Biologic drugs have provided excellent results in the treatment of moderate to severe psoriasis. Nevertheless, in routine clinical practice, combinations of biologic drugs with phototherapy or systemic drugs can increase efficacy, diminish toxicity, and reduce the cost of treatment. Published experience with these combinations is scarce, although the results are often satisfactory. This review examines the most relevant published experience in the combination of the most studied drug in this field—etanercept—with methotrexate, acitretin, ciclosporin, and narrowband UV-B phototherapy. Findings reported in the literature can help when taking major decisions on the management of biologic and systemic drugs in moderate to severe psoriasis.
Los fármacos biológicos han mostrado un excelente perfil en el tratamiento de la psoriasis moderada-grave. Sin embargo, existen situaciones de la práctica clínica habitual que se pueden beneficiar de combinaciones con los diferentes fármacos sistémicos, asi como de la fototerapia, permitiendo mejorar la eficacia, disminuir toxicidades o reducir los costes de tratar la enfermedad psoriásica. Hasta ahora la experiencia publicada de estas combinaciones es escasa pero los resultados son en muchos casos satisfactorios. En esta revisión se repasan las experiencias publicadas más relevantes con respecto a la combinación del fármaco biológico con más experiencia en este campo, etanercept, con metotrexato, acitretina, ciclosporina y fototerapia con ultravioleta B de banda estrecha. La experiencia procedente de la literatura puede ayudar a la hora de tomar decisiones importantes en el manejo de fármacos biológicos y sistémicos en la psoriasis moderada-grave.
When biologic agents began to be used in the management of psoriatic disease, it appeared that the traditional systemic drugs approved for the control of moderate to severe psoriasis would become a second line option and a mere prelude to biologic therapy. However, with the passage of time, and notwithstanding the excellent results achieved with biologic therapy in psoriatic disease, clinical experience has shown that combining a biologic agent with a traditional systemic drug or phototherapy can provide solutions to situations that cannot be managed with a biologic drug alone. Although the potential role of such combinations has already been considered in the context of other specialties in which the use of biologic therapy was introduced earlier, such as rheumatology, there are particular situations pertinent to psoriasis that need to