritated seborrheic keratosis, adnexal tumors, and some dermatofibromas (Fig. 7).
Melanoma MetastasisVascular areas are more common in cutaneous melanoma metastasis than in primary cutaneous melanomas. The predominant pattern depends on the thickness of the tumor, with dotted vessels predominating in thin lesions, and corkscrew or saccular vessels predominating in thick lesions. Corkscrew vessels, which have a helical or spiral form, are a hallmark of metastasis, appearing in an estimated 83.3% of cases; while they are specific to melanoma metastasis, they can also be observed in amelanotic areas of primary nodular tumors (Fig. 11).35 The saccular pattern is characterized by the presence of large round or oval structures, which generally have a brown-reddish, red, or black-bluish color.36 The saccular pattern in metastatic lesions can be confused with the lacunae seen in angiomas, as both have a highly uniform color and structure and abrupt border cutoff. The color of this pattern can also resemble that of blue nevi. In such cases, the presence of a reddish halo around the lesion, which is highly specific for metastasis, can help in the differential diagnosis.37
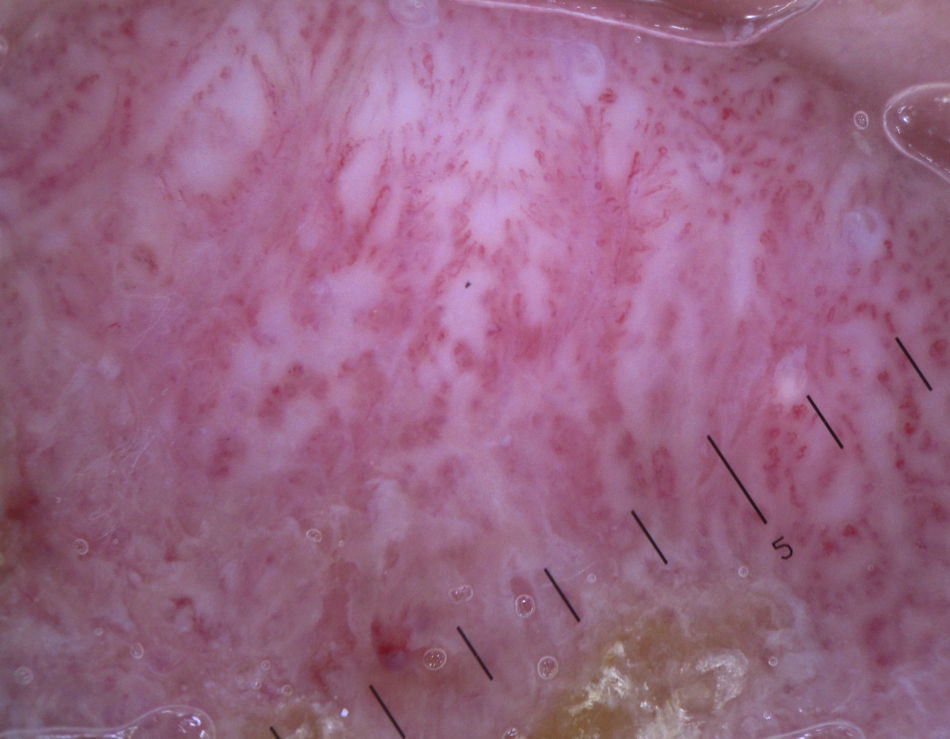
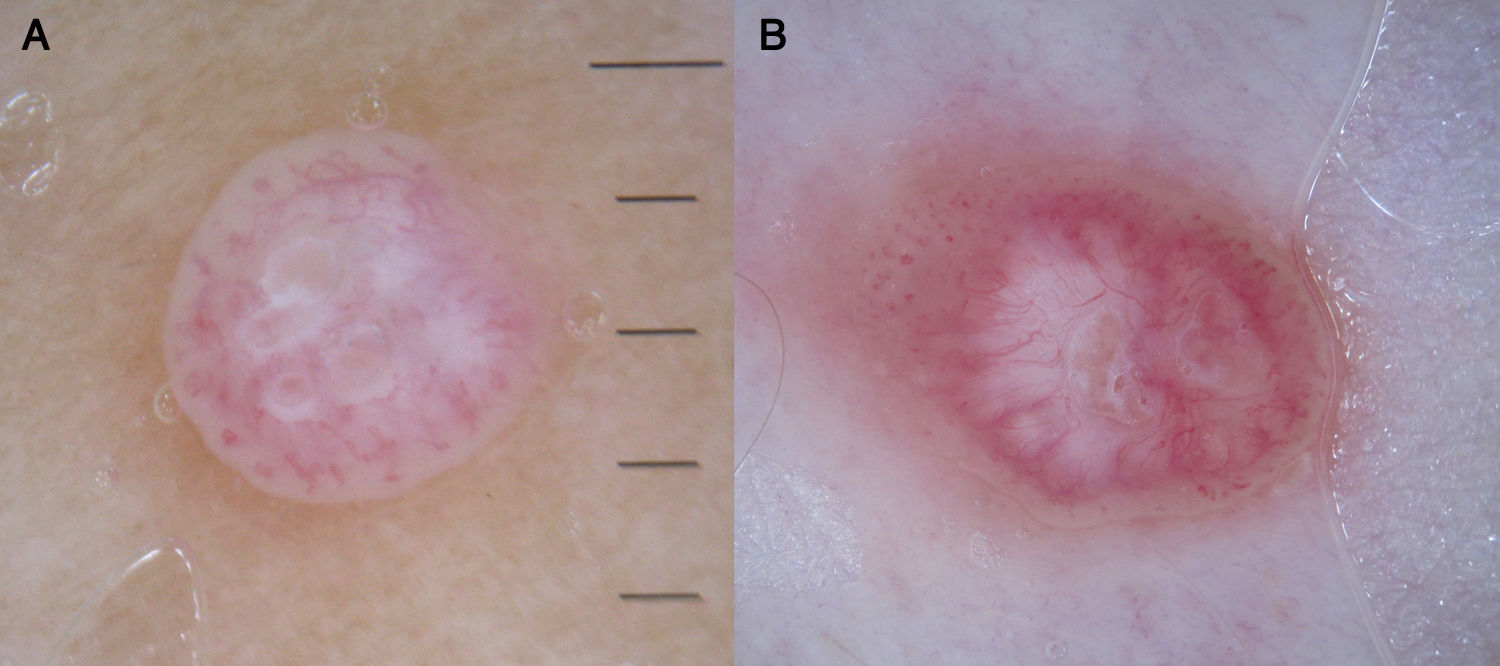
Keratinizing TumorsSeborrheic KeratosisWhile hairpin vessels are present in most keratinizing tumors, they are a hallmark of seborrheic keratosis (Fig. 12). Their positive predictive value for this condition is 70%, contrasting with just 13.3% for squamous cell carcinoma.12 They are most often seen in tumors of the head and neck and are characterized by a regular distribution, a monomorphous appearance, and often a whitish halo, which is a sign of keratinization. An exception is irritated seborrheic keratosis, in which dermoscopy often shows a polymorphous vascular pattern, with long, irregular, bent, twisted, or double-stranded hairpin vessels.
Additional features that can aid diagnosis are milia-like cysts, comedo-like openings, and abrupt border cutoff6,38 (Fig. 13).
