Clinical research is the study of patients with the aim of improving care. Our objectives were to calculate the percentage of presentations at territorial section meetings of the Spanish Academy of Dermatology and Venereology (AEDV) that described clinical research, to assess the level of evidence the research provided, and to analyze change in clinical research volume over time.
Material and methodsWe reviewed supplements of the journal Actas Dermo-Sifiliográficas for 2000 through 2015 that contained abstracts of presentations given at the AEDV section meetings in Galicia; the area comprising Asturias, Cantabria, and Castile-Leon (ACCL); and Andalusia. We selected abstracts that met a previously validated definition of clinical research and categorized each according to level of evidence. We also analyzed how the weight of clinical research presentations changed over time.
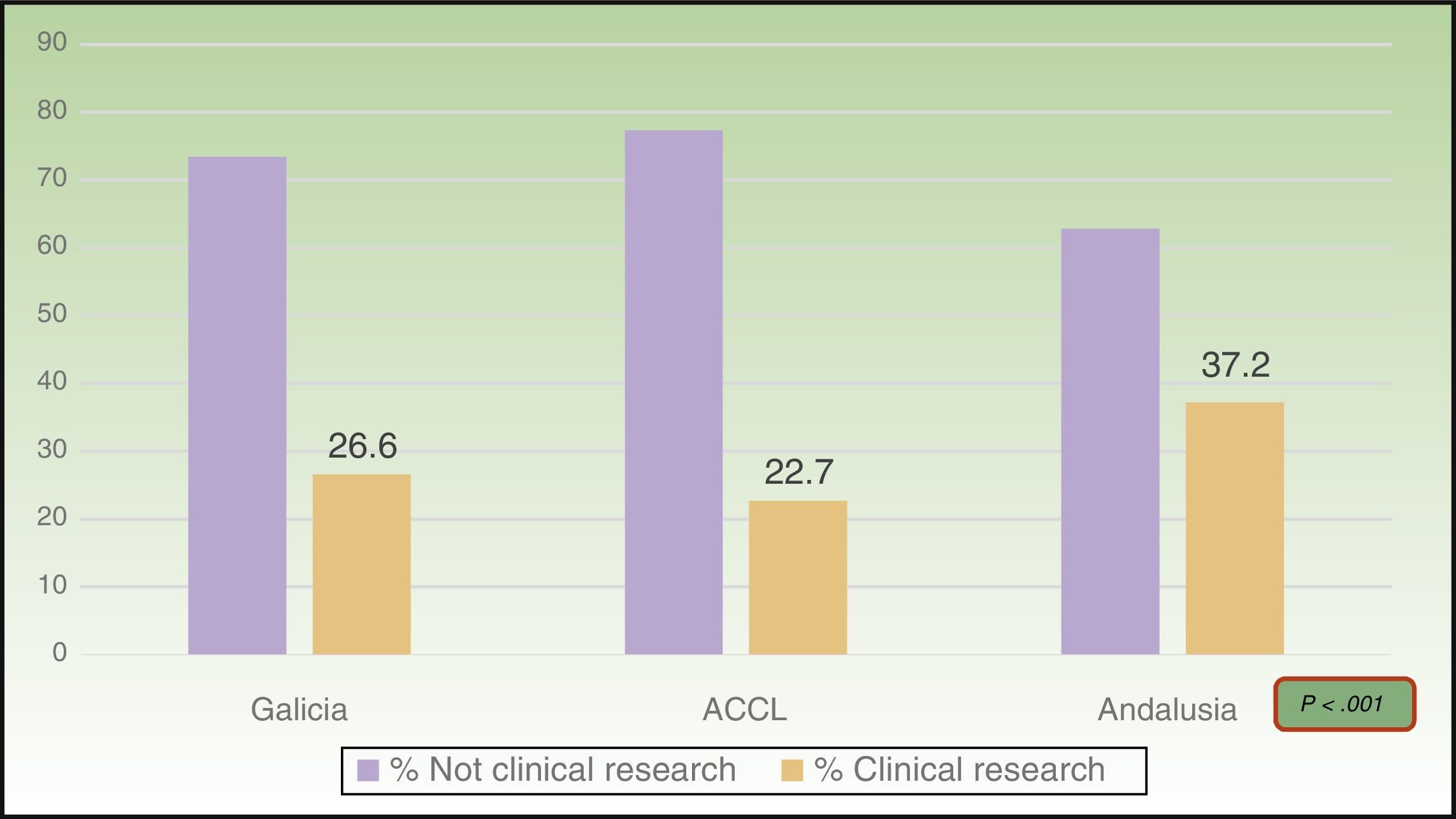
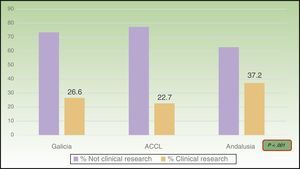
ResultsOf the total of 1,188 presentations, 29.6% met the criteria that defined clinical research. Most provided level-4 evidence (95.2%); 44.2% of those presentations reported cross-sectional studies and 55.8% analyzed case series. Clinical research accounted for 26.6% of the presentations in Galicia (94.7% of them, level 4), 22.7% of those at the ACCL meeting (97.6%, level 4), and 37.2% of those in Andalusia (94.3%, level 4). The proportion of clinical research increased significantly over the years studied.