be specifically evaluated. The success of combination therapy is based on the additive or synergistic effect achieved by combining 2 drugs with different mechanisms of action. This additive effect makes it possible to either achieve better efficacy than either one of the 2 drugs would produce in monotherapy or to achieve similar efficacy but with lower doses of both drugs. In moderate to severe psoriasis, regimens combining biologic and systemic drugs have the potential to increase effectiveness while decreasing toxicity and cost of treatment.1–3
This review addresses the combination of etanercept (ETN) with phototherapy and with various traditional systemic drugs used in the management of moderate to severe psoriasis.4,5
Etanercept and MethotrexateMethotrexate (MTX) has demonstrated great effectiveness in the management of psoriasis. It is one of the drugs most frequently used in the treatment of patients with moderate to severe plaque psoriasis in Europe.6 Nevertheless, some 50% of patients do not respond to MTX in monotherapy, and the drug's cumulative toxicity, which increases the risk of hepatotoxicity and hematological toxicity, limits its long-term use.7
The combination of ETN and MTX is supported primarily by studies in patients with rheumatoid arthritis, which demonstrate the superiority of the combined treatment over monotherapy with either ETN or MTX.8–10 Experience with this combination in psoriasis is more limited. In the EDUCATE study, one of the first clinical trials to assess the use of ETN in psoriatic arthritis, 77 patients were treated with MTX at baseline. After the introduction of ETN, 29% were able to discontinue MTX, and 7% were able to reduce the dose.11 In a series of 6 patients with psoriasis, Yamauchi et al.12 reported that ETN made possible gradual withdrawal of MTX while achieving a sustained response and without clinical worsening or rebound of the psoriasis.
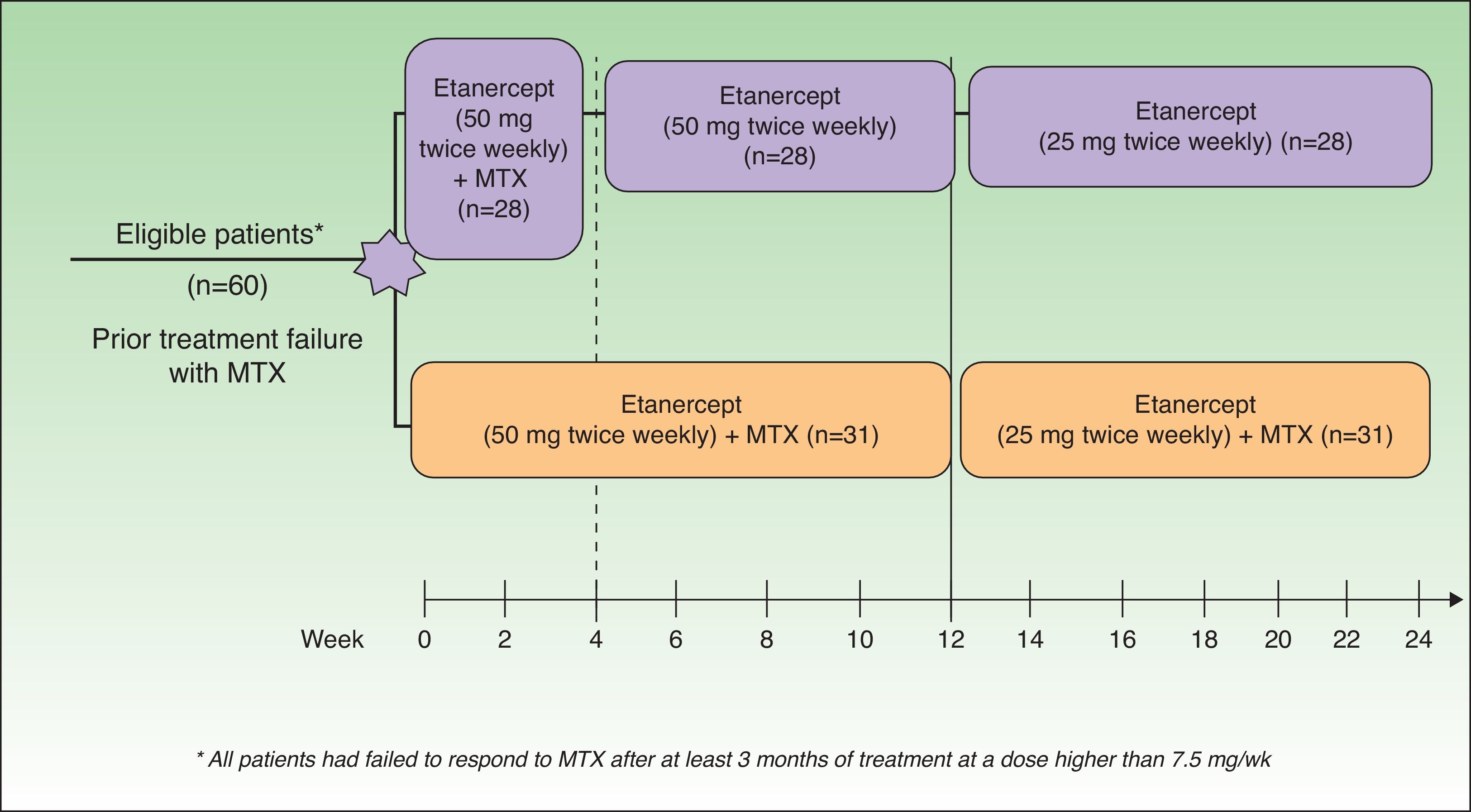
In 2008, 2 important studies were published by Zachariae13 and Driessen.14 In a study of patients with an inadequate response to MTX, Zachariae et al.13 compared the effectiveness of ETN plus continuous MTX with that of monotherapy with ETN after the withdrawal of MTX at 4 weeks. (Figure 1). After 24 weeks of treatment, 66.7% of the group receiving ETN plus MTX achieved a PGA 0-1 response as compared to 37% of the patients on ETN monotherapy. Moreover, 70% of the combination group achieved a 75% reduction in baseline Psoriasis Area Severity Index (PASI 75) after 24 weeks. The results of