Reflectance confocal microscopy is currently the most promising noninvasive diagnostic tool for studying cutaneous structures between the stratum corneum and the superficial reticular dermis. This tool gives real-time images parallel to the skin surface; the microscopic resolution is similar to that of conventional histology. Numerous studies have identified the main confocal features of various inflammatory skin diseases and tumors, demonstrating the good correlation of these features with certain dermatoscopic patterns and histologic findings. Confocal patterns and diagnostic algorithms have been shown to have high sensitivity and specificity in melanoma and nonmelanoma skin cancer. Possible present and future applications of this noninvasive technology are wide ranging and reach beyond its use in noninvasive diagnosis. This tool can also be used, for example, to evaluate dynamic skin processes that occur after UV exposure or to assess tumor response to noninvasive treatments such as photodynamic therapy. We explain the characteristic confocal features found in the main nonmelanoma skin tumors and discuss possible applications for this novel diagnostic technique in routine dermatology practice.
Actualmente la microscopia confocal reflectante es la técnica diagnóstica no invasiva más prometedora para el estudio de estructuras cutáneas situadas entre la capa córnea y la dermis reticular superficial, obteniendo imágenes paralelas a la superficie cutánea en tiempo real y con una resolución microscópica similar a la observada en la histología convencional. Numerosos estudios han señalado las principales características confocales que se observan en distintas enfermedades cutáneas, tanto tumorales como inflamatorias, demostrando una buena correlación con ciertos patrones dermatoscópicos, así como con el examen histológico. Además, se han descrito algoritmos diagnósticos y patrones confocales que han demostrado unas altas tasas de sensibilidad y especificidad para el diagnóstico de tumores cutáneos de tipo melanoma y no melanoma. Las posibles aplicaciones presentes y futuras de esta tecnología son muy amplias, no solo como herramienta diagnóstica no invasiva, sino también para la evaluación de distintos procesos dinámicos como aquellos que ocurren tras la exposición de la piel a la radiación ultravioleta, o la respuesta de los tumores a terapias no invasivas como la terapia fotodinámica. Explicamos con detalle los hallazgos confocales característicos de los principales tumores cutáneos de tipo no melanoma y discutimos las posibles aplicaciones de esta novedosa técnica diagnóstica en la práctica diaria de la consulta dermatológica.
In recent years, we have witnessed a true technological revolution in the field of dermatology, with implications for both clinical diagnosis and treatment. The growing preference for less invasive procedures has driven the development of new imaging technologies1–7 and even drugs that in many cases eliminate the need for skin tumor surgery.8–10
Nonmelanoma skin cancer is the most common cancer in white individuals and its incidence continues to rise.11 Histologic examination is the gold standard for the diagnosis of skin cancer, but it has limitations, such as the need to remove small amounts of tissue for ex-vivo analysis, which is usually performed at a later stage. These limitations have led to the development of new noninvasive diagnostic methods that offer real-time, in vivo, and in situ results without scarring. One of the main advantages of these new methods is that they provide dynamic information that can be used in the longitudinal monitoring of different skin conditions. Examples of these noninvasive technologies are magnetic resonance imaging, high-frequency ultrasound imaging, optical coherence tomography, and, more recently, reflectance confocal microscopy (RCM).12,13
Of the above techniques, RCM provides the best imaging-histologic correlation and has both high sensitivity and specificity for the diagnosis of nonmelanoma skin cancer.14–16
Researchers have described a range of RCM features and patterns that can be used to identify a range of skin tumors and differentiate these from normal skin.
RCM and Confocal Patterns of Normal SkinOf the numerous diagnostic imaging technologies to emerge in recent years, RCM has been studied most in clinical trials. It has also resulted in real clinical applications, particularly in the field of skin cancer. Among its strengths are its noninvasive nature and the fact that it provides real-time, in vivo images with a microscopic resolution similar to that of conventional histology (lateral and axial resolution of up to 1μm and 3μm, respectively).13
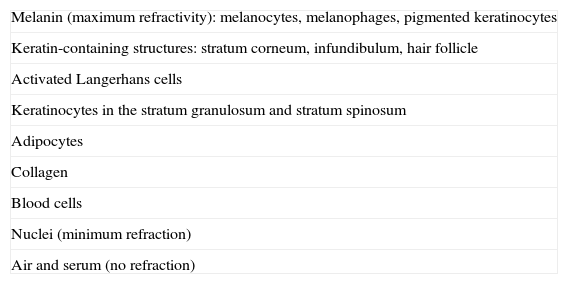
Like any optical system, the confocal microscope consists of a light source (generally a low-power diode laser), a condenser lens, an objective lens, a pinhole, and a detector. It is called confocal because the tissue plane studied is conjugate to the light source plane and the pinhole in front of the detector.4 RCM provides en face (horizontal) real-time images formed by light reflected from a focal plane. In other words, all light reflected by structures located outside this plane is rejected by the pinhole. Image contrast is produced by differences in the refractive indices of the varying tissue and cell structures. Melanin-containing structures (melanosomes, melanocytes, melanophages, and pigmented keratinocytes, among others) have the highest refractivity, followed by keratin-containing structures, such as the stratum corneum, the infundibulum, and the hair follicle. Nuclei, air, and serum exhibit minimum reflectivity.17 Microscopic structures, which are of a similar size to the wavelength of the incident light, have the highest refractive indices (Table 1). The maximum imaging depth that has been achieved to date with RCM is approximately 300μm,13 although this may increase in the near future.
Reflection of Skin Structures Seen by Reflectance Confocal Microscopy (From Highest to Lowest Refractivity).
| Melanin (maximum refractivity): melanocytes, melanophages, pigmented keratinocytes |
| Keratin-containing structures: stratum corneum, infundibulum, hair follicle |
| Activated Langerhans cells |
| Keratinocytes in the stratum granulosum and stratum spinosum |
| Adipocytes |
| Collagen |
| Blood cells |
| Nuclei (minimum refraction) |
| Air and serum (no refraction) |
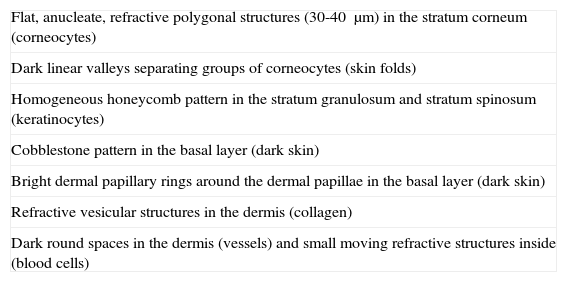
Before looking at the confocal patterns of different skin tumors, we will first describe the typical patterns seen in normal skin structures (Table 2). In brief, the epidermis is composed of 4 layers composed mainly of keratinocytes and 2 minority populations of dendritic cells: Langerhans cells and melanocytes. These layers can be distinguished from each other as they have different cytologic and architectural characteristics and are located at different depths.17 RCM imaging usually starts at the skin surface and progresses downwards. The first image seen thus is normally the stratum corneum as this has a different index of refraction to that of the immersion medium applied to the skin contact ring (generally water). The stratum corneum appears as a surface with variable reflectivity composed of polygonal, flat, anucleate cells ranging in size between 30 and 40μm. The total thickness of the corneal layer depends on topographic variations, sun exposure, and skin type. These factors also directly determine the depth of the skin folds, which are visualized as dark furrows separating the corneocytes into islands.