ConclusionsClinical research accounted for 29.6% of the communications presented at the meetings we reviewed. Most of these presentations concerned case series or cross-sectional studies. The prevalence of clinical research presentations differed between the 3 territories studied, but the levels of evidence were similar. The proportion of clinical research in the programs of these meetings has increased over time.
La investigación clínica (IC) se centra en el estudio de los pacientes para mejorar su atención. Nuestro objetivo fue conocer qué porcentaje de las comunicaciones presentadas a las reuniones de tres secciones territoriales de la AEDV cumplen criterios de IC, su nivel de evidencia y su evolución temporal.
Material y métodosSe revisaron las comunicaciones científicas de las reuniones territoriales gallega, astur-cántabro-castellano-leonesa (ACL) y andaluza publicadas en los suplementos de la revista Actas Dermo-Sifiliográficas (años 2000-2015). Utilizando una definición de IC previamente validada, se estableció cuáles de los resúmenes se ajustaban a dicha definición, se determinó su nivel de evidencia y la evolución temporal del porcentaje de estudios de investigación.
ResultadosDe las 1.188 comunicaciones analizadas, el 29,6% cumplieron criterios de IC. La mayoría correspondían a un nivel de evidencia4 (95,2%), distribuidos en un 44,2% de estudios transversales y un 55,8% de series de casos. La prevalencia de la IC en las secciones gallega, ACL y andaluza fue del 26,6% (94,7%, nivel 4), del 22,7% (97,6%, nivel 4) y del 37,2% (94,3%, nivel 4), respectivamente. El porcentaje de trabajos de IC aumentó significativamente a lo largo de los años.
ConclusionesLa IC representa el 29,6% de las comunicaciones en las reuniones evaluadas. La mayoría de los trabajos corresponden a series de casos y estudios transversales. Los territorios estudiados muestran diferencias en cuanto al porcentaje de IC, pero siguen una distribución similar de los niveles de evidencia. En el periodo de tiempo evaluado, el porcentaje de comunicaciones sobre IC se ha incrementado.
The purpose of clinical research is to answer frequently asked questions in medical practice in the interest of improving health care quality.1 Clinical questions often arise during routine care, and once a question is formulated it can become the object of research. Such research is usually presented at a national or international conference or other gathering.
We have little information about the characteristics of clinical research presented at dermatology conferences or the level of evidence provided in such presentations.2 Our aims were to calculate the percentage of presentations at the annual territorial section meetings of the Spanish Academy of Dermatology and Venereology (AEDV) that can be considered clinical research, to assess the level of evidence they provided, and to explore change over time.
Material and MethodsWe carried out a cross-sectional study of oral presentations given at territorial section meetings of the AEDV from 2000 through 2015. We chose to study Galicia, where we work, and to compare and contrast our situation with that of other territories in the north, center, and south of Spain by also studying the areas comprising Asturias, Cantabria, and Castile-Leon (ACCL) and Andalusia.
Abstracts for oral presentations at conferences were extracted from annual supplements of the journal Actas Dermo-Sifiliográficas.