that study suggest that the combined use of ETN and MTX in patients with a poor response to MTX in monotherapy might be a more effective option than a switch to single-drug therapy with ETN, and that it could offer the benefit of a synergistic effect without any increase in adverse events. Driessen et al.14 analyzed data from a small series of 14 patients treated with simultaneous ETN and MTX in clinical practice. The combination treatment was administered either because of an insufficient response to monotherapy with ETN or MTX or to prevent rebound of psoriasis during a switch from MTX to ETN. The satisfactory response observed in some of these patients whose response to monotherapy with either MTX or ETN had been insufficient may once again indicate a synergistic effect attributable to the different mechanisms of action of the 2 drugs. The authors observed no changes in the safety profile except elevated transaminase levels in 5 patients, which in no case required withdrawal of treatment. They concluded that the combination of ETN and MTX is a reasonable therapeutic option when the efficacy of ETN in monotherapy is unacceptable or when a rapid worsening of psoriasis is anticipated if MTX is discontinued during the transition to another therapy.
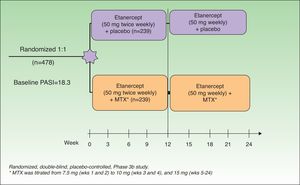
Study design. MTX indicates methotrexate.
Source: Zachariae et al.13
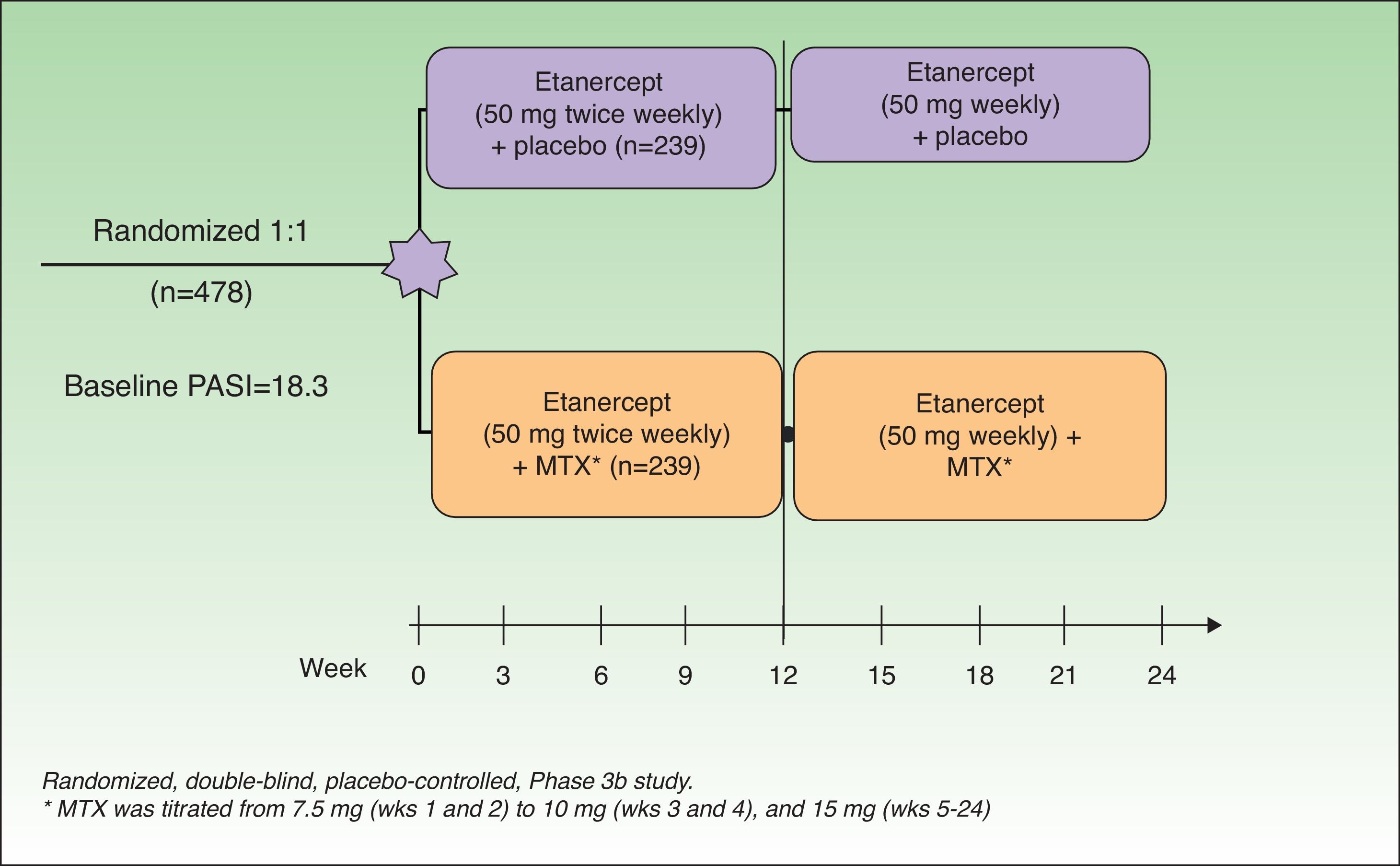
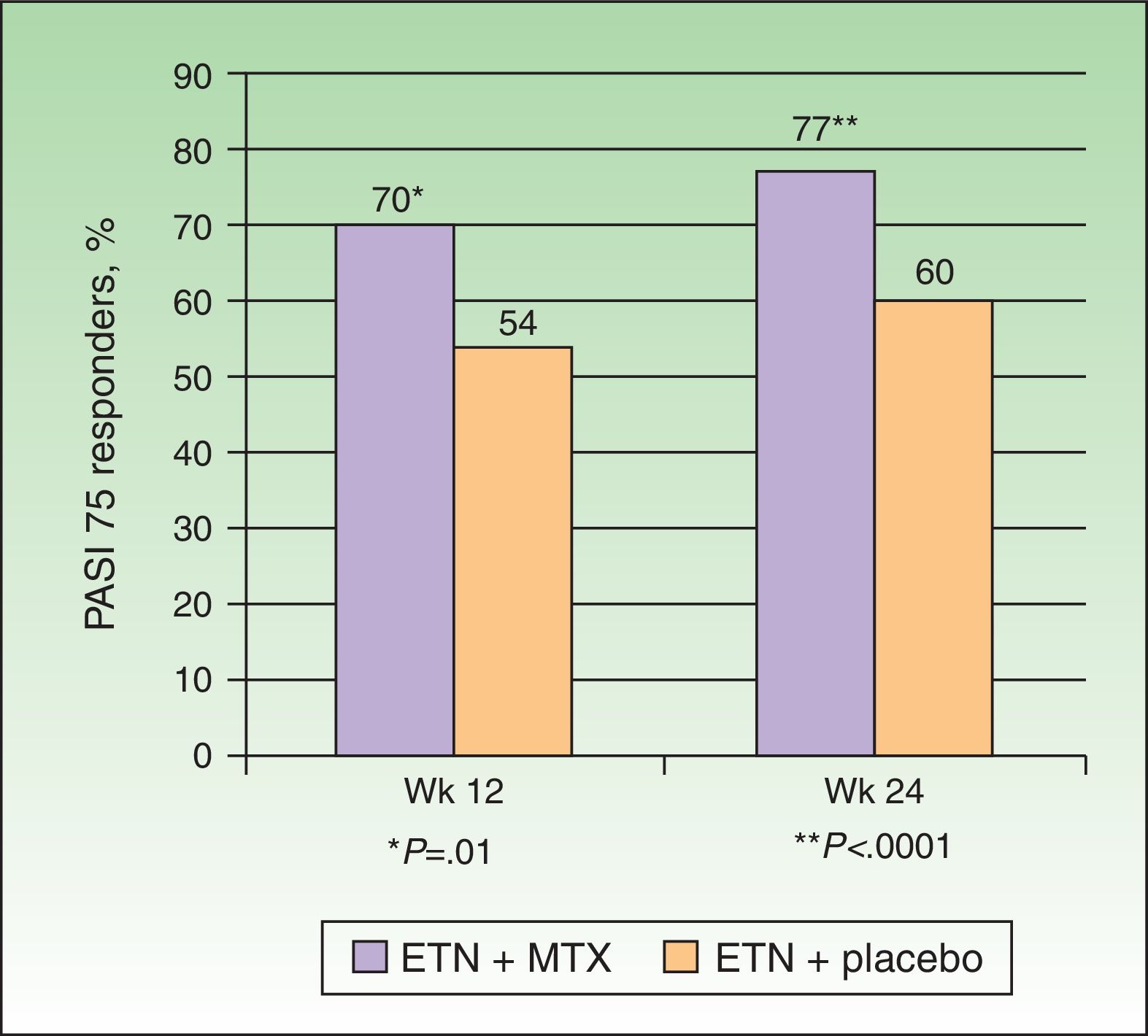
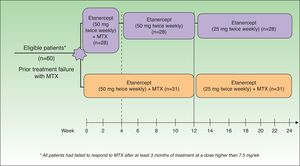
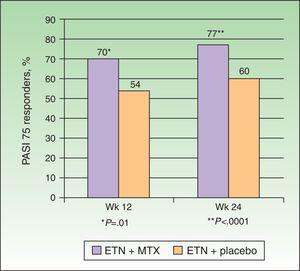
In 2012, in a randomized, double-blind, placebo-controlled study, Gottlieb et al.15 evaluated the safety and efficacy of a regimen combining ETN and MTX compared to that of ETN monotherapy in patients with no prior history of failure to respond to monotherapy with either drug. The patients in the monotherapy group (n = 239) received ETN 50 mg plus placebo twice weekly for 12 weeks followed by ETN 50 mg once a week and placebo for a further 12 weeks. The patients in the combination therapy group (n = 239) received the same dose of ETN but placebo was substituted by MTX titrated from 7.5 mg (weeks 1 and 2) to 10 mg (weeks 3 and 4) and up to a maximum of 15 mg or the maximum dose tolerated (weeks 5 to 24) (Figure 2). At week 24, the PASI 75 response rate was significantly higher in the combination group (77.3%) than in the monotherapy group (60.3%). This difference in favor of combination therapy was already observed in the PASI 75 response at week 12 (70.2% vs 54.3%) (Figure 3) and in the PASI 90 response