Merkel cell carcinoma is a rare yet aggressive cutaneous tumor with a poor prognosis. Few studies have analyzed series of patients from the same hospital.
MethodologyWe performed a retrospective, descriptive, observational study of all patients diagnosed with Merkel cell carcinoma at a tertiary care hospital between 2002 and 2017. We recorded epidemiological, clinical, and histologic data and information on treatments and survival. For analysis, the sample was divided into 2 groups from different periods: 2002-2009 and 2010-2017. We performed survival analysis using Kaplan-Meier curves and multivariate analysis using a Cox proportional hazards model.
ResultsThirty-eight patients (24 men and 14 women) with a mean age of 77.76 years were included. Mean follow-up time was 30.11 months. On comparing 2010-2017 with 2002-2009, we observed a 116% increase in the number of Merkel cell carcinoma cases (26 vs. 12), an older mean age at diagnosis (80.92 vs. 70.92 years, P < .05), and an increase in lesions located on the trunk and lower limbs (0% vs. 34.62%). Eleven patients died of Merkel cell carcinoma. Overall survival was 78.2% at 12 months and 69.3% at 24 months. In the univariate analysis, age over 70 years and lymph node involvement were associated with mortality, while tumor location on the upper extremities and wide surgical excision were associated with improved survival. Only lymph node involvement retained its prognostic significance in the multivariate analysis.
ConclusionsIn this series, we observed that Merkel cell carcinoma has become more common in recent years and is now diagnosed at an older age and found in new anatomic locations.
El carcinoma de células de Merkel (CCM) es un tumor cutáneo muy agresivo y de mal pronóstico, aunque la incidencia es muy baja. Existen pocas series que analicen la experiencia en un mismo centro.
MetodologíaEstudio observacional, descriptivo y retrospectivo de todos los pacientes diagnosticados en un hospital de tercer nivel entre 2002 y 2017. Se recogieron las características epidemiológicas, clínicas, histológicas, el tratamiento y la supervivencia, y se dividió la muestra en 2períodos para el análisis (2002-2009 y 2010-2017). Se realizó un análisis de supervivencia mediante el modelo de Kaplan-Meier y un análisis multivariante mediante el modelo de riesgos proporcionales de Cox.
ResultadosSe incluyó a 38 pacientes, 24 hombres y 14 mujeres, con una edad media 77,76 años. El período medio de seguimiento fue de 30,11 meses. Se observó un aumento del 116% (12 vs. 26) entre los años 2002-2009 y 2010-2017, así como una edad media más avanzada (70,92 vs. 80,92; p<0,05) y un incremento de lesiones en tronco y miembros inferiores (34,62% vs. 0%). Once pacientes fallecieron debido al CCM. La supervivencia global a los 12 meses en la serie fue del 78,2% y a los 24 meses del 69,3%. Los factores asociados a mortalidad fueron la edad mayor de 70 años y la afectación ganglionar, mientras que la localización en miembros superiores y la realización de ampliación de márgenes aumentó la supervivencia. Al realizar el análisis multivariante, solo la afectación de ganglios permaneció como factor pronóstico.
ConclusionesSe ha observado un aumento de la frecuencia en los últimos años y un cambio en la forma de presentación a edades más avanzadas y en otras localizaciones diferentes a las clásicas.
Merkel cell carcinoma (MCC), or primary cutaneous endocrine carcinoma, is a very aggressive tumor with a poor prognosis and a tendency for locoregional recurrence and distant metastasis. Its mortality is twice as high as that of melanoma.1
Unfortunately, because MCC is so rare, few large single-center series have been published.2–4
MCC is clinically characterized by fast-growing, asymptomatic tumors that are predominant in sun-exposed areas and more common in elderly and immunosuppressed patients. This clinical picture is reflected in the acronym AEIOU (Asymptomatic, Expanding rapidly, Immune suppression, Older than 50, and UV-exposed site).5
Data from recent studies indicate that MCC is on the rise, with some series reporting a triple-fold increase in the number of new cases, which is attributable to an increase in known risk factors (advanced age and immunosuppression) and probably also to a greater awareness of the disease among dermatologists and pathologists.1,6,7
Immunohistochemistry is essential for diagnosis, as numerous findings can help differentiate MCC from other blue round-cell skin tumors. These include positive staining for intermediate filaments, mainly cytokeratin (CK) 20 and neuroendocrine markers such as CD56, chromogranin, and synaptophysin, and negativity for thyroid transcription factor (TTF) 1, achaete-scute complex-like 1 (MASH1, ASCL1), CK7, protein S100, and leukocyte common antigen.8
The aims of this study were to describe the demographic and clinical features of patients with MCC in our setting, determine the increase in incident cases, and analyze clinical predictors of survival.