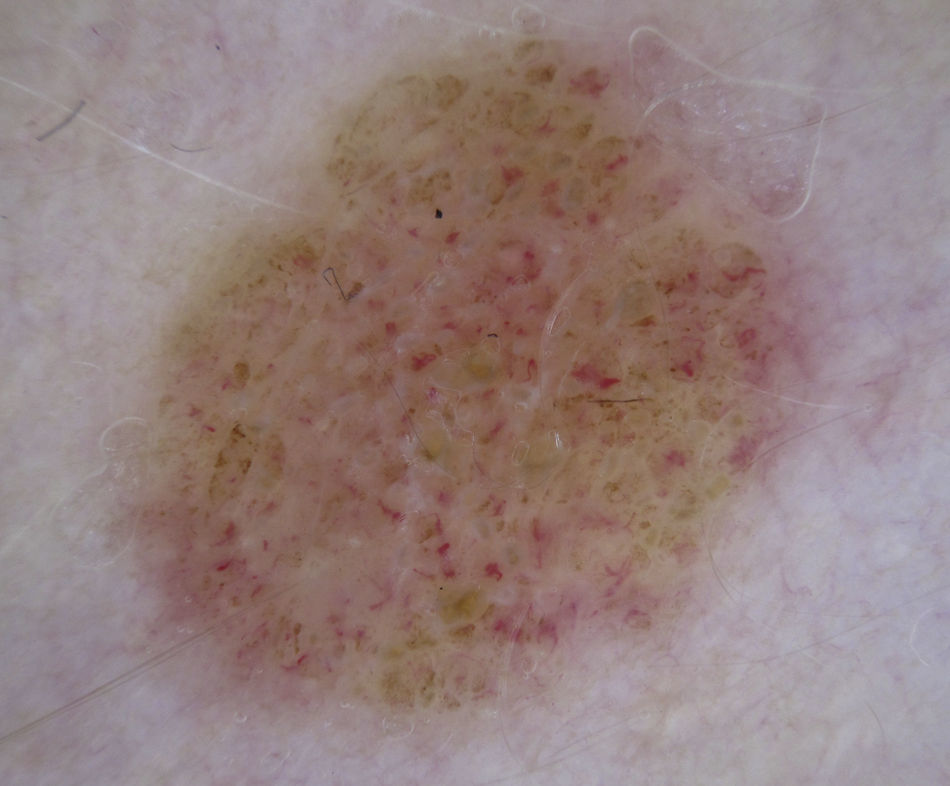
Clear-Cell AcanthomaDotted vessels are strong predictors of all melanocytic tumors except clear-cell acanthoma. In these tumors, the key to a correct diagnosis is the distribution of the vessels (Fig. 14).
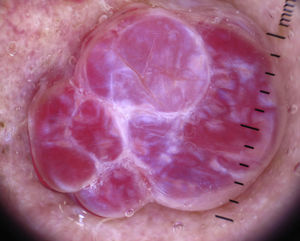
The dermoscopic features of clear-cell acanthoma are highly characteristic, with globular vessels arranged in a linear, string of pearls distribution or, on occasions, a reticular distribution.39,40 Common findings include a whitish background and a peripheral collarette, attributable to the rapid growth of the lesion41 (Fig. 7); similar features are observed in pyogenic granuloma.
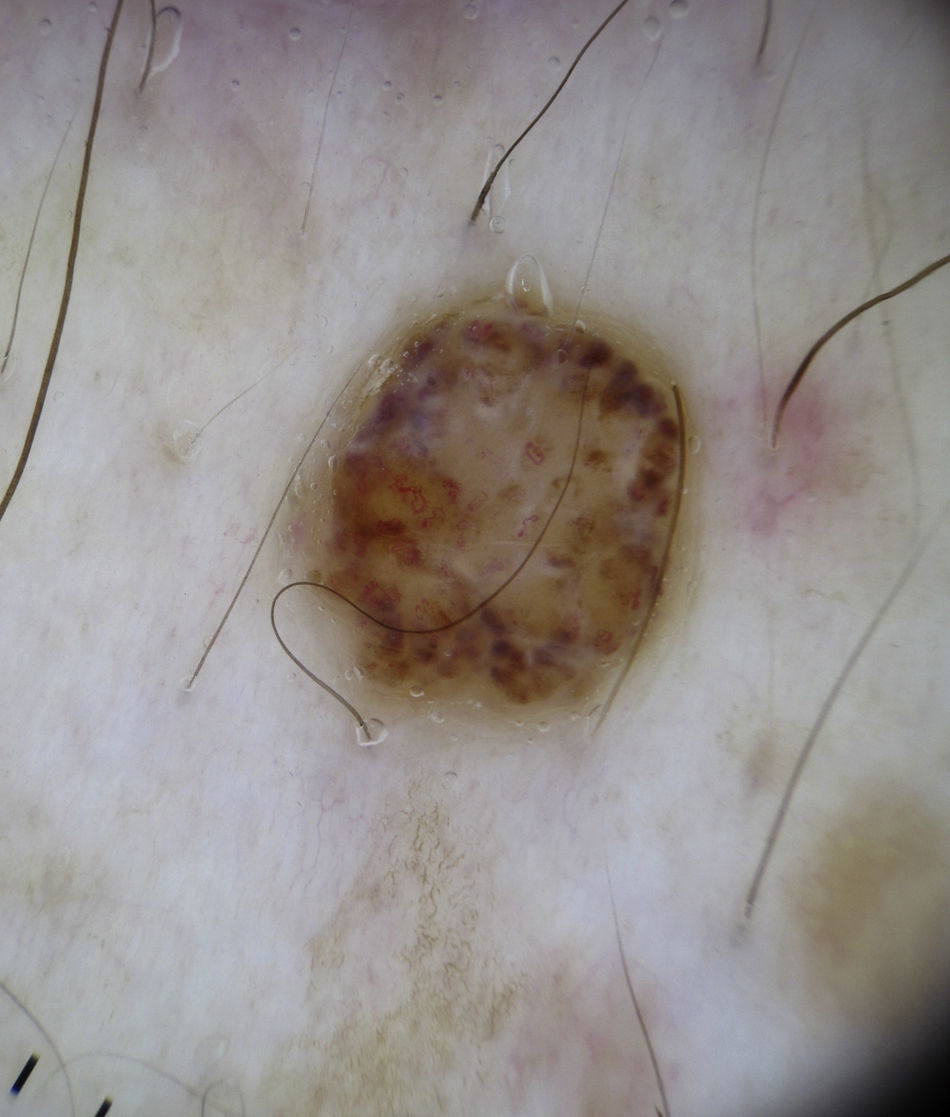
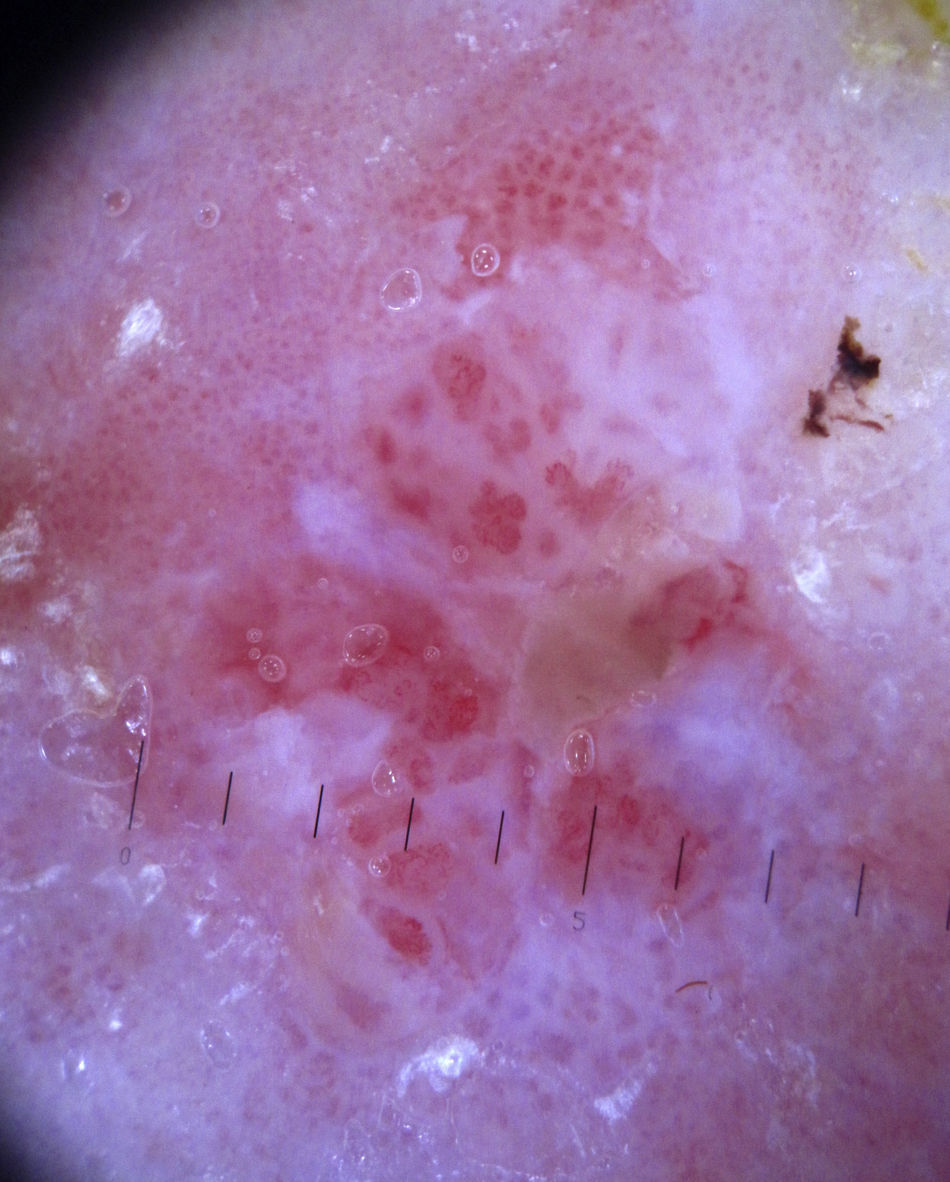
Bowen DiseaseBowen disease exhibits 2 types of vessels on dermoscopy: dotted vessels and glomerular vessels, both of which form small, tightly packed clusters in association with surface scales (Fig. 15).