at weeks 12 (34% vs 23.1%) and 24 (53.8% vs 34.2%). In terms of safety, while a higher proportion of patients in the combination therapy group experienced more adverse events than those in the monotherapy group (74.9% vs 59.8%), most of these events were mild or moderate. Elevations in transaminase levels occurred more frequently in the combination therapy group (7 patients) than in the monotherapy group (4 patients), and the number of patients who withdrew because of increased transaminase levels was also higher in the combined (4) than in the monotherapy (2) group. However, these elevations were not considered severe in any of the patients, and it was impossible to determine whether they were related solely to MTX or also due to the additive effect of ETN. In both groups there were very few withdrawals due to adverse effects and none of these was due to serious adverse effects or severe infections. No opportunistic infections or deaths occurred during the study.
Study design. MTX indicates methotrexate.
Source: Gottlieb et al.15
Percentage of PASI 75 responders at weeks 12 and 24 receiving etanercept (ETN)+methotrexate (MTX) or ETN+placebo.
Source: Gottlieb et al.15
In conclusion, on the basis of the outcomes measured in that study (PASI 50, 75 and 90 responses at weeks 12 and 24), the combination of ETN and MTX was more effective than ETN in monotherapy and the safety profile of the combination was acceptable. At week 24, 77.3% of the patients receiving combination therapy had achieved a PASI 75 response. Moreover, the addition of MTX during the 12-week ETN induction phase increased the PASI 75 response rate to 70%.
Several authors have reported that the addition of MTX to ETN therapy has been associated with increased drug survival in patients being treated for psoriatic arthritis. In a study of patients with psoriatic arthritis included in a Swedish registry, Kristensen et al.16 observed that treatment with MTX and a tumor necrosis factor (TNF) inhibitor was associated with better overall drug survival of the TNF blocker. They attributed this effect to the lower rate of dropouts due to adverse events in the group of patients receiving the combined regimen. However, the authors of a study based on data from a Danish database of patients with psoriasis treated with a biologic agent (DERMBIO) did not find better overall drug survival among patients on a combination regimen with MTX; however, they did observe better drug survival in a subgroup of patients who, after failure to respond to anti-TNF therapy, received MTX with the following biologic agent.17