Material and MethodsWe performed an observational, descriptive, retrospective study of all patients diagnosed with MCC at a tertiary care hospital over a period of 16 years (2002-2017). Data were obtained from electronic medical records and the hospital's pathology database. Diagnosis was confirmed by histology in all cases. We recorded data on epidemiological, histologic, and clinical variables (including AEIOU features), treatment, recurrence, and survival up to 28 February, 2018. Cause of death was noted where appropriate. All the patients were treated according to usual clinical practice. Most of the patients who, in the absence of a clinical diagnosis, were diagnosed after biopsy underwent a second operation with wide local excision (margins of 1-2cm). This second procedure was not possible in some patients due to their general state of health and advanced age. Sentinel lymph node (SLN) biopsy was not performed as a routine procedure, and as there were so few cases, they were not included in our analysis. Lymph node dissection was performed in patients with a positive SLN biopsy or enlarged lymph nodes. Advanced, unresectable local disease was treated with locoregional radiation therapy, while disseminated disease was treated with carboplatin and etoposide chemotherapy regimens. In the last year of the study, nonresponders to chemotherapy were treated with avelumab. Qualitative variables were expressed as absolute numbers and relative frequencies (percentages) and quantitative variables as mean (SD) and median (range). To check for differences over time, the sample was divided into two 8-year periods: 2002-2009 and 2010-2017. Survival analysis was performed using Kaplan-Meier curves and multivariate analysis using the Cox proportional hazards model. All data were collected and processed using version 21.0.0.0 of the IBM Statistical Package for Social Sciences.
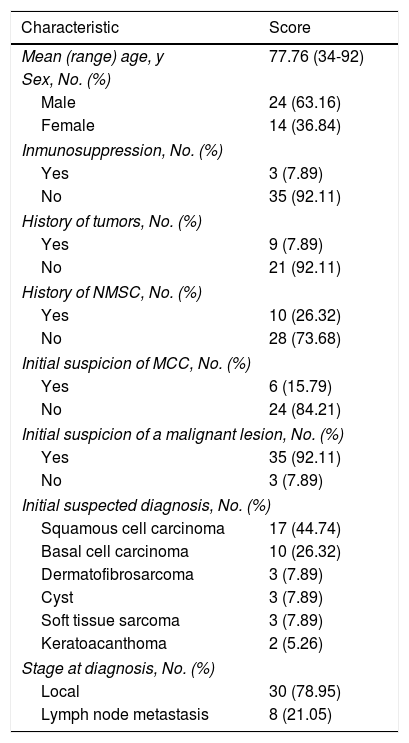
ResultsPatients. Thirty-eight patients with MCC were included. Their characteristics are summarized in Table 1. There were 24 men and 14 women (63.16% vs. 37.84%; ratio, 1.7:1) and all the patients were white. Mean age at diagnosis was 77.76 (12.07) years and there was no significant difference in age between men and women. Median age at diagnosis was 82 years (range, 34-92 years) and mean follow-up time was 30.11 (36.83) months (range, 0-114 months). Only 3 patients (7.89%) had some form of immunosuppression (lymphoproliferative syndrome, myeloproliferative syndrome, and heart transplant) and 9 (23.67%) had a past history of cancer (4 hematological malignancies, 3 colorectal cancers, 1 salivary gland cancer, and 1 case of prostate, bladder, and laryngeal adenocarcinoma). In addition, 10 patients (26.32%) had a personal history of nonmelanoma cutaneous cancer.