Glomerular vessels are a variation of dotted vessels; they are larger, tortuous capillaries, curled up into a ball and distributed in regular clusters.42 Their form resembles the histologic appearance of the glomerular apparatus of the kidneys. Additional features in pigmented Bowen disease include regular small brown globules in a patchy distribution (90% of cases) and homogeneous brown to gray pigmentation (80% of cases)43 (Fig. 13).
It has been reported that the concomitant presence of a clustered glomerular pattern and hyperkeratosis is 98% predictive for Bowen disease.44
The differential diagnosis for a dermoscopic finding of glomerular vessels should include stasis dermatitis of the legs.45
Invasive Squamous Cell CarcinomaInvasive squamous cell carcinoma exhibits highly polymorphous vascular structures, with hairpin vessels of varying shape and distribution that are often long, together with dotted and/or linear irregular vessels. The vessels appear on a whitish background with a central area of keratin scales and crusts.7 Ulceration may also be seen (Fig. 13).
KeratoacanthomaDermoscopically, keratoacanthoma shows a central mass of yellow-brown keratin frequently surrounded by long, and sometimes enlarged, hairpin vessels, which are typically surrounded by a whitish halo. They have a radial distribution and may occasionally be accompanied by atypical linear or glomerular vessels.
The peripheral arrangement of hairpin vessels in keratoacanthoma is a very useful finding for distinguishing this condition from seborrheic keratosis, where the vessels are evenly distributed throughout the lesion.7
Actinic KeratosisThe hallmark dermoscopic finding in nonpigmented actinic keratosis of the face is the strawberry pattern, seen in over 90% of cases46; this pattern is characterized by erythema or a reddish pseudonetwork and large out-of-focus vessels situated between prominent follicular openings and surrounded by a whitish halo. A rosette sign has also been described when lesions have been visualized by polarized light dermoscopy. This sign is a geometric figure with 4 transparent whitish symmetric points form a square in the center of the follicular openings. Although their origin is unknown, they might be due to the birefringent character of keratin in actinic keratinoses.6
Bowenoid actinic keratosis typically shows regularly distributed glomerular vessels that, unlike in Bowen disease, do not form clusters (Fig. 13).
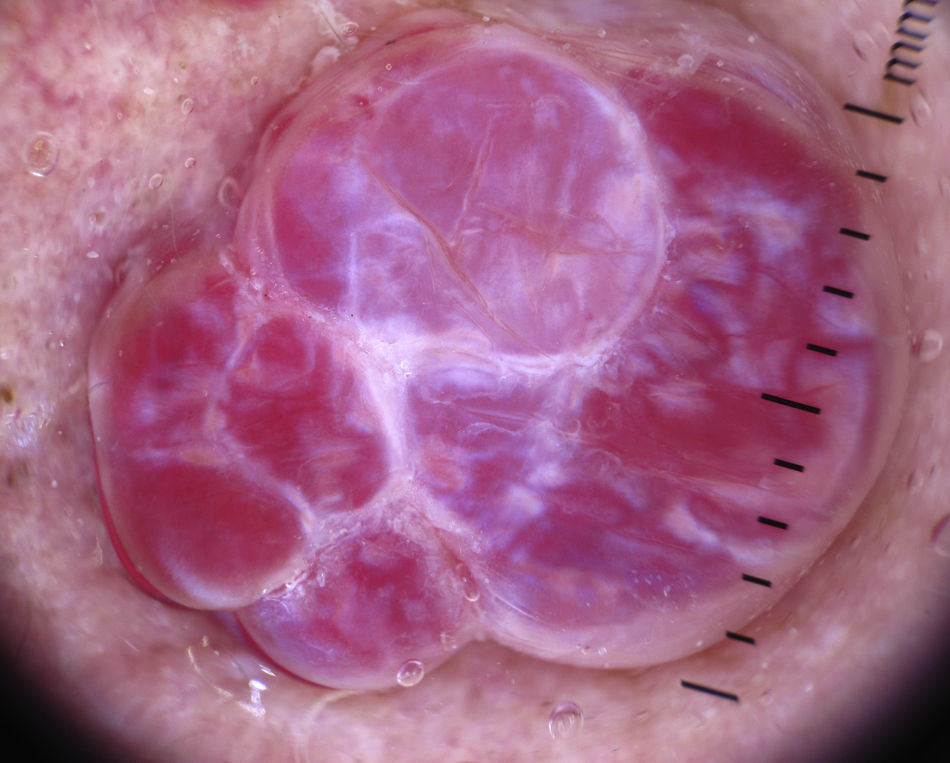
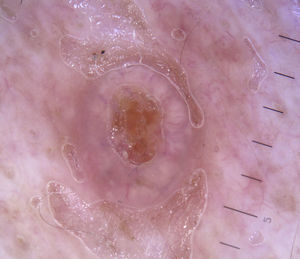
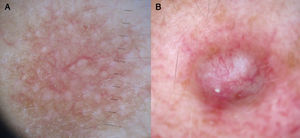
Other Tumor LesionsSebaceous HyperplasiaCrown vessels are a characteristic dermoscopic finding in sebaceous hyperplasia; they appear as long, out-of-focus telangiectasias, with tortuous yet regular barely branching vessels surrounding a central polylobulated whitish-yellowish area; they characteristically do not cross the center of the lesion,47,48 where the ostium of the gland is seen as a small crater (Figs. 13 and 16).49
Basal Cell CarcinomaThe key to the diagnosis of basal cell carcinoma is often the identification of vessels as between 80% and 90% of these tumors are nonpigmented.50
Arborizing telangiectasias is the most characteristic vascular pattern seen in nodular basal cell carcinoma, with a positive predictive value of 94%.12
These vessels are large-diameter vessels that branch at irregular intervals into increasingly thin capillaries; they are bright red, have abrupt border cutoffs, and are in sharp focus.15
They are located immediately below the epidermis, on the surface of the tumor, and throughout the tumor. They are histologically correlated with the presence of dilated vessels on the tumor surface and are specific to nodular lesions, which require a greater blood supply (Fig. 17).
The following features can help to diagnose pigmented basal cell carcinoma: blue-gray ovoid nests, multiple blue-gray globules, maple leaf–like areas, and spoke wheel areas.15,51
The characteristic findings in superficial basal cell carcinoma are shiny whitish red structureless areas, multiple small erosions, and short fine telangiectasias. The background consists of a transparent to opaque structureless white-reddish area with multiple small erosions corresponding to the hypervascularized area of the tumor.52
Short fine telangiectasias consist of small tortuous vessels, twisted together, measuring less than 1mm in length and displaying little or no branching. They are in sharp focus and are found in approximately 90% of superficial basal cell carcinomas.
In sclerodermiform basal cell carcinoma, the vessels are generally thinner, more diffuse, and have less branching; the background is also whiter, with diffuse, poorly demarcated borders.
In fibroepithelioma of Pinkus, typical findings include fine arborizing vessels, either alone or in association with dotted vessels and whitish streaks. The arborizing vessels are not particularly large and typically have less evident branching. Additional features include a structureless gray-brown area and numerous gray or brown dots53,54 (Fig. 7).
Adnexal TumorsAdnexal tumors can be mistaken for basal cell carcinoma as both entities have almost identical arborizing telangiectasias (Fig. 18); they may also be confused with melanoma because of the presence of highly polymorphous vessels.