In the studies cited above, the combination of ETN and MTX was found to be safe and well tolerated in patients with psoriasis. These results support the evidence from studies in rheumatoid arthritis, which involved large numbers of patients and follow-up periods of up to 10 years.8–10 The authors of the CORRONA study, who assessed the adverse effects of the combination of MTX and anti-TNF agents in patients with rheumatoid arthritis, found no increased risk of infections in patients on a combination regimen compared to those receiving monotherapy.18
Etanercept and AcitretinAcitretin (ACI) is considered by some authors to be the ideal drug for use in combination with biologic agents because it is not an immunosuppressant and may therefore produce a synergistic effect with the biologics without increasing the risk of toxicity.1,4 Moreover, the fact that ACI has preventive effects against skin cancer may be an additional benefit in patients with moderate to severe psoriasis in whom ETN, like other systemic drugs used to treat psoriasis, may increase the risk of squamous cell carcinoma.19–21
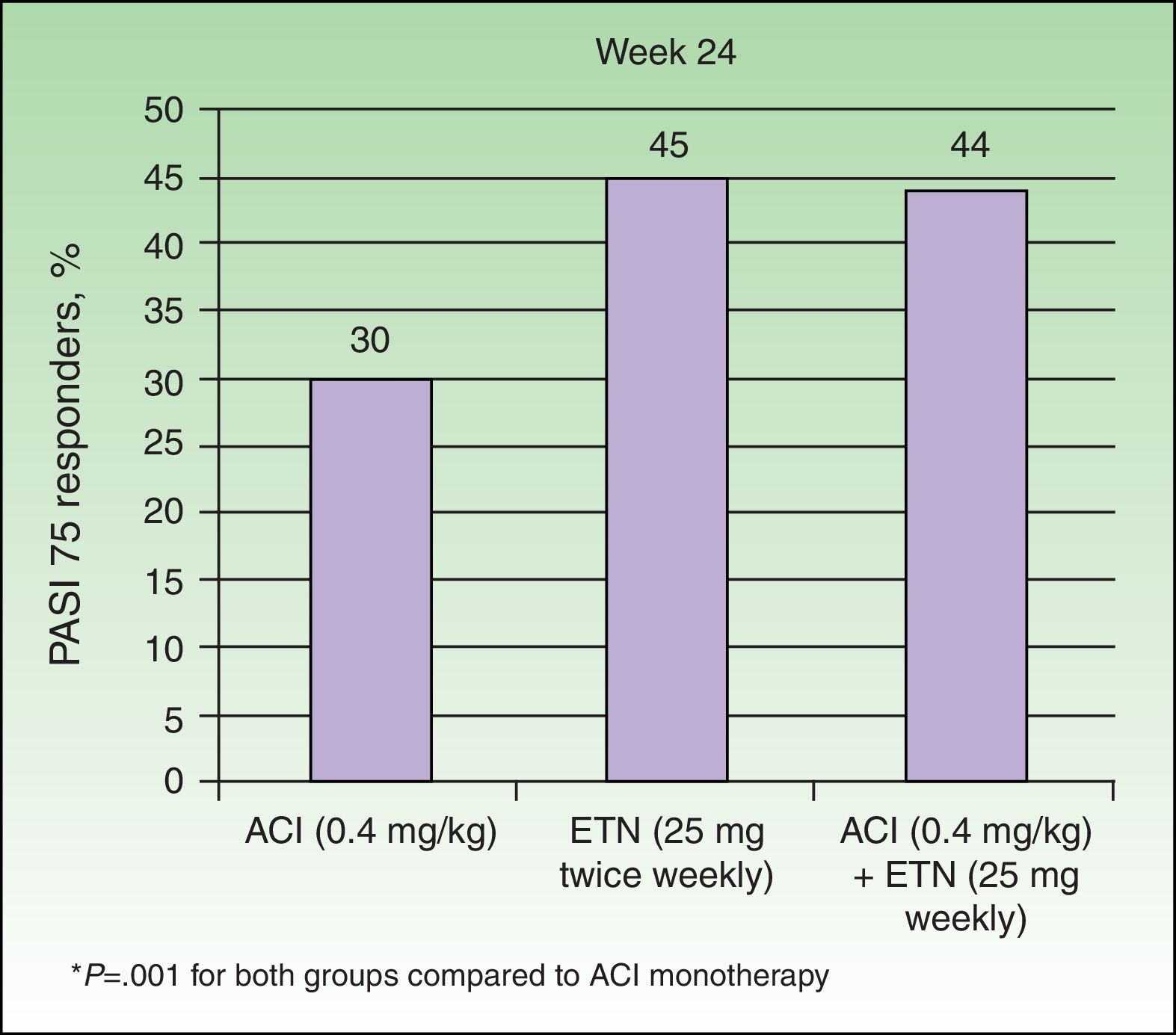
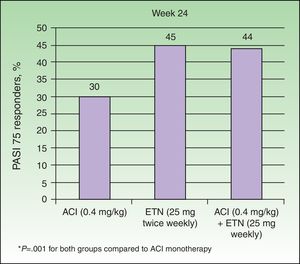
In a study of 60 patients with moderate to severe psoriasis, Gisondi et al.22 found that the combination of low doses of ETN (for example, 25mg once weekly) with oral ACI (0.4 mg/kg/d) achieved results similar to those obtained with ETN 25 mg twice weekly.22 At week 24, the proportion of patients who achieved a PASI 75 response in the groups treated with ETN monotherapy and ETN plus ACI were similar (45% and 44%, respectively), while the efficacy of treatment with ACI alone was significantly lower (30%) (Figure 4). The safety profile was similar in all 3 groups. These results support the hypothesis that the combination could reduce the cost of treatment since a regimen of ETN 25mg weekly could achieve a response rate similar to that normally obtained with the standard dose of 50 mg weekly. Another question that is posed is whether the addition of ACI to ETN therapy at the standard dose would enhance the effectiveness of the ETN therapy, improving either the initial response or the response of nonresponders.4,22 In terms of safety, the combination therapy was not associated with a higher rate of adverse events such as alterations in serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol, or triglycerides.
Percentage of PASI 75 responders at week 24 by group. ACI indicates acitretin; ETN, etanercept.