All presentations given from 2000 through 2015 were included. Excluded were any that were not published in the annual supplements. Thus, there were no abstracts for 2004 or 2005 from Galicia, none for 2003 or 2010 from the ACCL area, and none for 2002, 2003 or 2005 from Andalusia.
For each abstract we recorded the following information: year and location of the conference, authors, full text of the abstract, and whether clinical research was or was not presented. Presentations were classified according to a previously validated definition of clinical research (Table 1).3 Presentations that did not meet the stipulated criteria were considered nonclinical research topics. The identified clinical research presentations were then ranked by level of evidence according to the system of the Oxford Centre for Evidence-Based Medicine (CEBM),4 which distinguishes 5 levels. Level-1 studies provide the highest level of evidence, reporting results and knowledge that are the most reliable. Level-5 studies are the least reliable and most subject to random error, bias, and confounding factors. A case series was defined as a report of 2 or more cases.3
Definition of Clinical Research.
| An article was considered to report clinical research and was included in this study if it was planned, organized, and met the following 3 criteria: |
| 1) The study was performed in patients, other persons or health care systems or was based on patients. Included would be research on tissue samples from patients or healthy individuals (eg, biopsies, dermoscopic images, laboratory findings, etc.). |
| 2) The study set out to answer a question about clinical practice in order to solve practical problems of patient management. Included would be research on the prevalence, etiology, diagnosis, prognosis, prevention, and treatment of diseases, as well as studies on economic aspects of disease or health care systems. Systematic reviews on such topics were also included. |
| 3) The study had at least a level-4 evidence grade according to the Oxford Centre for Evidence-Based Medicine. Presentations reporting studies with a level of evidence of 3 or higher were considered high-quality research. |