Irregular milky-red areas, red lacunae, and linear irregular vessels can be seen in nonpigmented eccrine porocarcinoma,55 and there have also been reports of glomerular vessels, hairpin vessels, and irregular linear vessels with a white-pink halo and structureless pink areas.56 Finally, Merkel cell tumor exhibits atypical linear vessels against a reddish background.6
Kaposi SarcomaThe most characteristic dermoscopic finding in Kaposi sarcoma is the rainbow pattern, a term used to describe circumscribed structureless areas that display a spectrum of colors similar to those of a rainbow (Fig. 19).
The rainbow pattern was originally believed to be specific to Kaposi sarcoma,57 but similar patterns have since been described in other lesions, including dermatofibroma, pyogenic granuloma, melanoma, stasis dermatitis, and lichen planus.58
Other characteristic features of Kaposi sarcoma are homogeneous red-bluish pigmentation, lacunae, scaling, and brown globules6 (Fig. 20).
DermatofibromaVascular structures have been identified in approximately 50% of all dermatofibromas.59 Dotted vessels are the most common finding (30%), followed by comma vessels (17.3%) and hairpin vessels (15.3%). There have also been rarer reports of linear vessels and polymorphous atypical vessels (typically associated with melanoma), telangiectasias, and glomerular vessels (Fig. 21).
The identification of features that are specific to dermatofibroma, such as white central areas and a fine peripheral pigmented network, can aid diagnosis (Fig. 13).
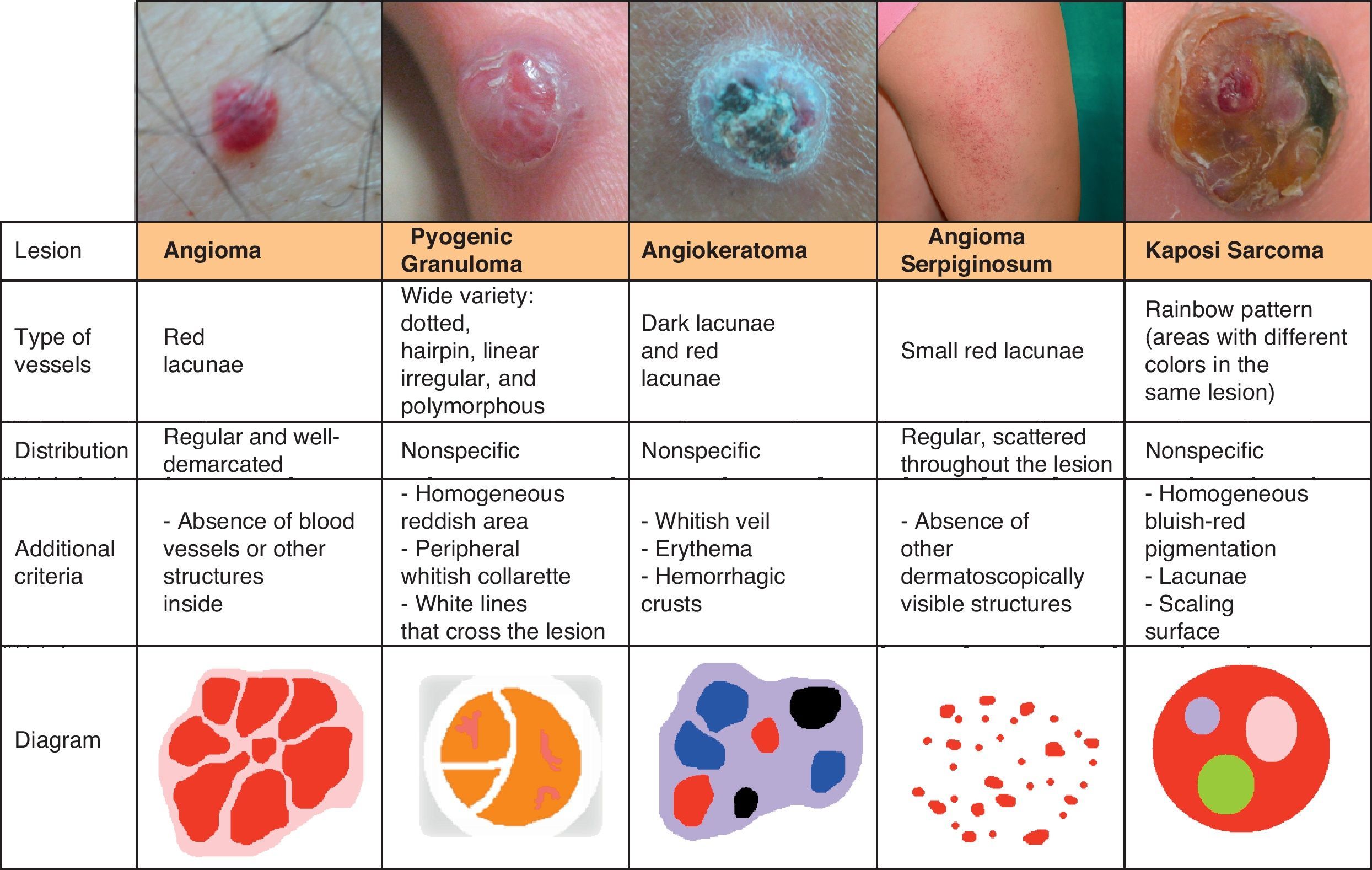
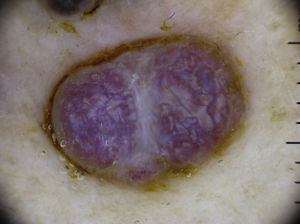
Vascular LesionsPyogenic GranulomaPyogenic granuloma is dermoscopically characterized by homogeneous reddish or white-reddish areas surrounded by a whitish collarette in up to 85% of cases. The areas correlate with a histologic finding of proliferating capillaries and veins, while the collarette corresponds to the epidermal collarette that often surrounds the proliferation of blood vessels in pyogenic granuloma.60
Additional criteria are white rail lines that intersect the homogeneous reddish area (Fig. 22), various vascular structures (hairpin, dotted, linear irregular, and polymorphous vessels), and hemorrhagic crusts.61 Histologically, the white rail lines correspond to the fibrous bands that distinguish lobular capillary hemangiomas from more advanced forms of pyogenic granuloma.
Nonetheless, none of the above features distinguish pyogenic granuloma from amelanotic nodular melanoma, hence the recommendation to excise and histologically examine all lesions that are clinically and dermoscopically suggestive of pyogenic granuloma23 (Fig. 20).