Reflectance Confocal Microscopy Features of Normal Skin.
| Flat, anucleate, refractive polygonal structures (30-40 μm) in the stratum corneum (corneocytes) |
| Dark linear valleys separating groups of corneocytes (skin folds) |
| Homogeneous honeycomb pattern in the stratum granulosum and stratum spinosum (keratinocytes) |
| Cobblestone pattern in the basal layer (dark skin) |
| Bright dermal papillary rings around the dermal papillae in the basal layer (dark skin) |
| Refractive vesicular structures in the dermis (collagen) |
| Dark round spaces in the dermis (vessels) and small moving refractive structures inside (blood cells) |
The next layer is the stratum granulosum. This is visualized as an evenly distributed structure at a depth of approximately 20μm, with a thickness of between 3 and 10 cells depending on the anatomic location. The keratinocytes in the granular layer measure approximately 25μm and their nucleus appears as a dark oval central structure surrounded by a cytoplasm with a granular appearance due to the presence of bright multiple structures (0.1 to 1.0μm) that correspond to keratohyalin granules. The granulocytes have clear outlines that form the characteristic confocal pattern known as the honeycomb pattern.
The stratum spinosum, located at approximately 30 to 100μm beneath the stratum corneum, is formed by polygonal keratinocytes measuring approximately 15μm that become progressively flatter towards the surface. The spinous layer has between 5 to 10 layers of cells, which have a dark oval central area (the nucleus) and clearly demarcated outlines that together with the stratum granulosum form the characteristic honeycomb pattern.
The dermal-epidermal junction, located at a depth of 50 to 100μm, contains the basal layer, which is formed by a single row of cuboidal cells and several scattered melanocytes with a round or dendritic morphology. The keratinocytes in this layer are smaller than those in the other layers of the epidermis. They have a diameter of approximately 10μm and are brighter due to their high melanin content, although this varies according to skin type. Melanin typically forms a protective cap over the nucleus of keratinocytes in the basal layer, whose brightness varies according to skin type and anatomic location. In fair skin, the basal keratinocytes do not appear very bright and are difficult to visualize, while in darker skin, they form a characteristic cobblestone pattern consisting of round cells that appear very bright due to the supranuclear melanin cap. In anatomic regions with rete ridges, these highly reflective cells form rings around dark areas that correspond to the dermal papillae, giving rise to another characteristic confocal pattern known as dermal papillary rings. Below this level, dermal papillae are seen to contain collagen bundles with varying brightness, together with round refractive structures with a dark center. These correspond to the blood capillaries inside which moving cells can be seen in real time during image capture.
Confocal Patterns and Characteristics of Basal Cell CarcinomaBasal cell carcinoma (BCC) is the most common skin cancer in the world and mostly affects fair-skinned adults. The main risk factors are exposure to UV radiation, genetic alterations, and compromised immune states. The clinical presentation is variable and is related to the main histologic subtype: superficial, nodular, or morpheaform.18 BCC is a slow-growing tumor that rarely metastasizes. It is one of the skin cancers that have been studied most by RCM,19–22 possibly because of its high incidence (80% of all nonmelanoma skin cancers). Several researchers have described the main confocal features of BCC, and one large multicenter study conducted in 2004 by Nori et al.14 showed high sensitivity and specificity for certain findings.