Source: Gisondi et al.22
In a retrospective review of 15 patients treated with ACI (10-50 mg/d) in combination with a biologic drug (4 were receiving ETN), Smith et al.23 concluded that the combination regimen was a valid therapeutic option for patients with psoriasis refractory to other treatments. Isolated cases have been reported of treatment with this combination, including one relating to a patient with palmoplantar pustulosis with arthro-osteitis treated with ETN and ACI.24
Ciclosporin and EtanerceptCiclosporin (CsA) is a drug used widely in patients with moderate to severe psoriasis because of its immunosuppressive effect on T cells. It is one of the therapies that offers the fastest and most effective action against psoriasis. However, the use of CsA is limited by its renal toxicity, carcinogenic potential and association with hypertension.1,25
In a review of possible combination regimens with ETN, Segaert4 concluded that—from the standpoint of safety—the combination of ETN with CsA is theoretically less recommended than other possible combinations because of its immunosuppressive effect, which may increase the risk of severe infection and malignancy. In a study of 11 patients with psoriatic arthritis in whom treatment with ETN had achieved good control of joint disease but unsatisfactory control of cutaneous symptoms, the safety and efficacy of adding ciclosporin to the regimen was studied.26 Of the 11 patients who were given ciclosporin (3 mg/kg/d) in addition to ETN, 9 achieved a PASI 75 response at week 24. Lee et al.27 administered a regimen combining a low dose of ciclosporin (200 mg/d) with ETN (50 mg weekly) to 7 patients with a history of poor response to systemic drugs in whom prior treatment had achieved only partial or poor control of psoriasis. After the disease was controlled in an initial phase, the dose of both ciclosporin and ETN were reduced during the maintenance phase (to 100 mg/d in the case of ciclosporin and 25 mg every other week for ETN). A PASI 90 response was achieved by 85% of the patients after a mean of 6.85 weeks. The mean follow-up of patients on maintenance therapy was 56.5 weeks. The mean reduction in PASI was 94.9% at the end of the initial therapy period and 93.2% following maintenance therapy at the decreased dose. Mean PASI was 0.94 at the end of the initial period and 1.25 during maintenance therapy. The combination treatment was well tolerated: no severe adverse events or clinically significant changes in laboratory values were reported, and the patients’ immune status was unchanged after 1 year of treatment. The authors concluded that, owing to the synergistic effects of using 2 drugs with different mechanisms of action, therapy with ciclosporin and ETN achieved a faster response and was more effective than ETN alone. Moreover, the combination would allow a dose reduction in both drugs, thereby reducing the toxicity of ciclosporin and the cost of ETN. Although there are no reports in the literature of severe infections or cases of malignancy, the immunosuppressive potential of this combination is cause for concern with respect to hematological malignancies and non-melanoma skin cancer. Consequently, long-term treatment with this regimen is not recommended, but it could be useful as a short-term solution in the management of refractory psoriasis, as shown by this study.27
In a group of 41 patients with moderate to severe psoriatic arthritis, Atzeni et al.28 found no differences between those treated with ETN (50 mg/wk) plus ciclosporin (3mg/kg/d) and those who received ETN (50 mg/wk)+MTX (7.5-15 mg/wk), although the combination of ETN and ciclosporin was more effective in reducing skin involvement.
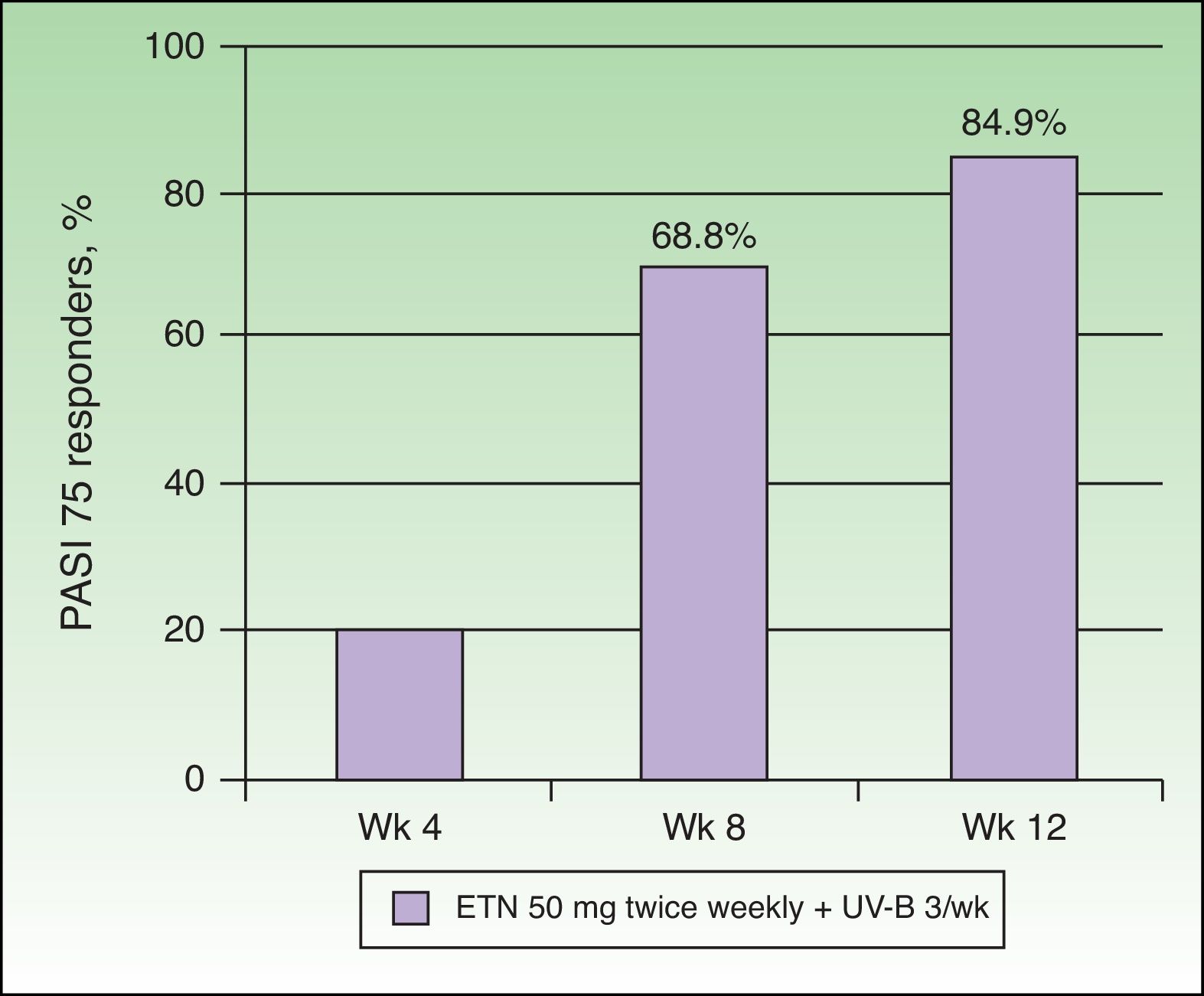
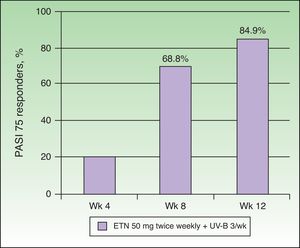
Etanercept and Narrowband UV-B PhototherapySimultaneous treatment with ETN and narrowband UV-B phototherapy has been assessed in 7 studies (Table 1). In an open-label, multicenter prospective study with a single treatment group (n = 86), Kircik et al.29 assessed the safety and efficacy of simultaneous treatment with ETN (50 mg twice weekly) and narrowband UV-B phototherapy (3 sessions per week) in patients with moderate to severe plaque psoriasis for 12 weeks (the UNITE study). At the end of week 12, 84.9% of the patients had achieved a PASI 75, 58.1% a PASI 90, and 26% a PASI 100 response. The mean time to PASI 75 was 57 days. It is interesting to note that 68.8% of the patients had already achieved a PASI 75 by week 8 (Figure 5). The authors attributed the high rate of efficacy achieved after 12 weeks (PASI 75 in 84.9% of patients) to the synergistic effect of the combination. Wolf et al.30 assessed the effect of adding narrowband UV-B therapy to the biologic regimen of 5 patients who had not achieved a PASI 75 response after 6 weeks of treatment with ETN (50 mg twice weekly) and in whom prior monotherapy with phototherapy had not been effective. For a