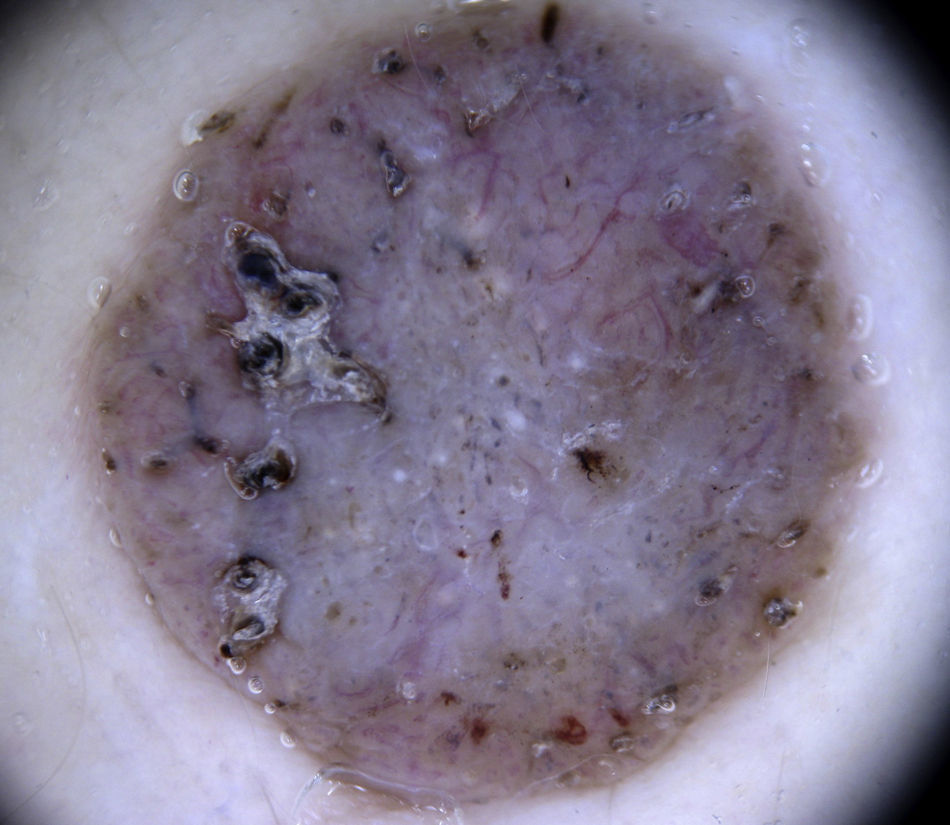
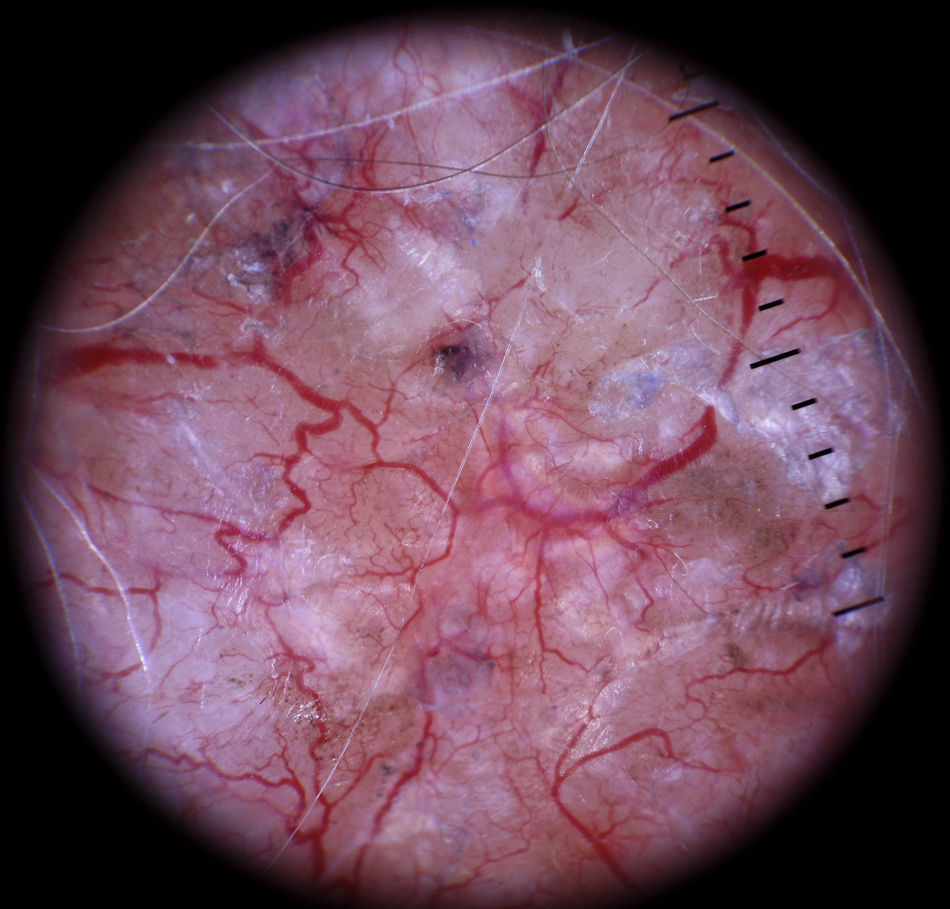
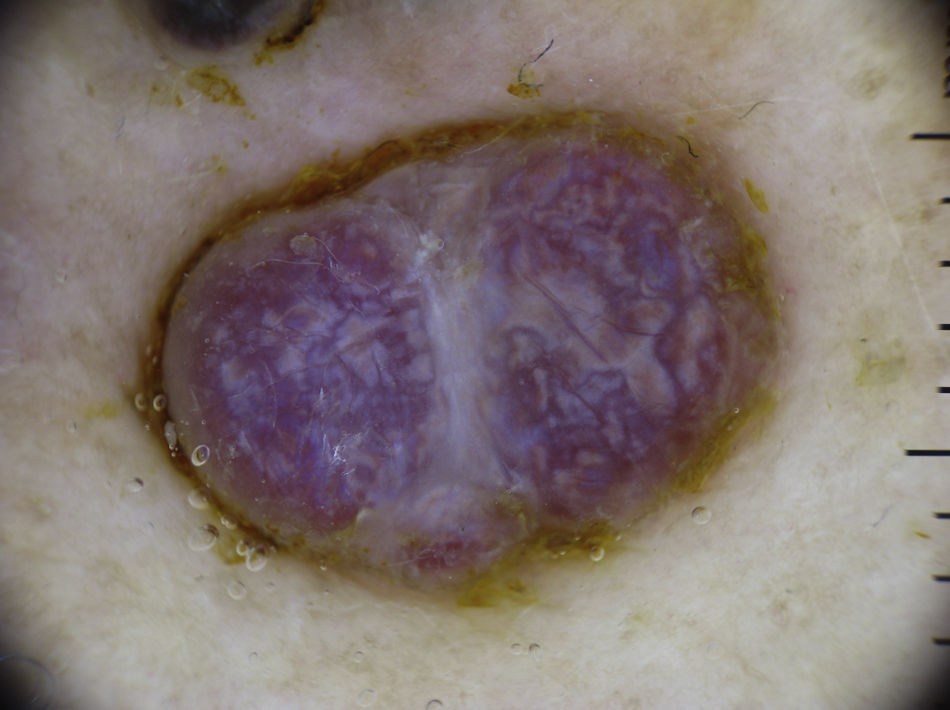
HemangiomasThe most characteristic dermoscopic feature of hemangiomas is the presence of numerous round or oval red-bluish lacunae that are generally well-demarcated. Histologically, these structures correspond to large dilated vascular spaces in the superficial dermis.62 No other vascular patterns are visible in the lacunae.62 The lacunae tend to vary in size and color within the same lesion and can appear alone or in clusters against a homogeneous reddish background. Partially or totally thrombosed hemangiomas tend to have dark, sometimes even black, lacunae, while partially involuted hemangiomas have whitish depigmented areas with a scar-like appearance (Fig. 20).
Vascular MalformationsDermoscopic findings for vascular malformations include a superficial pattern, known as a blob pattern, consisting of red globules and dots that correspond to dilated capillaries in the papillary dermis and a deeper pattern composed of reddish ring-like structures that correspond to ecstatic vessels located deep in the horizontal vascular plexus. The 2 patterns may be seen together.63–65
AngiokeratomaThe dermoscopic pattern most often seen in solitary angiokeratomas consists of dark lacunae and a whitish veil.66
Dark lacunae are the most frequent and specific dermoscopic finding for angiokeratoma and correspond histologically to partially or totally thrombosed dilated vessels in the dermis. The whitish veil corresponds to the characteristic histologic findings of hyperkeratosis and acanthosis. Red lacunae, erythema, and hemorrhagic crusts may also be seen (Fig. 20).
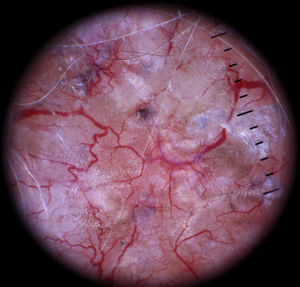
Other Rare Vascular LesionsMicrovenular hemangioma is dermoscopically characterized by diffuse erythema with multiple small red globules of varying size and a fine red peripheral network.67 Features of angioma serpiginosum include minute, well-demarcated round or oval lacunae distributed throughout the lesion (Figs. 20 and 23).68,69
The characteristic dermoscopic findings in lymphangioma circumscriptum are yellowish or light brown lacunae surrounded by whitish septa.