Nori et al.14 analyzed 152 BCC lesions and correlated RCM and histology findings. They described 5 major diagnostic criteria: elongated monomorphic nuclei, polarization of these nuclei along the same axis, a prominent inflammatory infiltrate, increased dermal vasculature, and pleomorphism of the overlying epidermis. They reported a sensitivity of 100% for the presence of 2 or more criteria and a specificity of 95.7% and a sensitivity of 82.9% for the presence of 4 or more criteria. The results showed little variability across different observers and BCC subtypes.
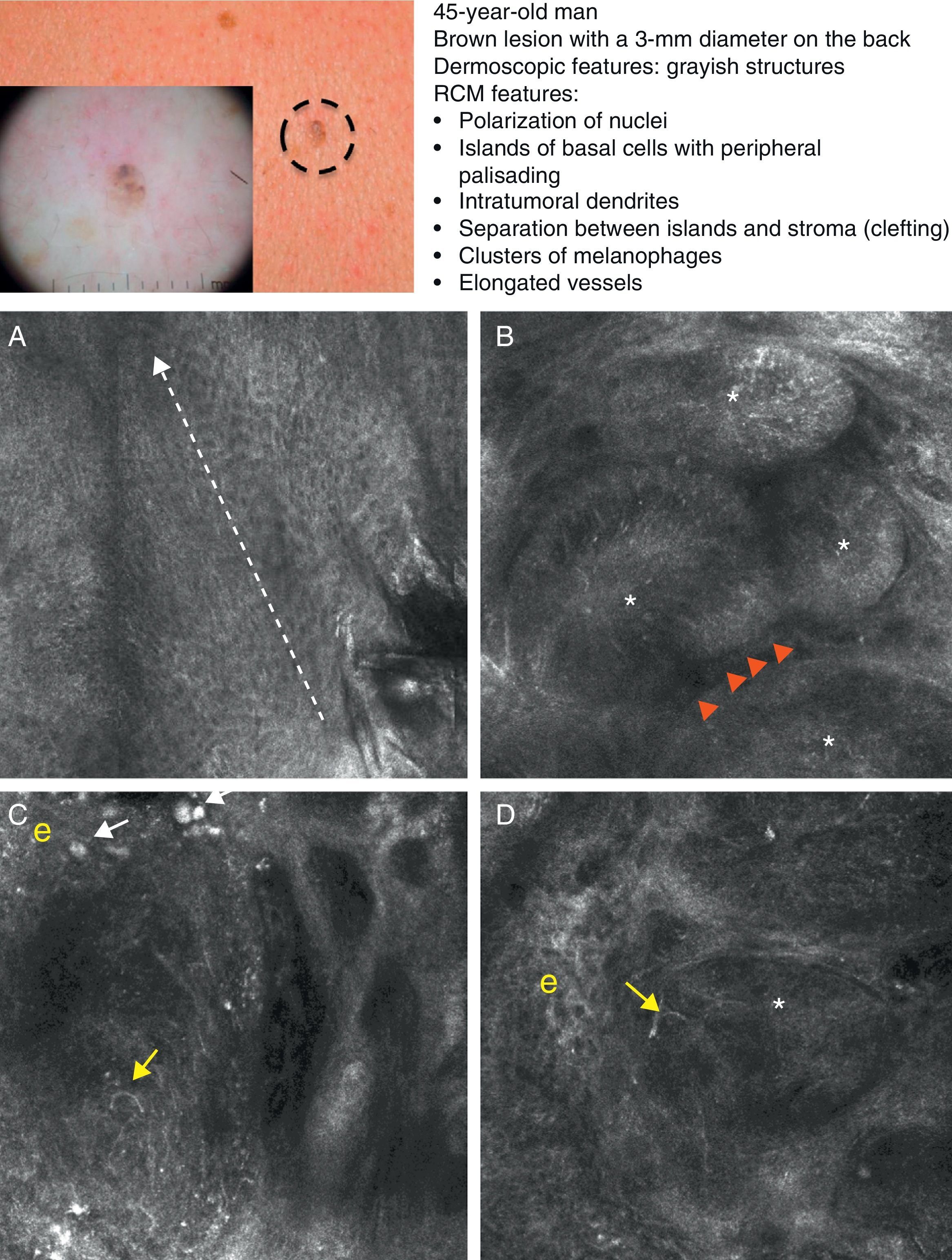
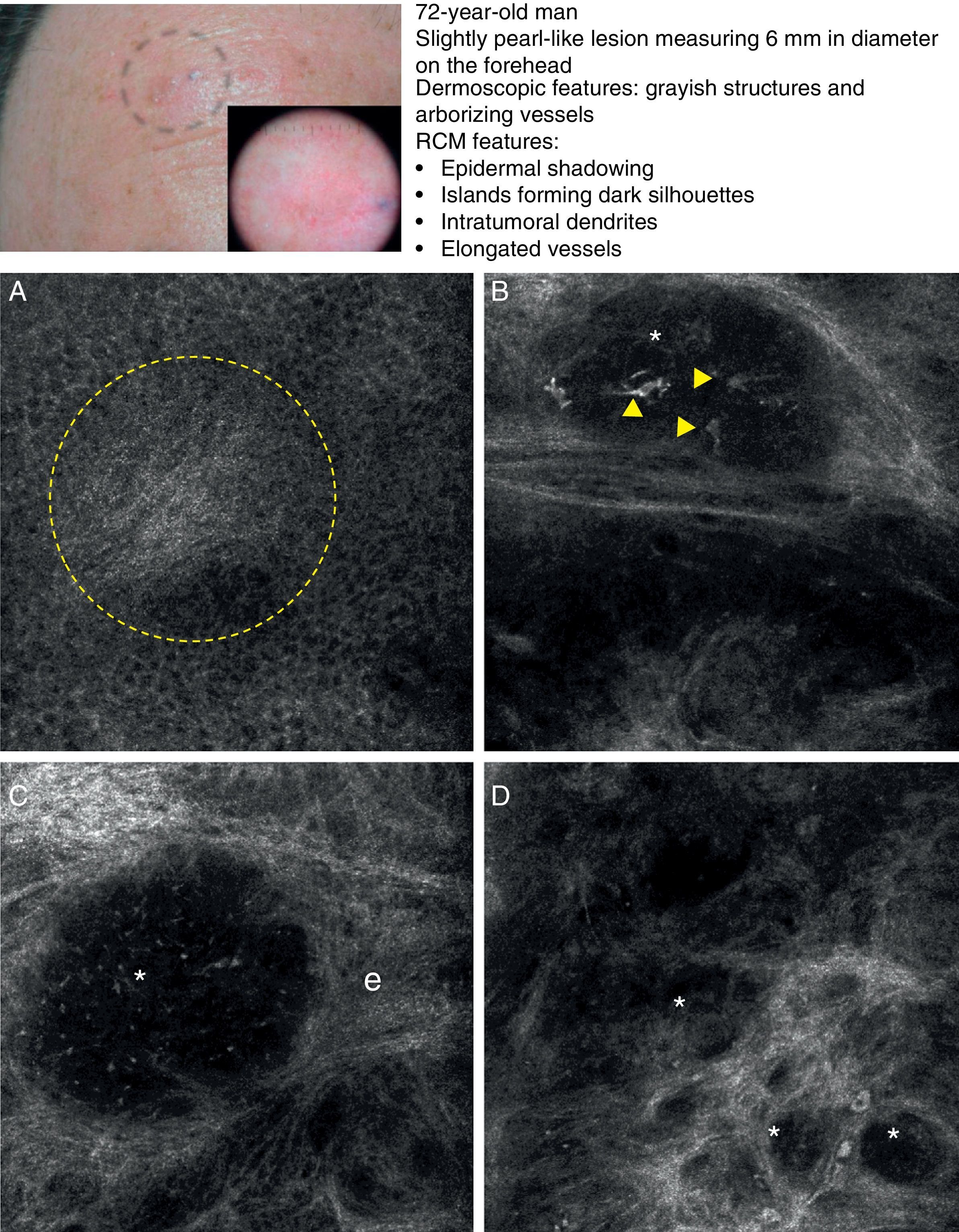
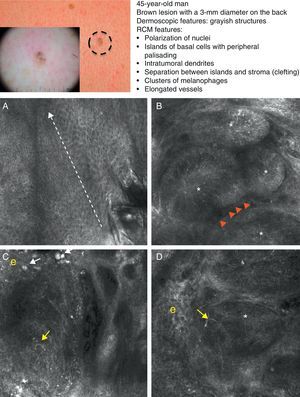
As occurs with histology, the different subtypes of BCC share certain confocal patterns (Table 3) that make it relatively easy to reach a diagnosis. The main defining pattern is the presence of dense structures formed by a homogeneous population of tumor cells with elongated nuclei oriented along the same axis (a phenomenon known as polarization of nuclei) (Fig. 1A).19 When this phenomenon occurs around an island of cells, it is known as peripheral palisading, as the elongated cells are oriented perpendicularly to the edge of the tumor. Another relatively common finding is a dark area surrounding aggregates of tumor cells, probably due to mucin deposits (Fig. 1B and D). This feature corresponds to the characteristic clefting (separation of tumor islands from the surrounding stroma) seen on histology. Some of the authors of the present study (MU, SG) and other colleagues studied 13 cases of BCC (including the 3 main histologic subtypes) and found good linear correlation between dark areas seen by RCM and peritumoral mucin thickness.22 The stroma sometimes appears brighter than the tumor islands, which are darker than usual (hyporeflective) and are referred to as dark silhouettes (Fig. 2B and C). This feature was first described by Ravinobitz (University of Miami, USA) and was recently described in detail.17 Another typical confocal pattern seen in BCC is epidermal disarray in the layers just above the tumor. The characteristic honeycomb pattern is typically altered and in its place are irregular keratinocytes, probably due to the chronic actinic damage typically seen in patients with BCC. The stroma surrounding the tumor nests may display some brightness due to the presence of collagen fibres and bundles (Figs. 1C and 2B and C). Blood flow is typically altered in BCC, probably due to angiogenesis, and is described as an elongation of blood capillaries, which are increased in number and size. RCM images show numerous dark, round spaces or branching (arborizing) lines, containing moving cells with different levels of refractivity (Fig. 1B). This phenomenon is known as trafficking of leukocytes.23 Small bright structures corresponding to inflammatory cells can also be seen in the dermis. The main confocal characteristics of pigmented BCC are similar to those of nonpigmented BCC, but in addition there are abundant bright, melanin-containing structures of different morphologies. Small, very bright amorphous grainy structures and dendritic structures, which appear to correspond to clusters of melanin and melanocytes, respectively, are visualized within the tumor islands.24
Reflectance Confocal Microscopy Features of Basal Cell Carcinoma.