further 6 weeks the selected patients were treated with narrowband UV-B phototherapy on a randomly selected body half 3 times a week and the patients continued to receive ETN at the same dose as before. After 6 weeks of the combined regimen, the mean PASI was 1.6 on the irradiated body halves versus 4.7 on the non-irradiated halves, a statistically significant reduction in PASI on the body half treated with narrowband UV-B phototherapy. The same authors recently published an update on the patients they have treated with narrowband UV-B phototherapy in combination with various biologic agents, including those treated with ETN.31 In those 5 patients on ETN, the reduction in PASI was 85% in the irradiated area compared to 55.2% in the non-irradiated area at week 6, and 90.7% in the area treated with UV-B compared to 81% in the area not treated at week 12. Thus, narrowband UV-B phototherapy increased the response to treatment with ETN in patients with moderate to severe psoriasis, especially in those who initially had a slow or inadequate response to treatment. However, the authors of that study emphasized the need for controlled clinical trials involving larger numbers of patients. They also suggested that it would be of great interest to determine whether the addition of phototherapy could significantly reduce the frequency of recurrence of the disease after withdrawal of the biologic therapy.
Published Studies on the Combination of Etanercept with Narrowband UV-B Phototherapy.
| Author | Year of Publication | Study Design | No. of Patients | Treatment Regimen |
|---|---|---|---|---|
| Kircik et al.29 | 2008 | Prospective, multicenter, single-arm, open-label study | 86 | Etanercept 50mg twice weekly+narrowband UV-B phototherapy 3 times/wk |
| Wolf et al.30,31 | 2009 | Prospective study, randomized comparison of both treatments in the same patient | 5 | Etanercept 50mg twice weekly±narrowband UV-B 3 times/wk for 6 weeks |
| Simone et al.32 | 2011 | Prospective, single-arm, open-label study | 33 | Etanercept 50mg/wk for 12 wks+narrowband UV-B 3 times/wk for 8 wks |
| Gambichler et al.33 | 2011 | Prospective, randomized, controlled, single-blind comparison of both treatments in the same patient | 14 | Etanercept 25mg twice weekly±narrowband UV-B 3 times/wk for 6 wks |
| Lynde et al.34 | 2011 | Prospective, multicenter, single-blind, open label study | 75 | Etanercept 50mg weekly±narrowband UV-B phototherapy 3 times/wk |
| Park et al.35 | 2012 | Prospective, randomized study in 2 phases (obese patients) | 30 | Etanercept 50 mg/wk±narrowband UV-B phototherapy 3 times/wk |
| Calzavara-Pinton et al.40 | 2013 | Prospective study | 20* | Etanercept 50mg twice weekly±narrowband UV-B phototherapy 3 times/wk |
Percentage of patients who achieved PASI 75 at 4, 8, and 12 weeks. ETN indicates etanercept.
Source: Kircik et al.29
De Simone et al.32 assessed the efficacy of combined treatment with ETN (50 mg once weekly) and narrowband UV-B phototherapy (3 sessions per wk) in a series of 33 patients. Patients received ETN for 12 weeks, while the phototherapy was only administered during the first 8 weeks. At weeks 4, 8 and 12, a PASI 75 response was achieved in 24.2%, 66.7% and 81.8% of patients, respectively. An interesting result reported in this study was that treatment with the usual maintenance dose of ETN (50 mg weekly) combined with narrowband UV-B phototherapy achieved better results than those reported in other studies for monotherapy with ETN 50 mg twice weekly or narrowband UV-B phototherapy without ETN. This treatment regimen may cost less than standard biologic therapy. In a prospective study by Gambichler et al.,33 14 patients were treated for 6 weeks with ETN (25 mg twice weekly) and narrowband UV-B phototherapy (3 sessions per wk with 311 nm) on selected lesions. In each patient, 2 psoriasis plaques—1 on the left and 1 on the right side of the trunk—were selected as marker lesions. One of these 2 lesions was then randomly assigned to be treated with ETN monotherapy and was occluded with an opaque patch during phototherapy sessions. The other marker lesion received combination therapy. The modified PASI was determined and biopsy samples for histological examination were obtained from both marker lesions in all patients. After 6 weeks of therapy, the mean (SD) reduction in modified PASI for the lesions treated with the combination of ETN plus narrowband UV-B was 64.7% (27.8%), a significantly greater reduction than that observed in the lesions treated with ETN monotherapy, which was 53.7% (36.9%). Furthermore, in the samples obtained following 6 weeks of treatment, both the histology score (calculated on the basis of the presence of hypogranulosis, parakeratosis, psoriasiform hyperplasia, and inflammatory infiltrate) and epidermal immunoreactivity for CD1a, CD4 and CD8 were significantly higher in the lesions treated with ETN monotherapy than in those treated with ETN plus narrowband UV-B.