When viewed dermoscopically, targetoid hemosiderotic hemangioma usually exhibits a central area of red or dark lacunae surrounded by a homogeneous reddish, violaceous, or brownish area, or in some cases, a fine pigmented network.70
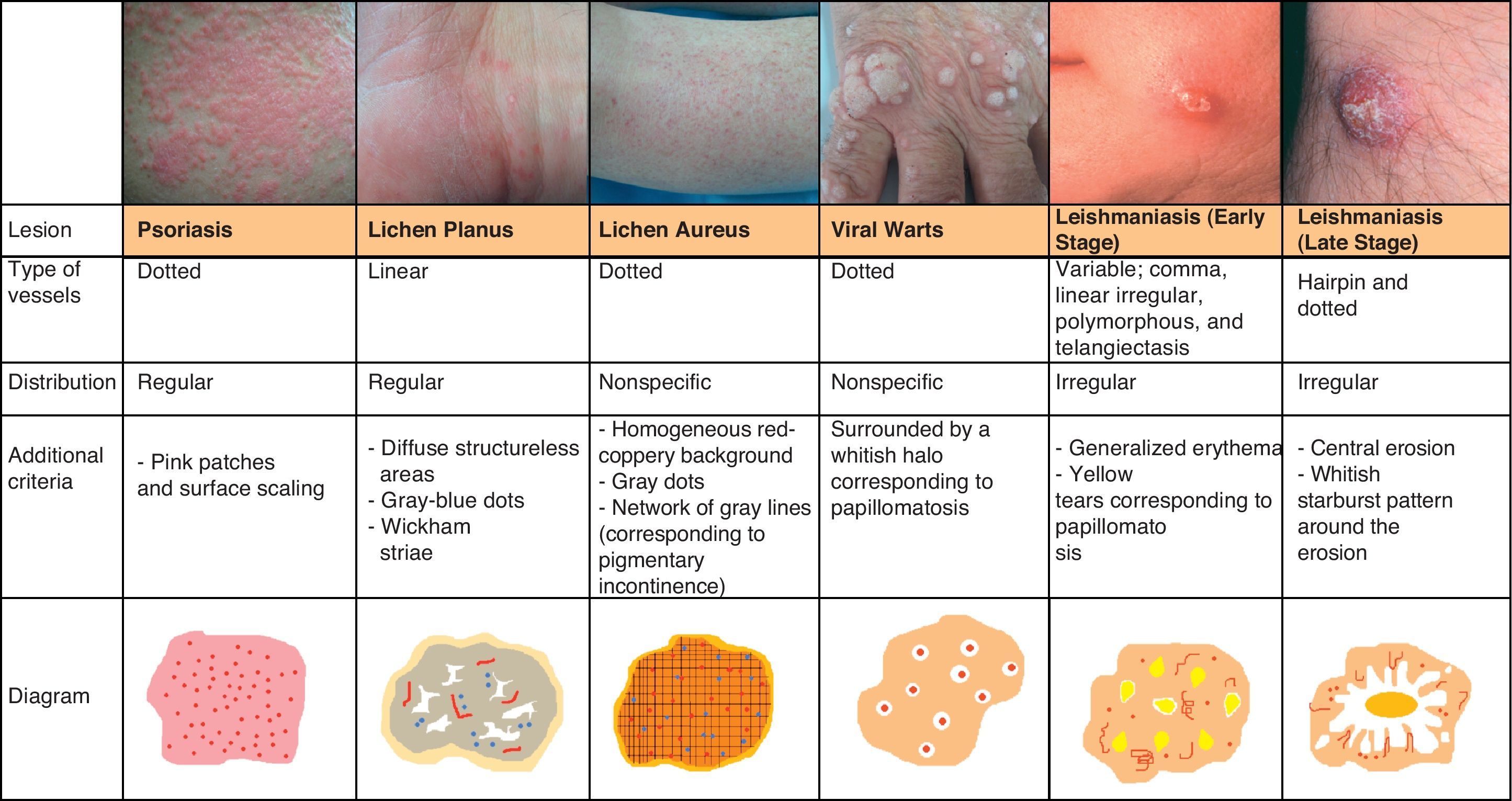
Inflammatory LesionsPsoriasisAlthough psoriasis lesions are generally easy to diagnose clinically, they can give rise to equivocal diagnoses, particularly in cases of atypical presentation.
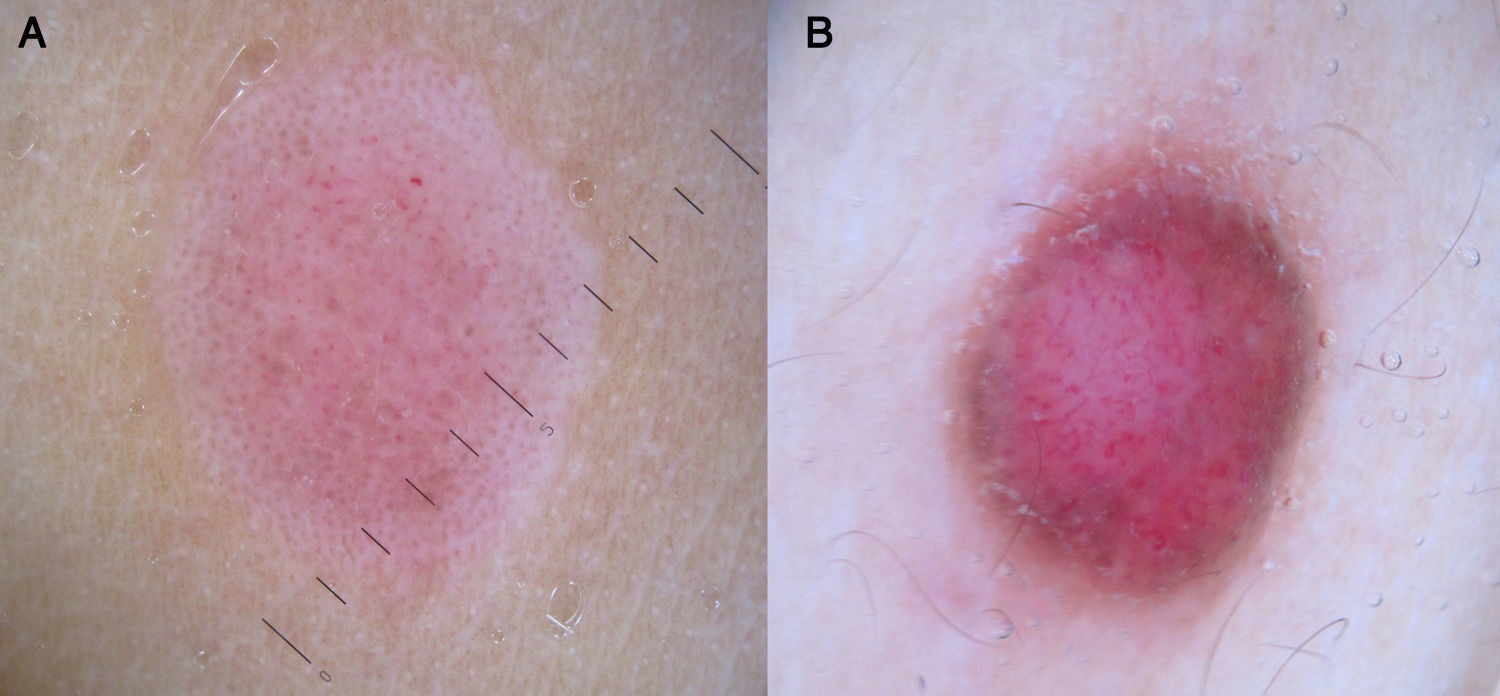
In recent years, dermoscopy has become an increasingly popular tool for the evaluation of psoriatic lesions thanks to the discovery that psoriasis has characteristic vascular patterns related to cutaneous microcirculation alterations, with larger vessels than those seen in healthy adjacent skin (caliber of 12-13μm vs 5-6μm).71,72
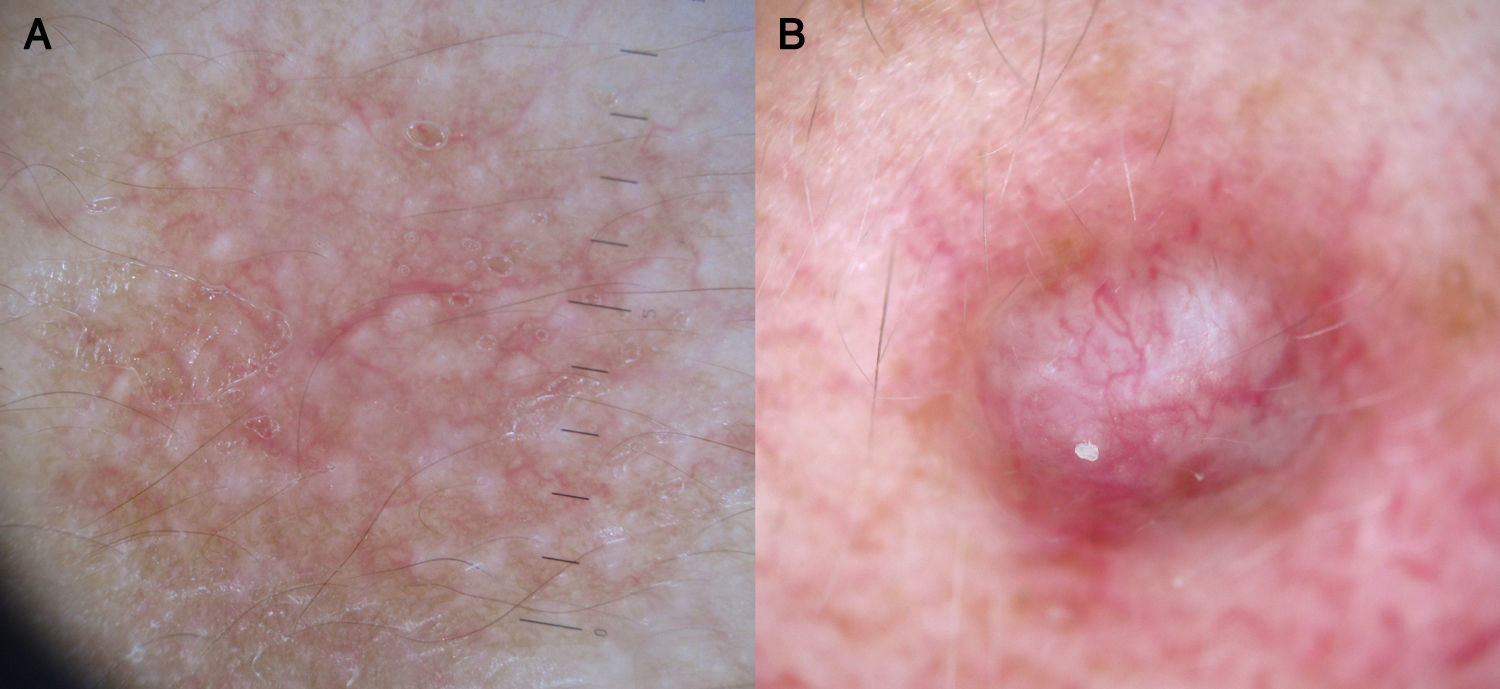
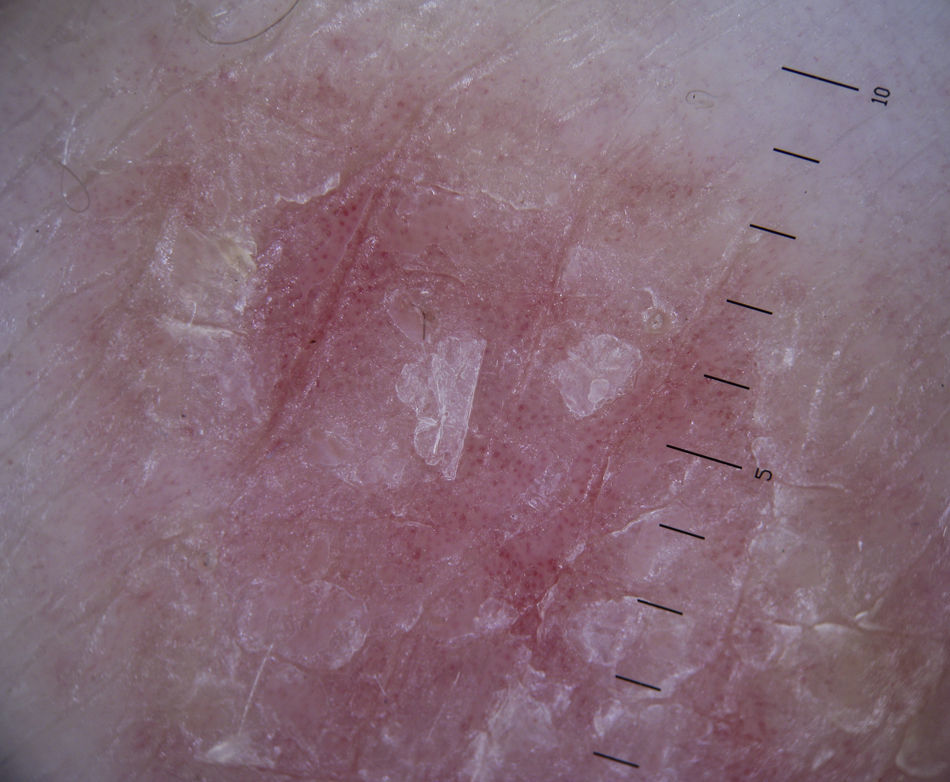
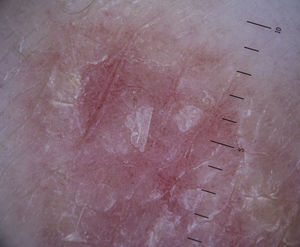
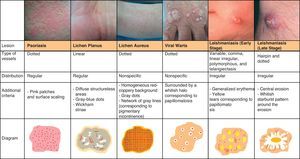
Psoriasis lesions exhibit a homogeneous globular pattern, with multiple uniformly sized dotted vessels in a patchy arrangement against a homogeneous pink background with a central scaling surface (Fig. 24).73
Dermoscopy is also useful for assessing the efficacy of treatment in psoriasis as clinicians can monitor changes in vascular patterns, which consist of a marked reduction in the density and length of vessels that is proportional to the clinical improvement seen in the lesions (Fig. 25).