| Elongation of nuclei |
| Polarization: elongation of cells along the same axis |
| Palisading: polarization of cells perpendicular to the edge of the tumor island |
| Clefting: dark space separating the tumor nests from the stroma |
| Atypical honeycomb pattern in the epidermis overlying the tumor |
| Dark round linear spaces containing abundant refractive structures in the papillary dermis (dilated, arborizing vessels) |
Clinical and dermoscopic images of pigmented basal cell carcinoma. A, RCM image (0.5×0.5mm) taken at a depth of approximately 30μm under the skin surface. Note the elongation (polarization) of the nuclei along the same axis (dashed line). B, RCM image (0.5×0.5mm) showing tumor islands with characteristic peripheral palisading (asterisks) and separation from stroma (e). C and D, Dilated vessels (arrows) nearby containing bright dendritic melanocytes. RCM images (0.5×0.5mm) showing the presence of a tumor (*) with intratumoral dendrites (yellow arrow). Note the clusters of melanophages (white arrows) in the stroma (e). RCM indicates reflectance confocal microscopy.
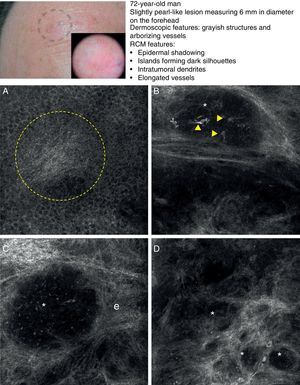
Clinical and dermoscopic images of basal cell carcinoma. A, RCM image (0.5×0.5mm) of lower part of the epidermis, showing epidermal shadowing (dashed circle). B, RCM image (0.5×0.5mm) of the dermis, showing nonrefractive tumor islands forming a dark silhouette pattern. Inside are dendritic cells (arrowheads) that appear to correspond to intratumoral melanocytes. C, RCM image (0.5×0.5mm) of the dermis, showing a nonrefractive tumor island (asterisk) forming a dark silhouette pattern surrounded by a reflective stroma (e). Note the presence of a refractive grainy material, possibly melanin, in the interior. D, RCM image (0.5×0.5mm) of the deep dermis, showing smaller nonrefractive tumor islands (asterisks). RCM indicates reflectance confocal microscopy.
The main stromal features stroma are large, bright, poorly demarcated structures that correspond to melanophages and smaller structures that correspond to melanin (Fig. 1C).
In a recently published large prospective study, Guitera et al.16 analyzed 710 consecutive equivocal lesions by RCM. The lesions included 216 melanomas, 266 nevi, 119 BCCs, 67 pigmented facial macules, and 42 lesions classified as other skin tumors. They studied 50% of the lesions (chosen randomly) by multivariate analysis and identified 8 independently significant diagnostic features for BCC, with a sensitivity of 97.1% and a specificity of 93.4%. Five of the factors were positive: polarized elongated structures in the superficial layer, linear telangiectasia-like horizontal vessels, compact nests of hyporeflective cells, peripheral palisading, and a new concept called epidermal shadowing (Fig. 2A), which they described as a large dark featureless area disrupting the epidermis due to en face clefting of the underlying tumor nests. The 3 negative features were the atypical honeycomb pattern, nonvisible papillae, and cerebriform nests.
Confocal Patterns and Characteristics of Squamous Cell CarcinomaActinic KeratosisActinic keratosis (AK), one of the most common skin tumors, is a proliferation of atypical keratinocytes confined to the epidermis. It is classified into 3 types depending on the degree of keratinocytic atypia.25 The main risk factors are fair skin, intermittent or prolonged exposure to UV radiation, genetic predisposition, and immunosuppression. AK has been associated with field cancerization, a concept that suggests that the whole anatomic region affected by actinic damage will show similar histologic changes before these become clinically visible.26–29 While histologic examination is the gold standard for the diagnosis of AK, most cases are diagnosed clinically by visual inspection and palpation. RCM, however, also reveals certain features that may help to establish a diagnosis of AK without the need for invasive methods29 (Table 4). The keratinocytes in the stratum corneum appear detached from one another and are seen as highly refractive polygonal structures. Another feature, nuclear retention, which corresponds to parakeratosis in histology, is visualized as dark round structures in the center of the corneocytes. RCM images of architectural disarray and cellular pleomorphism show an atypical honeycomb pattern (disruption of the characteristic honeycomb pattern) and cells with dark nuclei of irregular shapes and sizes. Images of the superficial dermis show different degrees of solar elastosis, which is visualized as bundles of lace-like collagen fibers with moderate to high reflectivity and small, roundish slightly elongated blood vessels.30,31
Reflectance Confocal Microscopy Features of Actinic Keratosis.
| Abundant independent bright polygonal structures containing dark spaces in the stratum corneum (parakeratotic hyperkeratosis) |
| Atypical honeycomb pattern in the epidermis |
| Characteristic irregular lace-like pattern in the dermis (solar elastosis) |
Bowen disease (BD) is a squamous cell carcinoma (SCC) in situ that is histologically characterized by a proliferation of atypical pleomorphic keratinocytes throughout the epidermis. Dyskeratosis, mitosis, and multinucleated cells are very common findings.32 Clinically, it can be confused with other nonmelanoma skin cancers or with certain inflammatory skin conditions, such as eczema and psoriasis.33 Ulrich et al.34 recently published a study of the confocal features of 10 BD lesions and described their correlation with routine histologic features. The most common confocal findings were disruption of the stratum corneum, an atypical honeycomb pattern in the epidermis with a greater degree of architectural disorder and cellular atypia than in AK, S-shaped blood vessels in the center of the dermal papillae, and 2 types of characteristic targetoid cells. The first type were large cells with a dark center, a bright rim, and a dark halo, and the second type were large cells with a bright center and a dark halo. The cells are thought to correspond to the different degrees of dyskeratosis seen by conventional histology. Other features observed were parakeratosis, multinucleated cells, and solar elastosis (Table 5).