Characteristics of 38 Patients With MCC.
| Characteristic | Score |
|---|---|
| Mean (range) age, y | 77.76 (34-92) |
| Sex, No. (%) | |
| Male | 24 (63.16) |
| Female | 14 (36.84) |
| Inmunosuppression, No. (%) | |
| Yes | 3 (7.89) |
| No | 35 (92.11) |
| History of tumors, No. (%) | |
| Yes | 9 (7.89) |
| No | 21 (92.11) |
| History of NMSC, No. (%) | |
| Yes | 10 (26.32) |
| No | 28 (73.68) |
| Initial suspicion of MCC, No. (%) | |
| Yes | 6 (15.79) |
| No | 24 (84.21) |
| Initial suspicion of a malignant lesion, No. (%) | |
| Yes | 35 (92.11) |
| No | 3 (7.89) |
| Initial suspected diagnosis, No. (%) | |
| Squamous cell carcinoma | 17 (44.74) |
| Basal cell carcinoma | 10 (26.32) |
| Dermatofibrosarcoma | 3 (7.89) |
| Cyst | 3 (7.89) |
| Soft tissue sarcoma | 3 (7.89) |
| Keratoacanthoma | 2 (5.26) |
| Stage at diagnosis, No. (%) | |
| Local | 30 (78.95) |
| Lymph node metastasis | 8 (21.05) |
Abbreviations: MCC, Merkel cell carcinoma; NMSC, nonmelanoma skin cancer.
MCC was suspected as the initial diagnosis in just 6 patients (15.79%). Overall, however, 92% of tumors were viewed as being malignant and just 8% were thought to be benign. The most common suspected diagnosis was squamous cell carcinoma (44.74%), followed by basal cell carcinoma (26.32%), dermatofibrosarcoma protuberans, a benign inflammatory lesion, soft tissue sarcoma (7.89%), and keratoacanthoma (5.26%).
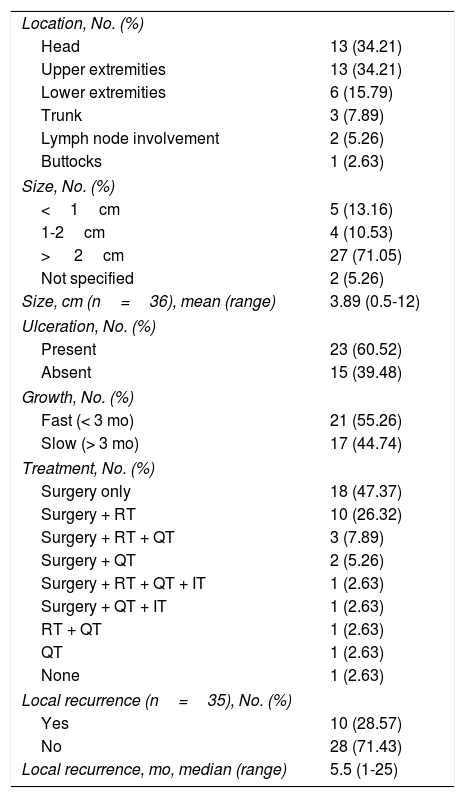
Tumor characteristics. In most cases, MCC presented as a solitary lesion with a mean size of 3.89 (2.90)cm and a median size of 2.8cm (range, 0.5-12cm). The tumors were asymptomatic in 90% of cases, ulcerated in 60.52%, and fast growing (over a period of 3 months) in 55.26% (Table 2).
Tumor Characteristics.
| Location, No. (%) | |
| Head | 13 (34.21) |
| Upper extremities | 13 (34.21) |
| Lower extremities | 6 (15.79) |
| Trunk | 3 (7.89) |
| Lymph node involvement | 2 (5.26) |
| Buttocks | 1 (2.63) |
| Size, No. (%) | |
| <1cm | 5 (13.16) |
| 1-2cm | 4 (10.53) |
| > 2cm | 27 (71.05) |
| Not specified | 2 (5.26) |
| Size, cm (n=36), mean (range) | 3.89 (0.5-12) |
| Ulceration, No. (%) | |
| Present | 23 (60.52) |
| Absent | 15 (39.48) |
| Growth, No. (%) | |
| Fast (< 3 mo) | 21 (55.26) |
| Slow (> 3 mo) | 17 (44.74) |
| Treatment, No. (%) | |
| Surgery only | 18 (47.37) |
| Surgery + RT | 10 (26.32) |
| Surgery + RT + QT | 3 (7.89) |
| Surgery + QT | 2 (5.26) |
| Surgery + RT + QT + IT | 1 (2.63) |
| Surgery + QT + IT | 1 (2.63) |
| RT + QT | 1 (2.63) |
| QT | 1 (2.63) |
| None | 1 (2.63) |
| Local recurrence (n=35), No. (%) | |
| Yes | 10 (28.57) |
| No | 28 (71.43) |
| Local recurrence, mo, median (range) | 5.5 (1-25) |
Abbreviations: IT, immunotherapy; QT, chemotherapy; RT, radiotherapy.