| Presentations were excluded from this classification (considered not to be clinical research) if they reported expert opinion based on an unspecified literature search method or they reported studies in physiology, pathogenesis, laboratory experiments, or other basic science models. |
All abstracts were independently evaluated by 2 authors (A.I.P. and A.B.). The first had no specific training in epidemiology; the second had taken courses in basic epidemiology as part of continuing professional development training. Classification discrepancies (6/1188, <0.5%) were resolved through discussion, leading to a classification accepted by both assessors.
Data were recorded in a spreadsheet (Excel) and analyzed with the statistical program R (version Ri 3863.3.0). The frequency distributions were calculated for all variables, expressed as percentages. The χ2 test was used to compare percentages of clinical research presented at each of the conferences between territorial sections and years. We considered a P value less than .05 to be significant.
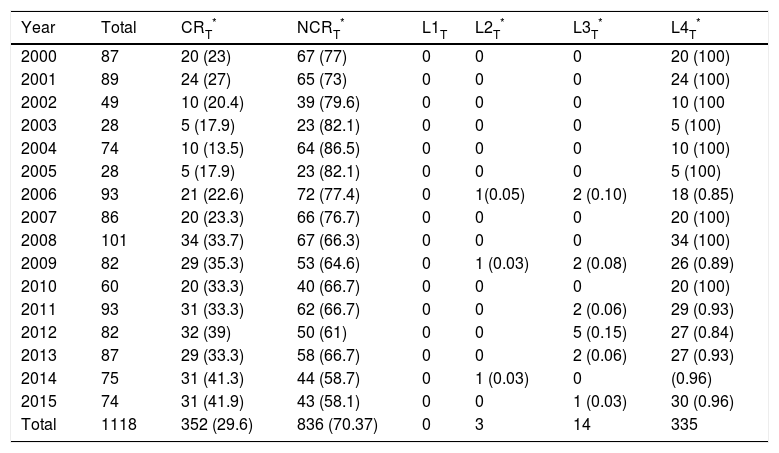
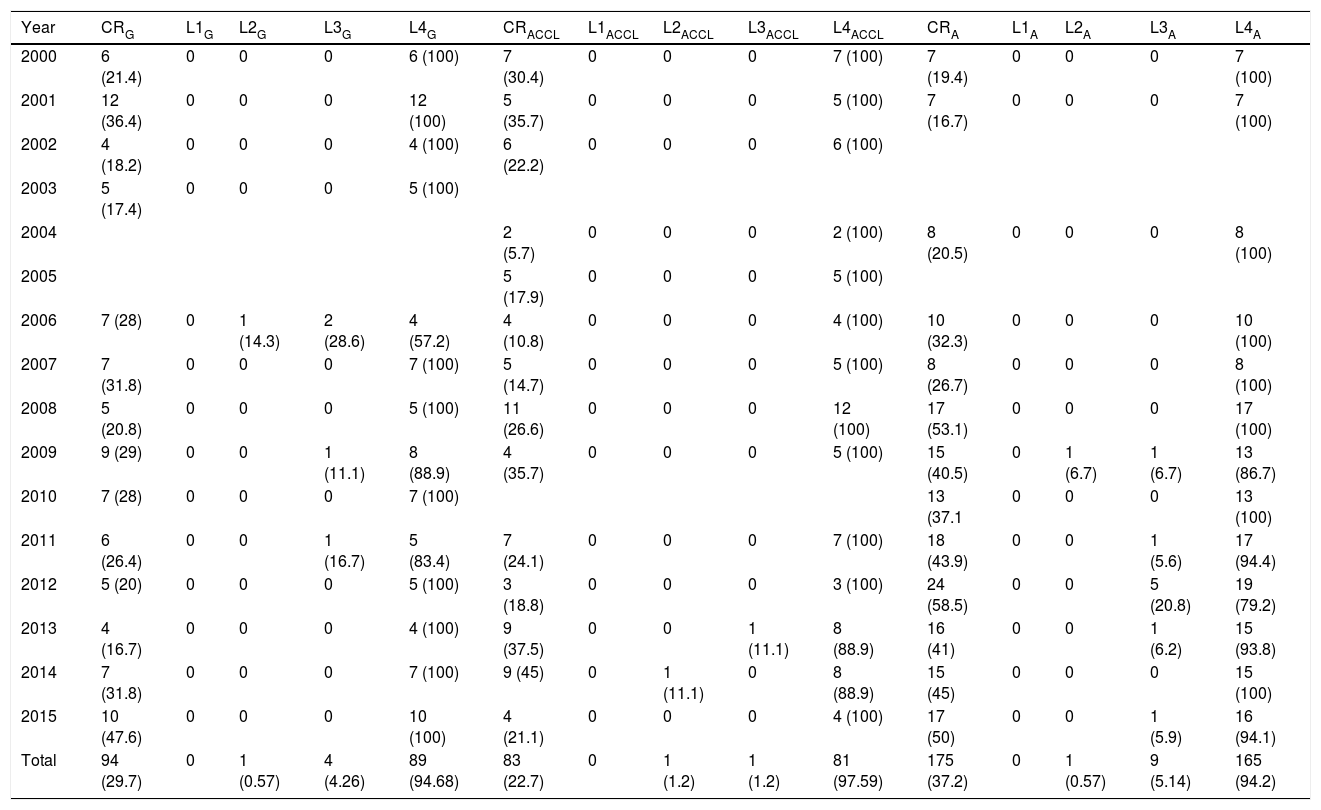
ResultsA total of 1188 presentation abstracts were analyzed: 353 (30%) from the conferences in Galicia, 365 (31%) from the ACCL area, and 470 (39%) from Andalusia. Three hundred fifty-two of the 1118 presentations (29.6%) described clinical research. Level-4 evidence was reported in 95.2% of the presentations, 4.0% reported level-3 evidence, and 0.8% reported level-2 evidence. No level-1 research was found. A majority (55.8%) of level-4 studies were case series. The remaining 44.2% were cross-sectional studies. Table 2 shows the number and percentage of presentations in each level of evidence for each year. In Galicia, clinical research presentations accounted for 26.6% of the total (94.7%, level4); 22.7% of the total in the ACCL areas (97.6%, level4); and 37.2% of the total in Andalusia (94.3%, level4) (Fig. 1). Table 3 shows the distribution of presentations by levels of evidence in each territory and year.