Reflectance Confocal Microscopy Features of Bowen Disease.
| Disruption of stratum corneum |
| Atypical honeycomb pattern in the epidermis |
| Large characteristic targetoid cells with a dark halo in the epidermis (dyskeratotic cells) |
| Dark circular spaces in the center of the papillary rings (characteristic S-shaped blood vessels) |
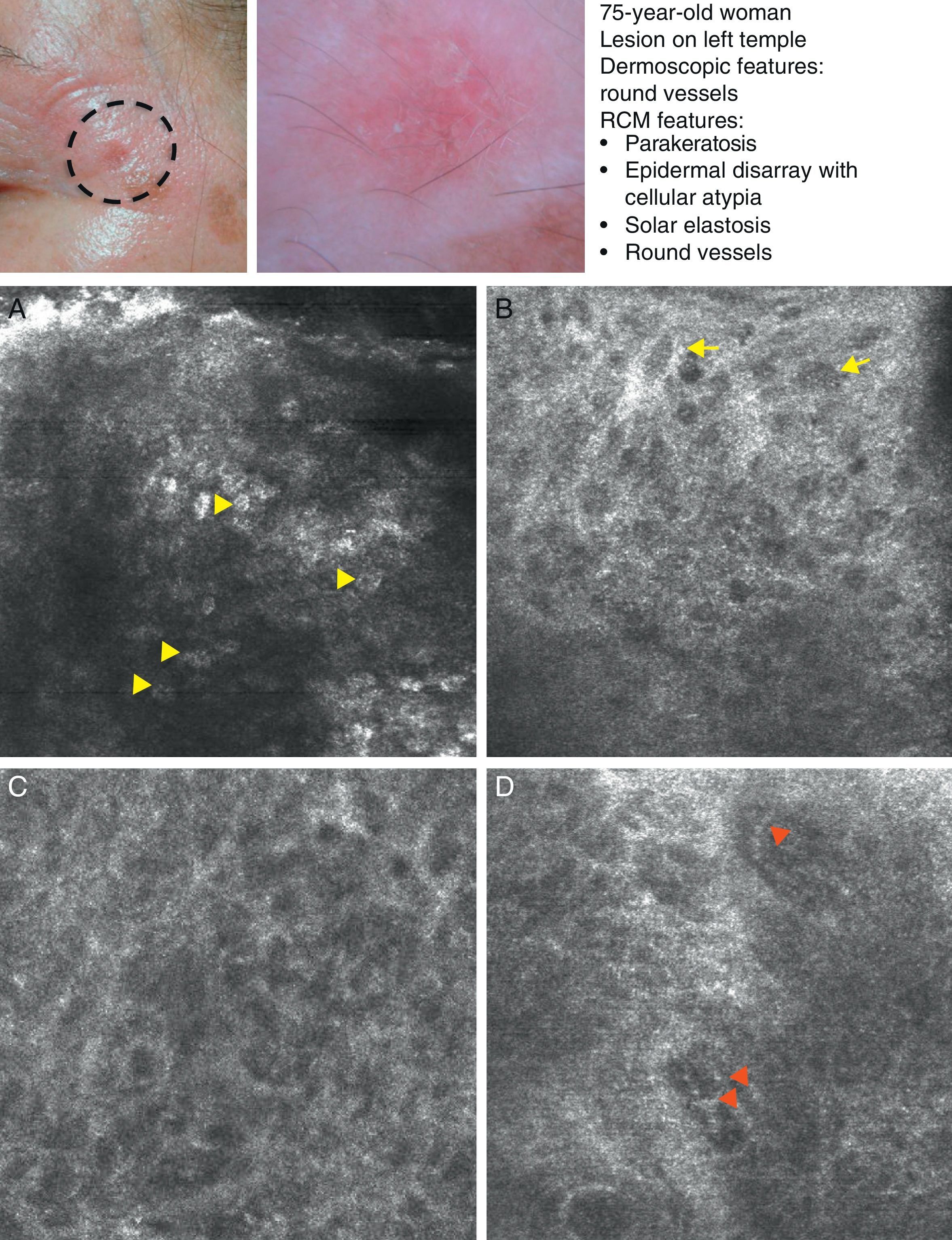
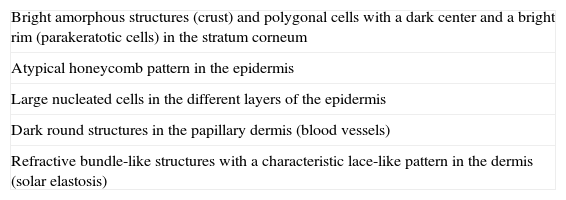
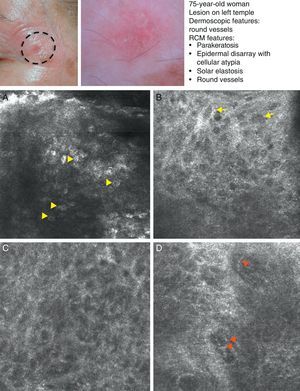
SCC is a common skin tumor derived from epidermal keratinocytes. It typically presents as a red scaling plaque, with or without ulceration, and can therefore be difficult to distinguish from other skin lesions.35 Dermoscopy and RCM can aid early diagnosis, which is very important in SCC considering the tumor's metastatic potential. Histologically, SCC is characterized by irregular keratinocytes with numerous pleomorphic nuclei and abundant eosinophilic cytoplasm, in addition to dyskeratosis and parakeratosis in the stratum corneum.36 The crusted surface often seen on SCC lesions can limit the diagnostic value of both dermoscopy and RCM, and it is generally necessary to remove the crust before examination. In 2009, Rishpon et al.37 published a study of the confocal characteristics of 38 clinically suspected SCC lesions that were subsequently confirmed by histology. The features identified were an atypical honeycomb or disarranged pattern in the epidermis (Fig. 3B and C), large round cells with nuclear atypia in the stratum spinosum and stratum granulosum (Fig. 3B and C), and round blood vessels crossing the dermal papillae (Fig. 3D). RCM images of the stratum corneum typically reveal bright amorphous structures that correspond to the presence of crusts on the tumor surface and polygonal nucleated cells with a bright rim around a dark nucleus (parakeratosis) (Fig. 3A). Hyperkeratosis and acanthosis permitting, RCM may also show increased dermal vasculature and solar elastosis as well as tumor islands in the case of invasive SCC38 (Table 6).