The most common locations were the head and upper limbs (34.21% in both cases), followed by the lower limbs (15.79%), the trunk (7.89%), and the buttocks (2.63%). Two patients (5.26%) did not have an identifiable primary cutaneous tumor. Two-thirds of the lesions were located in sun-exposed areas.
Immunohistochemically, the Merkel cells expressed epithelial and neuroendocrine markers, with positive staining for CK20 (31/33, 93.94%), chromogranin (25/31, 80.65%), and synaptophysin (19/25, 76.00%). CK7 and TTF1 expression was negative in most patients (16/21 [76.19%] and 20/22 [90.91%], respectively).
At the time of diagnosis, just 8 patients (21.05%) had lymph node involvement. The remaining 30 (78.95%) had local involvement only. None of the patients had distant metastasis.
Most patients underwent standard surgical excision (n=35) followed by wide local excision with margins of 1 to 2cm, unless contraindicated by age or tumor location (n=15). Fourteen patients received adjuvant locoregional radiotherapy and 7 patients with unresectable or partially resected tumors or lymph nodes or recurrent disease were treated with carboplatin and etoposide chemotherapy. Two patients who did not respond to chemotherapy were additionally treated with avelumab.
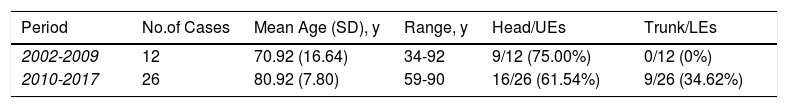
Trends over time. On comparing the 2 time periods, we observed a 116% increase in MCC cases diagnosed between 2002-2009 (n=12) and 2010-2017 (n=26). We also observed differences in clinical characteristics. On the one hand, the patients were significantly older in the second period (mean age, 70.92 vs. 80.92, P<.05) and on the other, the tumors were located at different sites. In the first period, all the lesions except 2 (75%) were located on the head or upper extremities and none were located on the trunk or lower extremities. In the second period, however, head and upper extremity tumors were still predominant (61.54%), but 34.62% of tumors were located on the trunk or lower extremities (Table 3).
Distribution of Cases and Age Between the 2 Study Periods.
| Period | No.of Cases | Mean Age (SD), y | Range, y | Head/UEs | Trunk/LEs |
|---|---|---|---|---|---|
| 2002-2009 | 12 | 70.92 (16.64) | 34-92 | 9/12 (75.00%) | 0/12 (0%) |
| 2010-2017 | 26 | 80.92 (7.80) | 59-90 | 16/26 (61.54%) | 9/26 (34.62%) |
Abbreviations: LEs, lower extremities; UEs, upper extremities.
Survival. Ten (28.57%) of the 35 patients who underwent surgery experienced recurrence during follow-up (median time to recurrence, 5.5 months).