Conference Presentations Distributed According to Levels of Evidence During the Period Studied.
| Year | Total | CRT* | NCRT* | L1T | L2T* | L3T* | L4T* |
|---|---|---|---|---|---|---|---|
| 2000 | 87 | 20 (23) | 67 (77) | 0 | 0 | 0 | 20 (100) |
| 2001 | 89 | 24 (27) | 65 (73) | 0 | 0 | 0 | 24 (100) |
| 2002 | 49 | 10 (20.4) | 39 (79.6) | 0 | 0 | 0 | 10 (100 |
| 2003 | 28 | 5 (17.9) | 23 (82.1) | 0 | 0 | 0 | 5 (100) |
| 2004 | 74 | 10 (13.5) | 64 (86.5) | 0 | 0 | 0 | 10 (100) |
| 2005 | 28 | 5 (17.9) | 23 (82.1) | 0 | 0 | 0 | 5 (100) |
| 2006 | 93 | 21 (22.6) | 72 (77.4) | 0 | 1(0.05) | 2 (0.10) | 18 (0.85) |
| 2007 | 86 | 20 (23.3) | 66 (76.7) | 0 | 0 | 0 | 20 (100) |
| 2008 | 101 | 34 (33.7) | 67 (66.3) | 0 | 0 | 0 | 34 (100) |
| 2009 | 82 | 29 (35.3) | 53 (64.6) | 0 | 1 (0.03) | 2 (0.08) | 26 (0.89) |
| 2010 | 60 | 20 (33.3) | 40 (66.7) | 0 | 0 | 0 | 20 (100) |
| 2011 | 93 | 31 (33.3) | 62 (66.7) | 0 | 0 | 2 (0.06) | 29 (0.93) |
| 2012 | 82 | 32 (39) | 50 (61) | 0 | 0 | 5 (0.15) | 27 (0.84) |
| 2013 | 87 | 29 (33.3) | 58 (66.7) | 0 | 0 | 2 (0.06) | 27 (0.93) |
| 2014 | 75 | 31 (41.3) | 44 (58.7) | 0 | 1 (0.03) | 0 | (0.96) |
| 2015 | 74 | 31 (41.9) | 43 (58.1) | 0 | 0 | 1 (0.03) | 30 (0.96) |
| Total | 1118 | 352 (29.6) | 836 (70.37) | 0 | 3 | 14 | 335 |
Values between parentheses (in columns 3 and 4) are percentages of the total for each year or (in columns 6 through 8) are percentages of the total number of presentations reporting at least level 4 evidence at each territorial conference.
ABBREVIATIONS: CR, clinical research; L1T, total number of level-1 presentations; L2T, total number of level-2 presentations; L3T, total number of level-3 presentations; L4T total number of level-4 presentations; NCR, not clinical research.
Presentations at Each Territorial Conference in Each Year Studied, Distributed According to Levels of Evidence*.
| Year | CRG | L1G | L2G | L3G | L4G | CRACCL | L1ACCL | L2ACCL | L3ACCL | L4ACCL | CRA | L1A | L2A | L3A | L4A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 6 (21.4) | 0 | 0 | 0 | 6 (100) | 7 (30.4) | 0 | 0 | 0 | 7 (100) | 7 (19.4) | 0 | 0 | 0 | 7 (100) |