Clinical and dermoscopic images of squamous cell carcinoma in situ. A, RCM image (0.5×0.5mm) of the stratum corneum, showing numerous very bright polyhedral cells (arrowheads). B, RCM image (0.5×0.5mm) of the stratum granulosum, showing the atypical honeycomb pattern with architectural disorder and cellular atypia (arrows). C, RCM image (0.5×0.5mm) of the stratum spinosum, showing considerable architectural disorder. D, RCM image (0.5×0.5mm) of the dermis, showing solar elastosis and round vessels (arrowheads). RCM indicates reflectance confocal microscopy.
Reflectance Confocal Microscopy Features of Squamous Cell Carcinoma.
| Bright amorphous structures (crust) and polygonal cells with a dark center and a bright rim (parakeratotic cells) in the stratum corneum |
| Atypical honeycomb pattern in the epidermis |
| Large nucleated cells in the different layers of the epidermis |
| Dark round structures in the papillary dermis (blood vessels) |
| Refractive bundle-like structures with a characteristic lace-like pattern in the dermis (solar elastosis) |
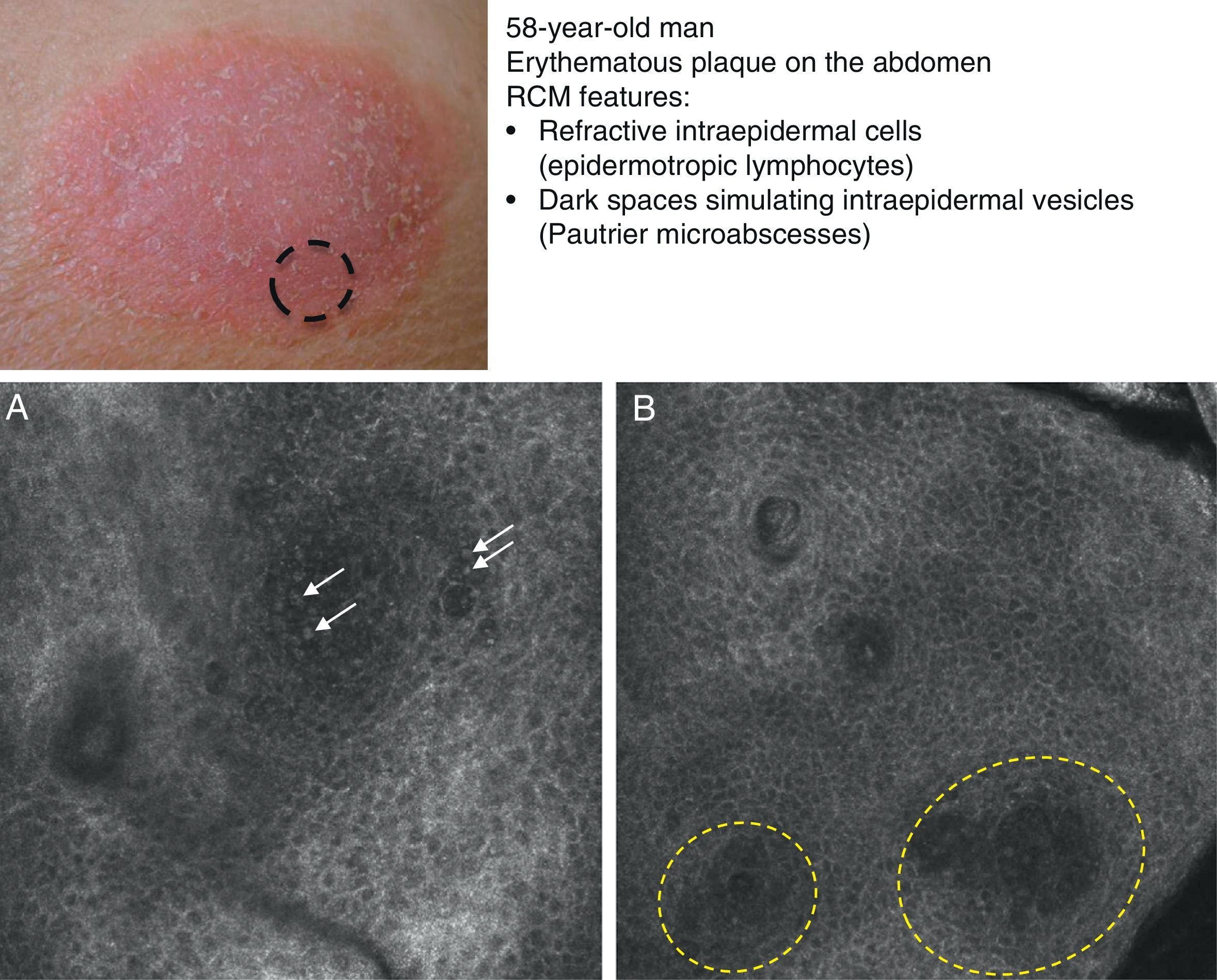
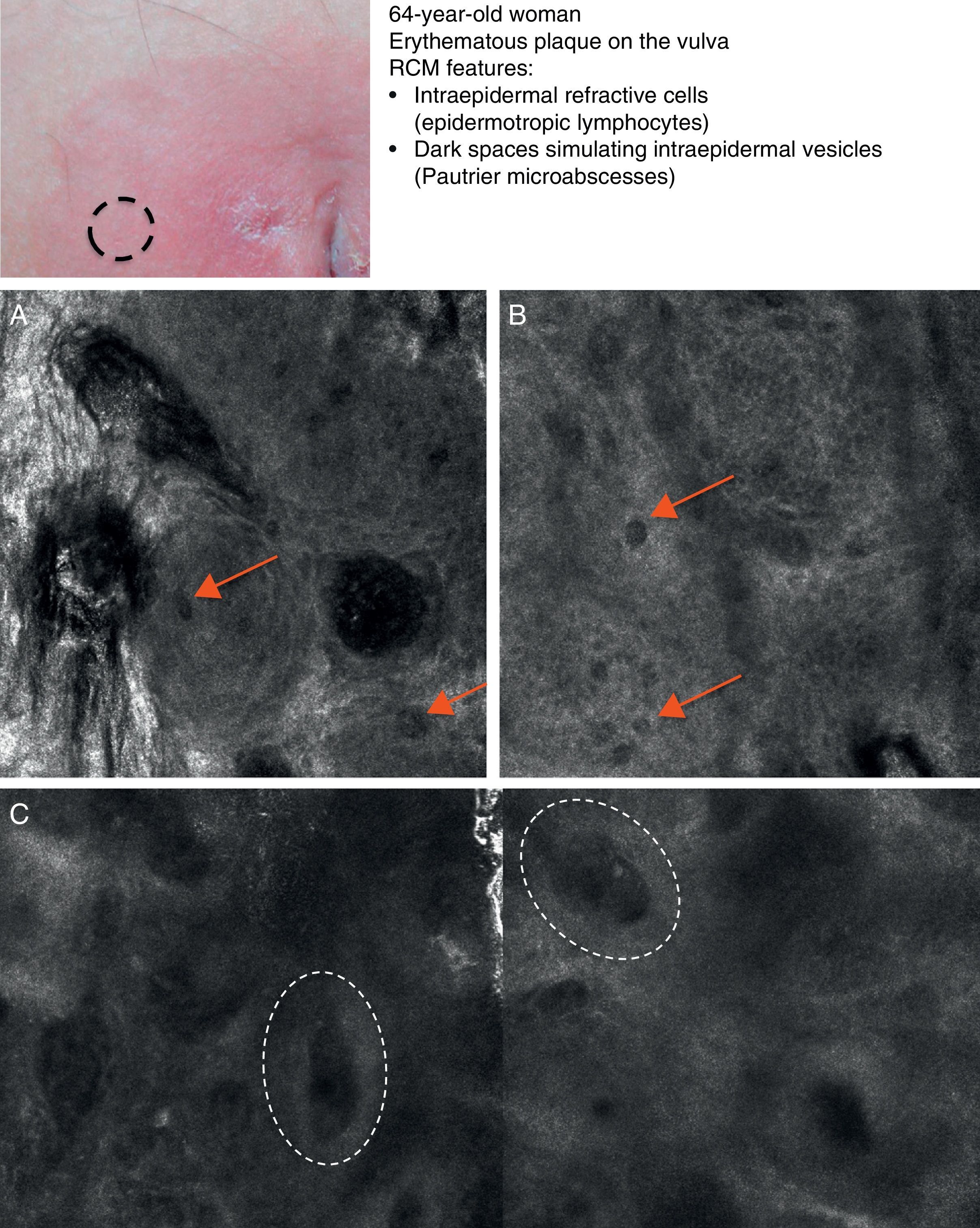
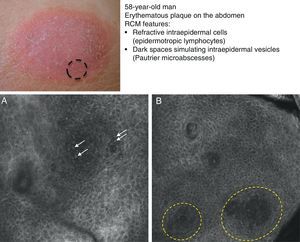
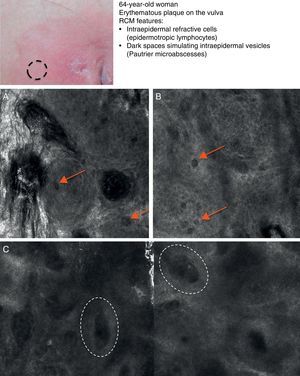
Mycosis fungoides (MF) is the most common cutaneous T-cell lymphoma. Lymphomas are generally classified based on the results of different tests, such as histology, molecular genetics, and immunophenotyping studies.39 The differential diagnosis is extensive and complex, and includes numerous common eczematous conditions such as psoriasis and chronic dermatitis. The histologic features of MF have been studied in detail, but only a few have been found to be distinguishing features.40 Key histologic findings for establishing an early diagnosis of MF are lymphocytes surrounded by a halo and a combination of criteria such as epidermotropism, a band-like inflammatory infiltrate along the dermal-epidermal junction, and fibrosis in the dermal papillae. Pautrier microabscesses, which are considered specific to MF, are only seen in a minority of cases (mostly in advanced plaque-stage MF). Numerous clinical and histologic examinations are often required before a diagnosis of MF is reached, and it can sometimes take years or even decades to make a correct diagnosis and start appropriate treatment. It has been shown that 2 or even more biopsies of different MF lesions taken from the same patient on the same day frequently display different histologic features.41 The selection of an appropriate lesion for biopsy is therefore essential for making a correct diagnosis. RCM can be of value in such cases as it can help to identify the best biopsy site and reduce the number of false negatives with histology. Agero et al.42 were the first authors to describe the confocal characteristics of MF and concluded that RCM was a useful tool for selecting an appropriate biopsy site. The main RCM features of cutaneous T-cell lymphoma are small weakly refractive cells in the epidermis (epidermotropic lymphocytes) (Fig. 4A), hyporeflective papillary rings due to the presence of atypical lymphocytes