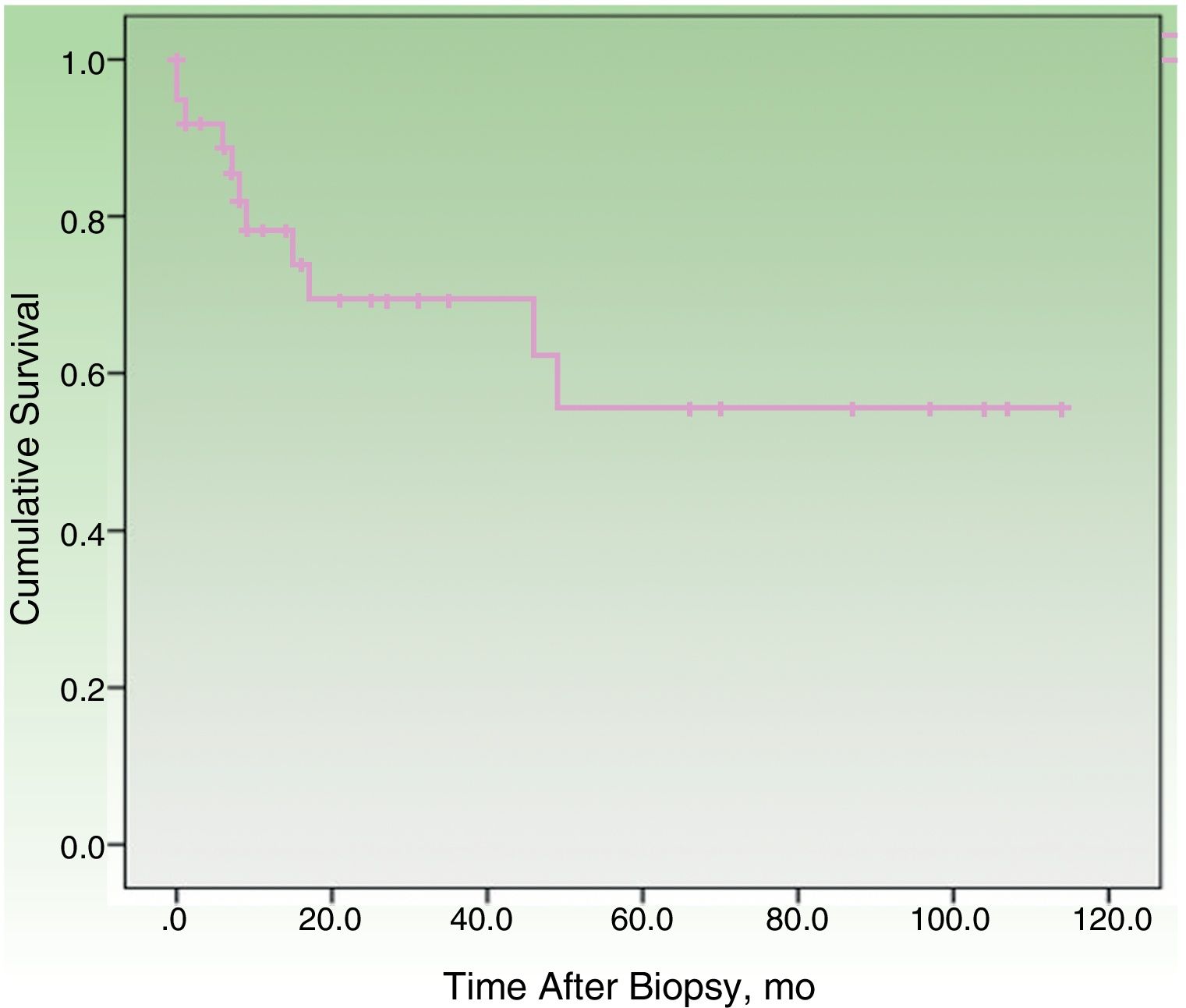
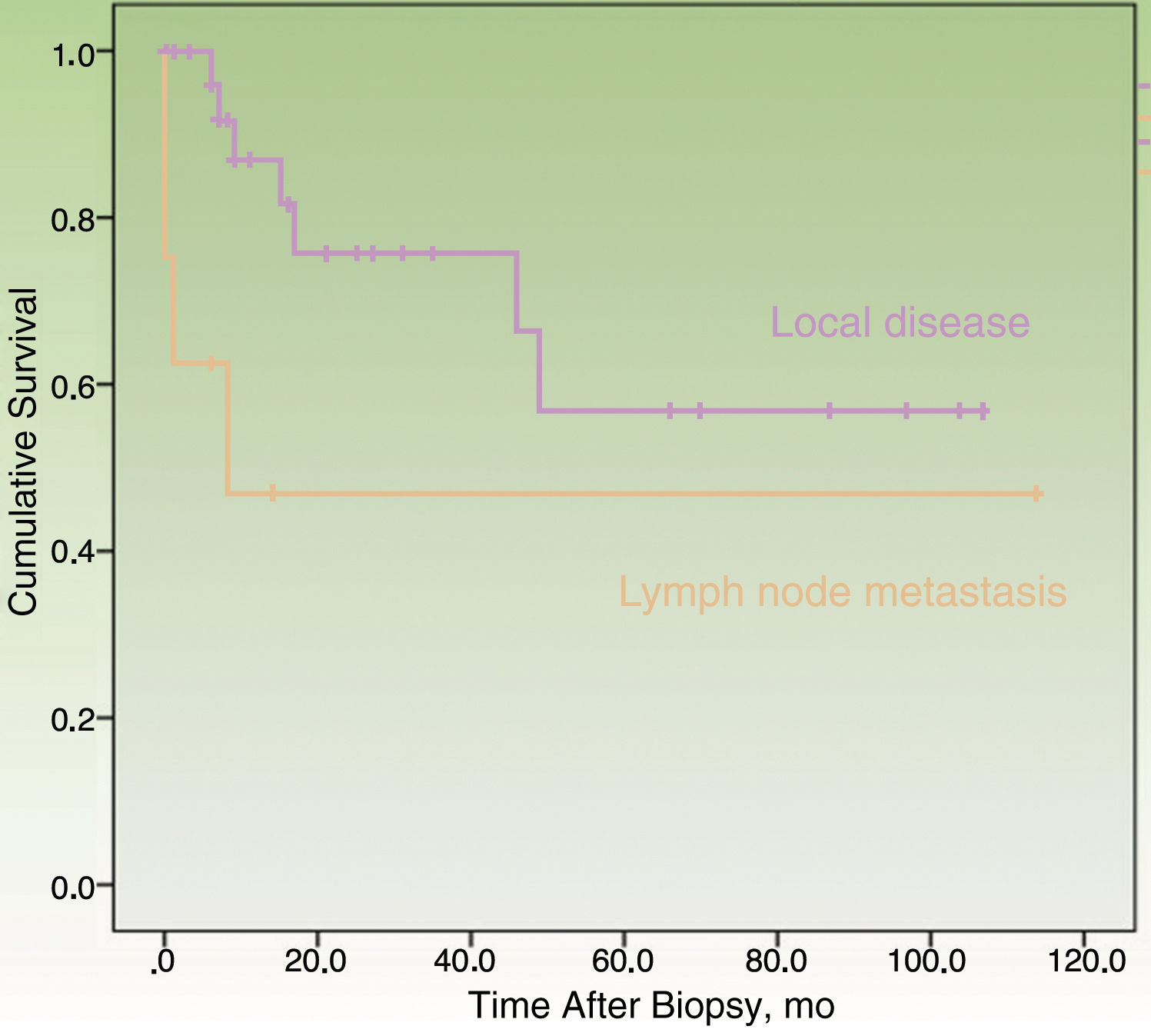
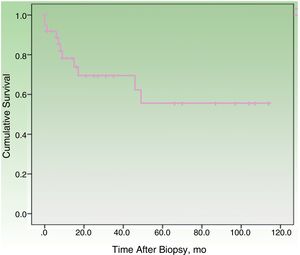
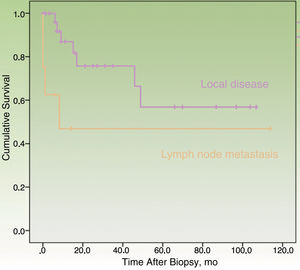
By the end of the follow-up period, 17 patients (44.74%) had died: 11 (28.95%) due to MCC and 6 (15.79%) due to other causes. MCC-specific survival was 78.2% at 12 months and 69.3% at 24 months (Fig. 1). On analyzing survival by stage at diagnosis, 12-month disease-specific survival was 87.0% for patients with localized disease and 46.90% for those with lymph node metastasis (Fig. 2).