| 2001 | 12 (36.4) | 0 | 0 | 0 | 12 (100) | 5 (35.7) | 0 | 0 | 0 | 5 (100) | 7 (16.7) | 0 | 0 | 0 | 7 (100) |
| 2002 | 4 (18.2) | 0 | 0 | 0 | 4 (100) | 6 (22.2) | 0 | 0 | 0 | 6 (100) | |||||
| 2003 | 5 (17.4) | 0 | 0 | 0 | 5 (100) | ||||||||||
| 2004 | 2 (5.7) | 0 | 0 | 0 | 2 (100) | 8 (20.5) | 0 | 0 | 0 | 8 (100) | |||||
| 2005 | 5 (17.9) | 0 | 0 | 0 | 5 (100) | ||||||||||
| 2006 | 7 (28) | 0 | 1 (14.3) | 2 (28.6) | 4 (57.2) | 4 (10.8) | 0 | 0 | 0 | 4 (100) | 10 (32.3) | 0 | 0 | 0 | 10 (100) |
| 2007 | 7 (31.8) | 0 | 0 | 0 | 7 (100) | 5 (14.7) | 0 | 0 | 0 | 5 (100) | 8 (26.7) | 0 | 0 | 0 | 8 (100) |
| 2008 | 5 (20.8) | 0 | 0 | 0 | 5 (100) | 11 (26.6) | 0 | 0 | 0 | 12 (100) | 17 (53.1) | 0 | 0 | 0 | 17 (100) |
| 2009 | 9 (29) | 0 | 0 | 1 (11.1) | 8 (88.9) | 4 (35.7) | 0 | 0 | 0 | 5 (100) | 15 (40.5) | 0 | 1 (6.7) | 1 (6.7) | 13 (86.7) |
| 2010 | 7 (28) | 0 | 0 | 0 | 7 (100) | 13 (37.1 | 0 | 0 | 0 | 13 (100) | |||||
| 2011 | 6 (26.4) | 0 | 0 | 1 (16.7) | 5 (83.4) | 7 (24.1) | 0 | 0 | 0 | 7 (100) | 18 (43.9) | 0 | 0 | 1 (5.6) | 17 (94.4) |
| 2012 | 5 (20) | 0 | 0 | 0 | 5 (100) | 3 (18.8) | 0 | 0 | 0 | 3 (100) | 24 (58.5) | 0 | 0 | 5 (20.8) | 19 (79.2) |
| 2013 | 4 (16.7) | 0 | 0 | 0 | 4 (100) | 9 (37.5) | 0 | 0 | 1 (11.1) | 8 (88.9) | 16 (41) | 0 | 0 | 1 (6.2) | 15 (93.8) |
| 2014 | 7 (31.8) | 0 | 0 | 0 | 7 (100) | 9 (45) | 0 | 1 (11.1) | 0 | 8 (88.9) | 15 (45) | 0 | 0 | 0 | 15 (100) |
| 2015 | 10 (47.6) | 0 | 0 | 0 | 10 (100) | 4 (21.1) | 0 | 0 | 0 | 4 (100) | 17 (50) | 0 | 0 | 1 (5.9) | 16 (94.1) |
| Total | 94 (29.7) | 0 | 1 (0.57) | 4 (4.26) | 89 (94.68) | 83 (22.7) | 0 | 1 (1.2) | 1 (1.2) | 81 (97.59) | 175 (37.2) | 0 | 1 (0.57) | 9 (5.14) | 165 (94.2) |
Values between parentheses in each evidence level column are percentages with respect to the total number of presentations reporting at least level 4 evidence for a territory and year.
ABBREVIATIONS: A (subscript), Andalusia; ACCL (subscript), Asturias, Cantabria, and Castile-Leon; CR, clinical research; G (subscript), Galicia; L1, level 1; L2, level 2, L3, level 4; L4, level 4; NCR, not clinical research.