at the dermal-epidermal junction, dark vesicle-like spaces (Pautrier microabscesses) in the epidermis (mainly in plaque-stage MF) (Fig. 4B), and dilated blood vessels with thick walls (Table 7). The vesicle-like spaces are distinguishable from those observed in acute eczema due to the absence of spongiosis and parakeratosis and the presence of other characteristics suggestive of MF, such as hyporeflective papillary rings.22,42 Lange-Asschenfeldt et al.43 recently published a study on the use of RCM in 39 lesions from 15 patients with histologically confirmed MF, parapsoriasis, Sézary syndrome, and lymphomatoid papulosis. They reviewed the confocal features of cutaneous T-cell lymphoma and demonstrated high interobserver agreement.
Clinical image of plaque-stage mycosis fungoides. A, RCM image (0.5×0.5mm) of the superficial stratum spinosum showing disruption and bright cells (epidermotropism, white arrows) between the keratinocytes. B, RCM image (0.5×0.5mm) of the stratum spinosum, showing small dark spaces corresponding to Pautrier microabscesses (dashed circles). Note the highly reflective cells (atypical lymphocytes) in the interior. RCM indicates reflectance confocal microscopy.
Reflectance Confocal Microscopy Features of Mycosis Fungoides.
| Small refractive structures in the epidermis (epidermotropic lymphocytes) |
| Dark intraepidermal spaces (Pautrier microabscesses) in plaque-stage mycosis fungoides |
| Hyporeflective papillary rings |
| Dark round linear structures, with hyper-reflective contours in the dermis (dilated blood vessels with thick walls) |
Mammary Paget disease (PD) and extramammary PD are uncommon intraepidermal adenocarcinomas with very similar clinical and histologic characteristics that mimic a range of inflammatory and infectious skin conditions. The origin of both diseases, and extramammary PD, in particular, is controversial.44 The most widely accepted hypothesis regarding the etiology of mammary PD is that it is an epidermal extension of an invasive ductal carcinoma of the underlying mammary tissue, as the 2 diseases co-occur in most cases. Associated internal malignancies are much less common (9%-32%) in extramammary PD, which is currently considered a primary intraepidermal tumor whose cells may originate either in the ducts of the apocrine glands or in certain pluripotential keratinocytic cells (Toker cells). PD cells have been well characterized immunohistochemically and there are clear indications of glandular differentiation. While there are large similarities between mammary and extramammary PD, subtle differences exist that suggest that they may have different origins.44
Mammary PD exclusively affects the nipple and/or the areola. Clinically, it presents as a slow-growing unilateral erythematous, scaling plaque with well-defined irregular borders that frequently causes itching or a burning sensation. It can pose a substantial diagnostic challenge as it can clinically mimic common inflammatory or infectious diseases such as eczematous dermatitis of the nipple. Diagnosis—and consequently correct management—is thus often delayed.