Lichen PlanusDermoscopy can also be of value in the diagnosis of lichen planus, which is characterized by easily identifiable Wickham striae on the surface of the papules. Histologically, these striae correlate with areas of orthokeratosis, which are among the most characteristic histologic features of lichen planus.74,75
The vascular pattern can be more or less evident, and is characterized by the presence of predominantly linear regular vessels and diffuse brownish structureless areas that correspond to pigment in the epidermis. Other common findings are fine gray-blue or dark brown dots of globules that correspond to pigment deposits within dermal melanophages76 (Fig. 25).
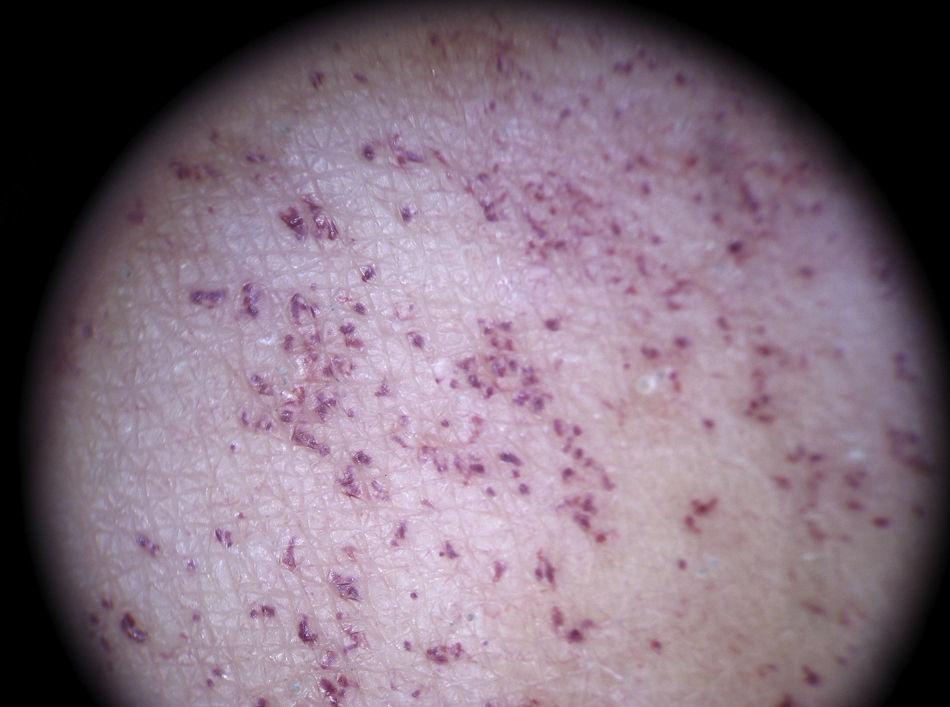
Pigmented Purpuric DermatitisThe term pigmented purpuric dermatitis refers to several entities, the most notable of which is lichen aureus. All the entities have overlapping clinical and histological features as they have a similar pathogenic mechanism, consisting of the extravasation of red blood cells and hemosiderin deposition in the papillary dermis.
The predominant dermoscopic pattern is a homogeneous red-coppery background with red dots or globules, gray dots, and a network of grayish lines.