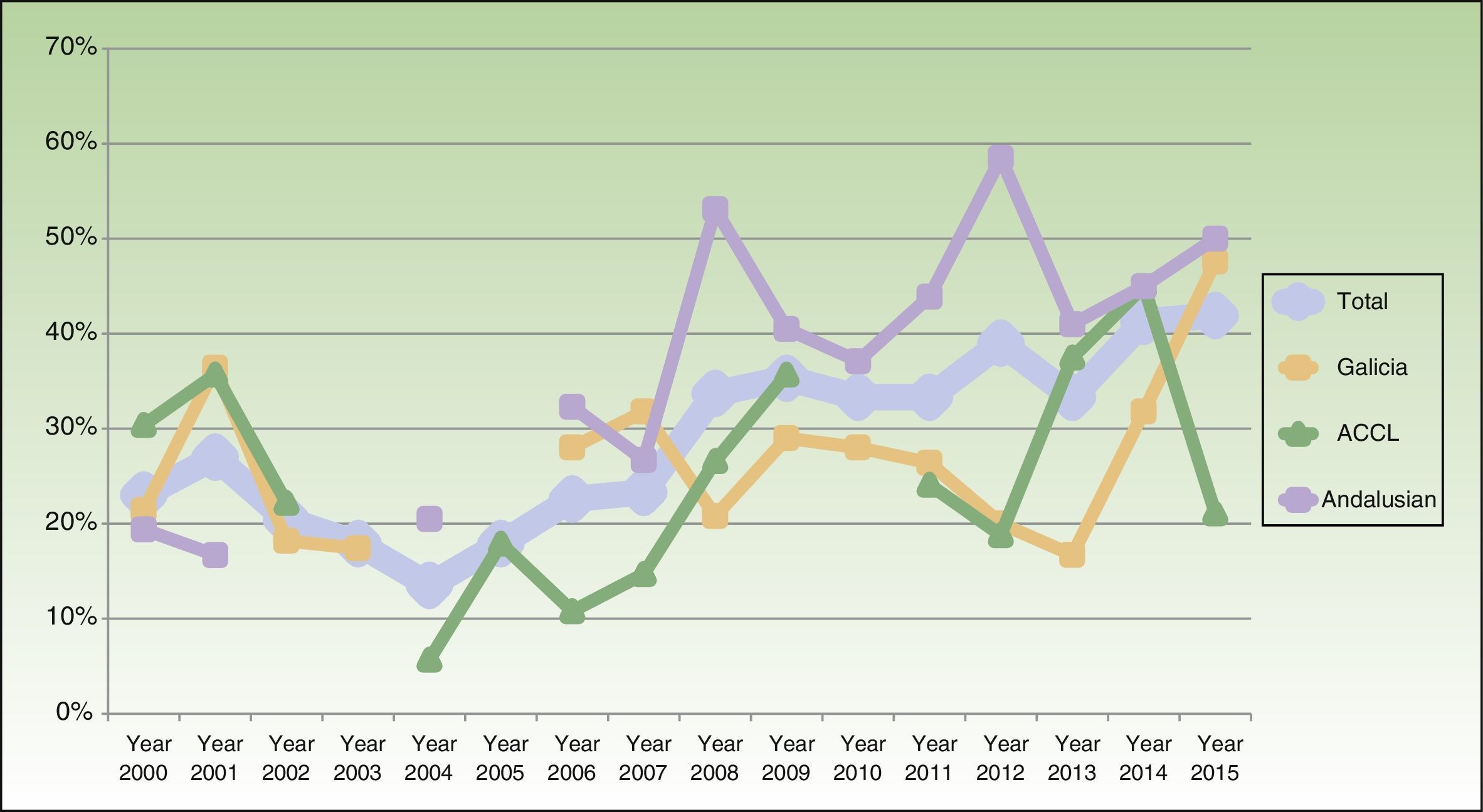
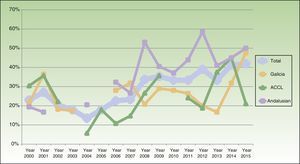
The percentage of clinical research presentations increased significantly over the study period, rising to a high of 41.9% in 2015 (P<.001). The time trends for percentage change in clinical research overall and within each of the geographic areas are shown in Fig. 2. When we divided the total number of years into 3 periods of roughly similar lengths and numbers of presentations, it was still possible to discern a trend toward a gradual increase in the proportion of clinical research. The differences were significant in the sample overall (the pool of presentations from all areas; P<.001) and in the Andalusian subsection (P<.001). In the subset of 17 presentations of studies with higher levels of evidence (at least level3), we also detected a significant increase in output over the years (P=.008).
DiscussionA medical specialty's annual conferences and other meetings serve as platforms for communicating and exchanging scientific knowledge. Presentations and other talks scheduled at such meetings concern case reports, case series, research with previously stipulated methods and, on occasion, talks based on a nonsystematic review of the literature or the personal opinions of an expert on a subject.
Few publications have analyzed the prevalence and level of evidence provided by presentations at medical conferences and other meetings.1,5
One study worth mentioning described the impact of work presented at the AEDV's national conferences in 2000 and 2003.2 The authors found that about 90% of the presentations were single case reports or case series and that few were multicenter studies coordinated or organized by groups. Although the study did not report the percentage of clinical research, its findings are consistent with our observation that single case reports or small series predominated over studies providing a higher level of evidence.