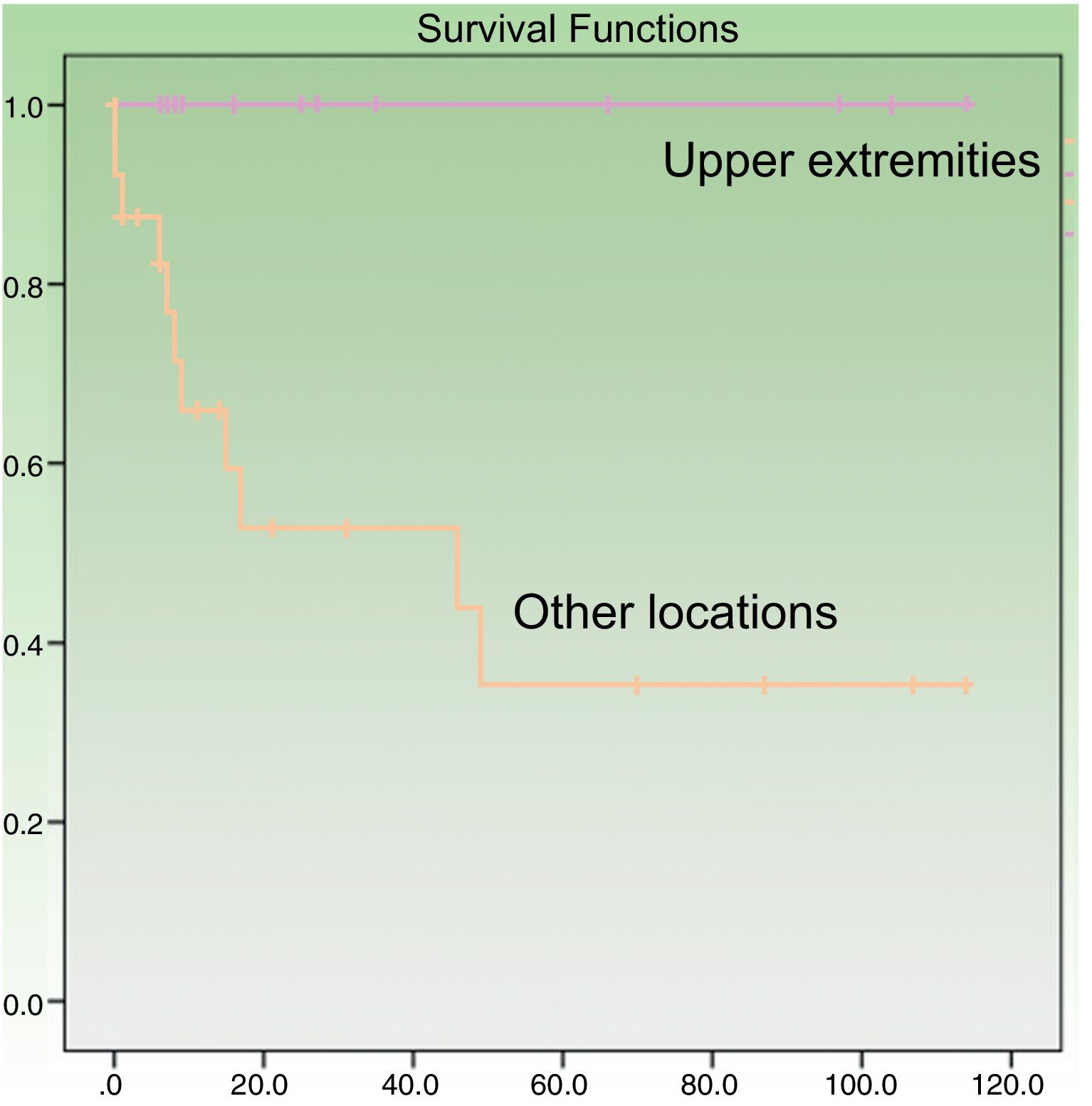
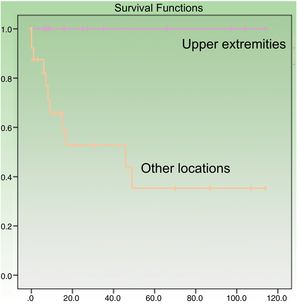
Age at diagnosis was a significant predictor of survival in the univariate analysis, with an age of over 70 years associated with higher mortality (P=.049). Patients with tumors on the upper extremities had a better prognosis (P=.005) (Fig. 3). Their survival rate was 100%, even though the tumors at this location were larger than at other sites (mean size, 4.64 [3.98] cm vs. 3.60 [2.41] cm). Other factors associated with better survival were wide local excision (with margins of any size) after the initial surgery (P=.011) and absence of lymph node metastasis at any stage of the disease (P=.023). Survival was also better in women, although the difference with men did not reach statistical significance. In the multivariate Cox regression analysis including the 4 significant variables, only lymph node metastasis retained its significance as an independent predictor of survival.