The red-coppery color is related to the presence of a dense lymphocytic and histiocytic infiltrate in the dermis caused by the extravasation of red blood cells and the presence of hemosiderin in the histiocytes. The red dots and globules correspond to extravasation of red blood cells from the dermal vessels, which are larger and more numerous than usual, and the gray dots are histologically correlated with the deposits of hemosiderin in the dermal macrophages. Finally, the network of grayish lines corresponds to hyperpigmentation of the basal layer and pigmentary incontinence in the upper layers of the dermis related to the lichenoid infiltrate in lichen planus lesions.77
The recognition of this pattern can be useful for differentiating lichen aureus from conditions with similar clinical manifestations, such as stasis dermatitis (caused by chronic venous insufficiency) and angioma serpinginosum72 (Fig. 25).
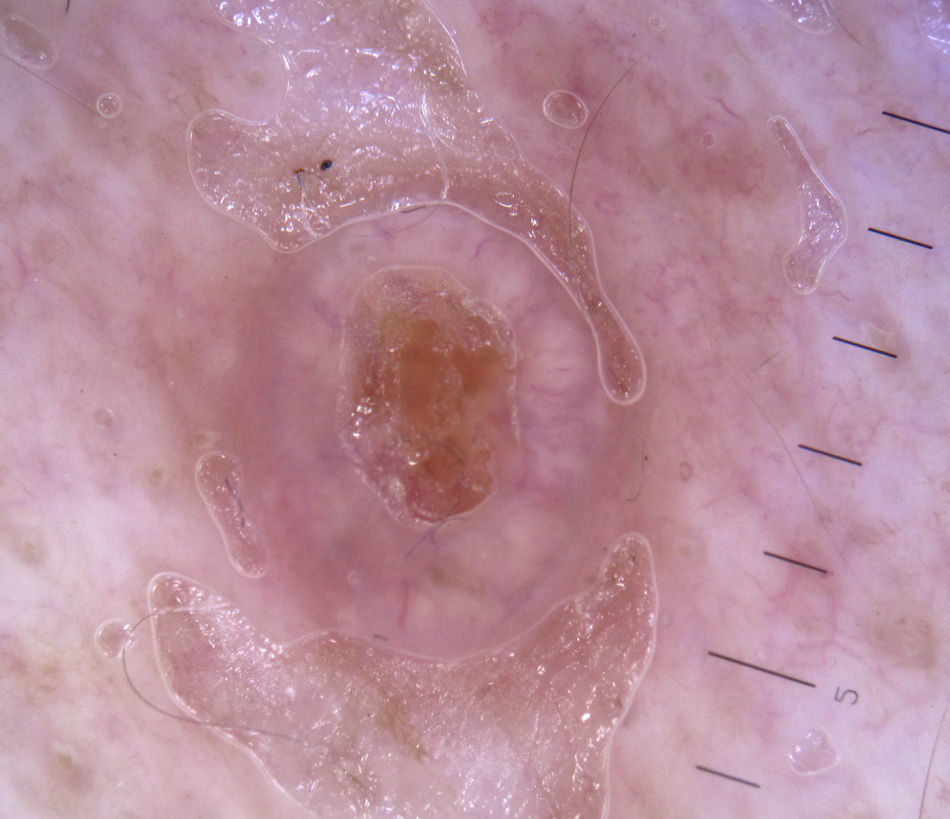
Infectious LesionsMolluscum ContagiosumThe hallmark dermoscopic pattern of molluscum contagiosum consists of crown vessels at the periphery of the lesion with a radial distribution (not crossing the center of the lobules) that resembles a red crown; a similar pattern is seen in sebaceous gland hyperplasia (Fig. 26).6,78
In the center there is a white-yellowish polylobular amorphous structure that correlates histologically with the presence of inverted lobules of hyperplastic squamous epithelium occupied by molluscum bodies that expand as the infection spreads, displacing the dermis and, consequently, the dermal vessels, to the periphery of the lesion (Fig. 13).
LeishmaniasisThe predominant vascular pattern in leishmaniasis varies according to the stage of the infection. Two clearly differentiated patterns have been described79 but they have in common generalized erythema (the most frequent finding) and a prominent vascular pattern.
In the early stages of infection the vascular morphology is highly varied, and there have been reports of 4 types of vessels (comma, linear irregular, polymorphous, and arborizing) occurring in the same lesion. Peripheral structures, such as hairpin and dotted vessels, predominate in more advanced stages of infection. Other, less common, findings are yellow tears (which correspond to follicular plugs) and areas of central erosion/ulceration surrounded by hyperkeratosis in the form of a whitish starburst pattern (Fig. 25).
Viral WartsThe dermoscopic vascular pattern of viral warts consists of multiple homogeneous dots and globules, ranging in color from red to black, surrounded by a whitish halo, which corresponds to the presence of papillomatosis. This halo may not be visible if there is significant vascular occlusion. The use of dermoscopy and the identification of the above structures in hyperkeratotic plantar lesions helps to distinguish warts from common conditions with a similar morphology, such as calluses and corns; the technique is also useful for evaluating the efficacy of treatment80 (Fig. 25).
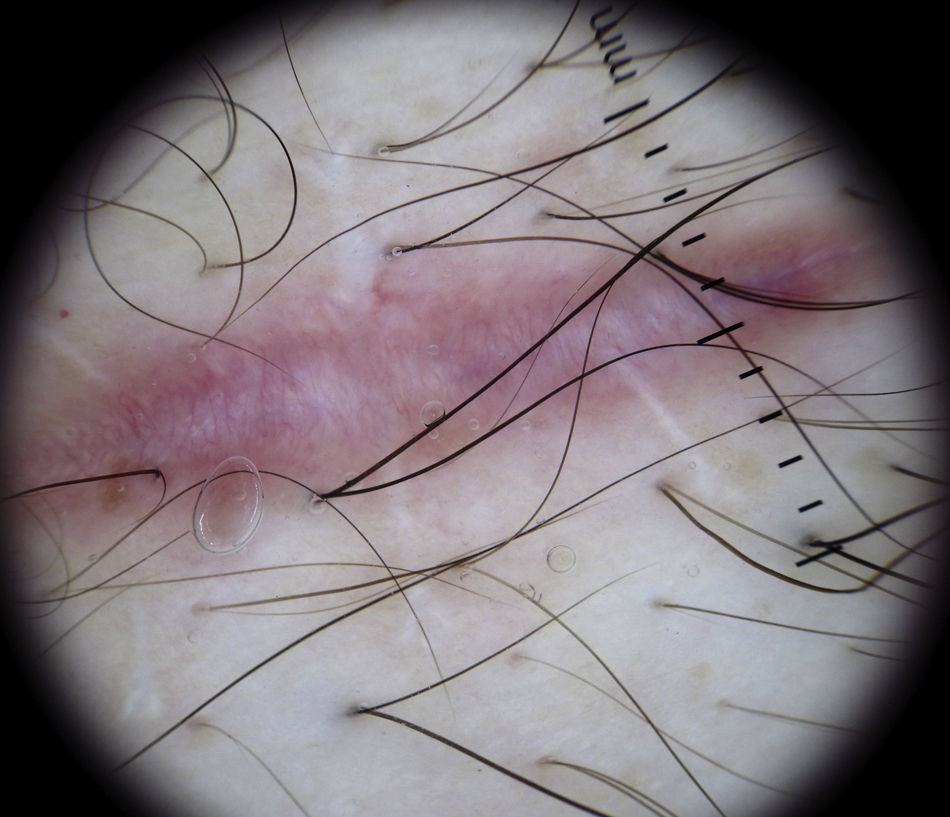
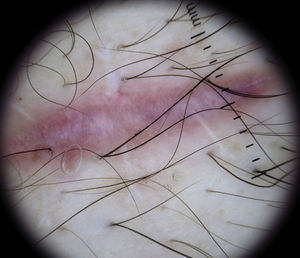
Special LesionsScarsFresh scars visualized under a dermoscope show short vessel loops emerging from the edges of normal tissue (Fig. 27). As the scar heals, the vessels grow and eventually come in contact with those emerging from the opposite side. They develop into fine vessels with a diameter of 20 to 30μm that cross the white scar tissue, creating a rope-ladder pattern.6
ConclusionsThe use of dermoscopy to recognize and identify vascular patterns can lead to a more accurate diagnosis and help to differentiate between clinically similar lesions (Fig. 28).
Conflict of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martín JM, et al. Vascularización en dermatoscopia. Actas Dermosifiliogr.2012;103:357-75.