When Aranegui et al3 evaluated the level of evidence provided by Spanish dermatologists’ publications indexed in MEDLINE (PubMed) in the years 1992, 1996, 2000, 2004, and 2008, they found that 36% of the articles reported clinical research, and that 7% provided high-quality evidence in 2008. Neither the proportion of clinical research nor the level of evidence varied significantly over the years studied. Our study evaluated conference presentations, not published articles, but we found that the percentage corresponding to clinical research was similar (29.6%) and that 4.8% of the presentations offered a high level of evidence. Aranegui and colleagues made interesting comparisons between Spanish dermatologists’ clinical research output and that of British and French dermatologists and of Spanish rheumatologists in 2008. A significantly higher percentage of the rheumatologists’ publications (54%) reported clinical research.
García-Muret and Pujol2 measured the impact of the Spanish national AEDV conference in dermatology by quantifying the number of oral presentations or posters that led to publications. They calculated an overall publication rate of 13.5% and found that the rates were similar in all the years studied (2000–2003). The lowest rate (11.4%) corresponded to 2003, attributable to the short space of time that had passed between the conference and the search for publications.3
Farley-Loftus et al6 analyzed changes between 1998 and 2007 in clinical research appearing in 2 journals published in the United States: the Journal of the American Academy of Dermatology and Archives of Dermatology. They saw a trend toward increasing numbers of single case reports and case series and a decrease in articles providing high levels of evidence, such as randomized controlled trials or metaanalyses. In our study, however, the level of evidence of clinical research presentations increased over the study period.
Various factors can explain the low percentage of clinical research we observed. Lack of funding for research and consequent lack of time often make it difficult to develop protocols with solid research designs or to recruit large numbers of patients. In some cases the low prevalence of certain conditions complicates the design of high-quality studies (defined as those that provide at least level 3 or 4 evidence). This situation favors the choice of the single case report as the preferred vehicle for reporting new therapeutic approaches or adverse effects.6
We observed a trend toward more methodologically complex designs, and we think that their inclusion in conference programs conveys to participants a message that such studies are feasible, encouraging others to undertake them. The launch of the AEDV's research group in 2012 has also been able to facilitate this trend by contributing to the design of new studies.7
We are aware that our study has a series of limitations.
First, we only looked at 3 areas of the country and cannot extrapolate to the rest of Spain.
Second, we only analyzed presentations whose abstracts were published in the journal Actas Dermo-Sifiliográficas. Nonpublication of abstracts usually corresponded to early years of our study period, when clinical research accounted for a lower percentage of presentations. Any possible bias, therefore, would underestimate the increase in clinical research output over the years, thus reinforcing our conclusions. In any case, very few abstracts would have been missed and their numbers would probably be distributed evenly over the different classifications.
The third limitation concerns the significant increase we observed in the number of studies offering a high level of evidence (level3 or higher) over the years. Because that observation was based on only 17 presentations in this category, it should be interpreted cautiously. Firm conclusions cannot be drawn.
The fourth limitation concerns the somewhat subjective classification of studies into CEBM evidence levels. We attempted to reduce the effect of this problem by using 2 assessors.
Finally, as discussed above, numerous articles and editorials have been published in Actas Dermo-Sifiliográficas to promote the importance of clinical research within the AEDV.1,3,7 Basic and advanced courses on research methods and an introduction to systematic reviewing have also been organized. These initiatives might be considered to have biased conferences toward encouraging work that provides higher levels of evidence in recent years, but they are also a means for strengthening the quality of clinical research in dermatology.
ConclusionsClinical research was reported in 29.6% of presentations at the dermatology conferences of the AEDV territorial sections we studied. Research providing high levels of evidence was described in 4.8% of the presentations. Most of the research involved cross-sectional studies or case series. We detected differences between geographic areas with regard to the prevalence of clinical research at meetings, but the levels of evidence provided were similar. Clinical research both increased over the years studied and improved in quality. However, our observations reflect a relatively low number of presentations given in 3 areas of Spain. More studies are therefore required to confirm these findings, analyze factors affecting them, and suggest approaches to increasing the level of evidence of research presented.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Iglesias-Puzas Á, Batalla A, Flórez Á. La investigación clínica en las secciones territoriales de la Academia Española de Dermatología y Venereología (AEDV). Actas Dermosifiliogr. 2018;109:148–154.