DiscussionMCC is a rare tumor and few single-center series have been published.9,10 The largest series to date analyzed 9387 patients from over 1000 hospitals in the United States.9 Very few cases in Spain have been described.11,12 The largest series is that published by Instituto Valenciano de Oncología, which reported on 48 cases in a poster at the 43rd National Dermatology Congress in 2013.13 The current series, which includes 38 cases diagnosed at our hospital over a period of 16 years, is one of the largest Spanish series published to date.
Apart from being rare, MCC has nonspecific clinical features and may even appear to be benign. It is therefore rarely suspected as an initial diagnosis. To aid earlier diagnosis, Heath et al.5 created the acronym AEIOU to summarize the main clinical features of MCC, where A refers to asymptomatic, E to expanding rapidly, I to immune suppression, O to older than 50 years, and U to UV-exposed site. The presence of 3 or more of these features has high diagnostic sensitivity: 89% of patients in the study by Heath at al. and 87% of those in our series had at least 3 features. The detection of polyomavirus sequences in 80% of MCC tumors in 2008 led to the discovery of this virus as a pathogenic factor.14
The basic demographic profile of the patients in our series is consistent with reports in the literature, although there was a higher proportion of upper extremity tumors (34% vs. 24%9) and ulcerated tumors (61% vs. 10.6%10). Localized stages were also more common (79% vs. 65%9). The increase in incidence observed in the second period (2010-2017) is noteworthy and supports reports from recent years.8,15,16 Other differences noted in the second period were a older mean age at diagnosis and the presence of tumors at sites other than the head and upper extremities; occurrence at these new sites was very rare in the first 8 years studied. The increased incidence of MCC would appear to be linked to an increase in the at-risk population (due to ageing and higher rates of immunosuppression and UV exposure) and greater awareness of MCC among dermatologists and pathologists thanks to the use of new immunohistochemical techniques.6 This is the first report of an older age at diagnosis and an increase in recent cases in the Spanish literature.
The main clinical factors associated with a favorable prognosis were female sex, tumors located on the upper extremities, primary nodal tumors in the absence of a primary cutaneous site, a tumor size less than 2cm, absence of lymph node or distant metastasis, and absence of immunosuppression.8 Patients with upper extremity tumors also had a more favorable prognosis, even the tumors were on average larger than those at other sites. Although this association has been previously described,10 the reasons for more favorable outcomes in upper extremity tumors have not yet been fully elucidated. Merkel cell polyomavirus-infected tumors, however, are more common in this location and have been linked to better survival.17 Higher mortality rates have been reported for patients older than 70 years and patients with lymph node metastasis.18 In our series, patients who underwent a second operation with wide local excision (margins of 1-2cm) also had better survival outcomes.
Until recently, patients with advanced MCC did not have access to specific medical treatment and were typically treated with conventional chemotherapy regimens, which had limited effectiveness in this setting. New immunotherapeutic agents, however, have since been approved for metastatic MCC and include pembrolizumab19 and, more recently, avelumab,20 which are associated with complete or partial response in up to 56% of patients. Two patients with lymph node metastasis in our series were treated with second-line avelumab after failing to respond to conventional chemotherapy. It is too early, however, to evaluate response, as they had been receiving the drug for less than 6 months at the time of this study.
In conclusion, our analysis of 38 patients with MCC shows a rise in incidence in recent years and a trend towards an older age at diagnosis and occurrence of tumors at nontypical sites. Our study is the first to report these trends for Spain. Age, tumor location, wide local excision, and lymph node metastasis were all predictors of survival. MCC must be contemplated as a possible diagnosis in patients who present with a lesion that meets 3 or more AEIOU criteria.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Dañino-García M, Domínguez-Cruz JJ, Pérez-Ruiz C, Conejo-Mir J, Pereyra-Rodríguez JJ. Características clínico-epidemiológicas del carcinoma de células de Merkel en una serie de 38 pacientes. Actas Dermosifiliogr. 2019;110:360–365.