Extramammary PD is more common in the anogenital region and other areas rich in apocrine glands, such as the axillae and the external ear. The most common clinical manifestation is intense pruritus. The lesions are similar in appearance to those seen in mammary PD, but there may also be hyperpigmentation or hypopigmentation.45
PD is typically multicentric and it is therefore generally necessary to perform several biopsies to map the contours of the lesion before starting treatment, which is usually surgical excision with wide margins.
The histologic features of mammary and extramammary PD are similar46 and include large Paget cells with a clear, finely granular cytoplasm and a wide central nucleus. The cells exhibit pronounced atypia and pleomorphism and are distributed singly or in small nests invading the various layers of the epidermis. This characteristic histologic pattern of epidermal infiltration is known as pagetoid spread. Pagetoid spread occurs in other conditions that must be included in the differential diagnosis, namely, superficial spreading melanoma, BD, MF, Langerhans cell histiocytosis, and Spitz nevus.44,47
RCM has also been used to study PD, probably because it has proven to be an excellent tool for identifying pagetoid spread in lentigo maligna melanoma and superficial spreading melanoma.48 In 2007, Longo et al.49 published a case of pigmented mammary PD with RCM findings suggestive of melanoma: pagetoid infiltration by large, round atypical cells with a bright cytoplasm and a dark nucleus together with numerous large cells with long dendritic branches in the stratum corneum and ill-defined papillary rings. More recently Guitera et al.50 published a series of 9 cases of extramammary PD and 1 case of mammary PD. All the lesions displayed large Paget cells distributed either singly or in small nests, which is a characteristic finding in PD. The Paget cells in the stratum granulosum were visualized as dark or hyporeflective structures. In 4 cases, the cells were seen as bright structures with a dark halo (targetoid lesion). This halo may correspond to the cleft-like separation of Paget cells from the epidermis that is seen on histology and is possibly due to mucin secreted by these cells or to intercellular fluid (Fig. 5A and B). While mucin appears hyporeflective in RCM images, Paget cells are frequently seen as hyper-reflective, probably due to the reflection of light from the secretory granules and to the prominent Golgi apparatus and numerous free ribosomes that these cells contain. An atypical or disarrayed honeycomb pattern indicating disruption of the epidermis is seen in many cases. The blood vessels are generally abundant, small, and vertically oriented, a typical finding for skin in the anogenital region. Considering the nonspecificity of the clinical manifestations of PD and the fact that this disease can mimic a range of inflammatory, allergic, and infectious conditions, RCM may facilitate earlier diagnosis and faster initiation of appropriate treatment.51 It can also be useful for selecting the most appropriate biopsy site and for mapping out margins prior to treatment52 (Table 8).
Clinical image of extramammary Paget disease. A, RCM image (0.5×0.5mm) (stratum corneum/stratum granulosum), showing infiltration by weakly refractive, dark Paget cells (arrows). B, RCM image (0.5×0.5mm) of the stratum spinosum, showing invasion by large Paget cells (arrows) that are twice or three times the size of the surrounding keratinocytes and disrupt the characteristic honeycomb pattern. C, RCM image (1×0.5mm) of the dermal-epidermal junction, showing blurring of rings (dashed circles) containing an inflammatory infiltrate. RCM indicates reflectance confocal microscopy.
Reflectance Confocal Microscopy Features of Paget Disease.
| Pagetoid invasion by large dark cells (twice as big as normal keratinocytes) arranged singly or in small clusters |
| Atypical honeycomb pattern, sometimes replaced by cell nests |
| On occasions targetoid cell structures or nests with a bright center and a dark halo |
RCM marks the beginning of a new era for dermatology, particularly in the field of skin cancer. This new technology offers substantial diagnostic advantages over routine histology, the gold standard for the diagnosis of skin disorders. Among its advantages are its noninvasive nature and the fact that it produces in vivo, in situ images. In other words, it offers immediate results and eliminates the need for prior interventions. Once pathologists or dermatologists become accustomed to viewing horizontal, gray-scale images of the skin and are adequately trained in recognizing characteristic features and patterns, they will be able to identify certain equivocal lesions with high rates of sensitivity and specificity. The current applications of RCM in clinical practice include mapping of presurgical margins in lesions with unclear clinical borders that are candidates for surgical excision with histologically clear margins. Examples are certain subtypes of BCC of the face and certain melanomas with poorly defined margins on the face and scalp, such as lentigo maligna/lentigo maligna melanoma, and melanocytic melanoma.53,54 RCM could also be used for the early detection of residual tumor55 or for distinguishing residual tumor from scarring.
Furthermore, the increasing use of noninvasive treatments for nonmelanoma skin cancer has attracted growing interest in RCM for the microscopic monitoring of response to these treatments, which include topical imiquimod, photodynamic therapy, and cryotherapy.56–58 RCM has already been incorporated into the clinical practice and its growing applications have led to the development of new, improved versions that offer faster image acquisition and have a smaller, more manageable handpiece for examining complicated locations such as the nose and ears (Vivascope 3000, Lucid, Inc.). RCM also has an indisputable role in the field of skin cancer surgery thanks to its in vivo imaging feature that can be used to map out appropriate presurgical margins and identify appropriate biopsy sites. The possibility of using RCM ex vivo during Mohs micrographic surgery has also opened up new, promising avenues of research. The value of RCM in inflammatory skin diseases is not limited to diagnosis, as the technique could also have an important role in predicting onset of disease or response to different treatments.59 The limitations of RCM are mainly related to the optical properties of the skin. Light is attenuated in the case of hyperkeratotic lesions and the deep dermis remains out of reach. Indeed it is sometimes difficult to differentiate between SCC in situ and invasive SCC by RCM due to inadequate visualization of the dermal-epidermal junction. Another considerable limitation is the fact that it is impossible to accurately distinguish between cells with a dendritic morphology, such as melanocytes and Langerhans cells. In conclusion, RCM, like dermoscopy, is a valuable tool for improving the diagnosis of skin conditions in the dermatology office.
In 2008, the International Confocal Working Group, formed by scientists, clinicians, and telemedicine experts was created to further the investigation and use of RCM in dermatology (www.skinconfocalmicroscopy.org). There is also an ongoing multicenter clinical trial of RCM in telemedicine via a platform known as Vivanet. It involves centers in different cities in Spain, France, and Italy, and its main aim is to develop a telediagnosis procedure based on RCM and dermoscopy by comparing the results of RCM vs dermoscopy, RCM vs dermoscopy plus RCM, and dermoscopy vs dermoscopy plus RCM.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: González S, Sánchez V, González-Rodríguez A, Parrado C, Ullrich M. Patrones de microscopia confocal para el cáncer cutáneo no melanoma y aplicaciones clínicas. Actas Dermosifiliogr. 2014;105:446–458.