Although a significant difference between the 2 treatment groups was observed in the clinical response, the findings relating to immunohistochemical markers of differentiation (involucrin) and proliferation (Ki67) were similar in the 2 groups, indicating that after 6 weeks of treatment the 2 regimens had not had a significantly different effect on premature keratinization or epidermal proliferation, 2 histopathological findings typical of psoriasis. The authors attributed the lower levels of CD1a, CD4 and CD8 found in the lesions treated with narrowband UV-B phototherapy compared to the non-irradiated lesions to the known effect of UV-B radiation on the apoptosis of Langerhans and T cells.
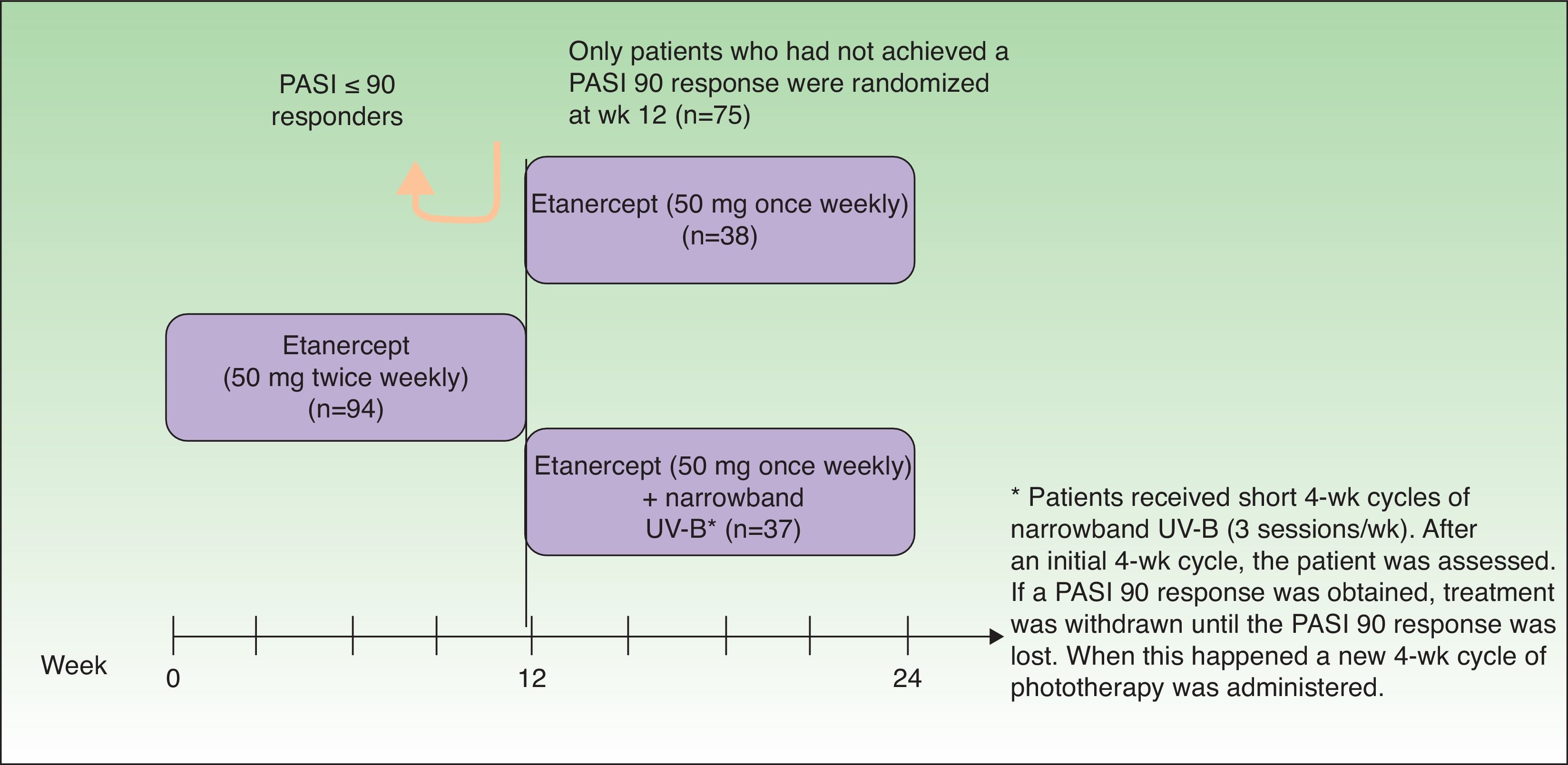
Lynde et al.34 studied 75 patients with moderate to severe psoriasis who had not reached PASI 90 after 12 weeks of ETN therapy at the normal induction dose (50 mg twice weekly) to ascertain whether the addition of short courses of narrowband UV-B could help these patients to achieve an optimal response (PASI 90) (Figure 6). Analysis of the results after 24 weeks of treatment revealed no significant differences in the proportion of patients who achieved PASI 90 between the ETN group (15.8%) and the group treated with ETN plus narrowband UV-B (16.2%), a finding that might appear to indicate that the combination did not improve the response. However, in the subset of patients who had received at least 90% of the ETN injections and at least 80% of the phototherapy sessions provided for in the study protocol, that is, the patients with high adherence to the treatment regimen (29 of those receiving ETN monotherapy and 7 patients in the combined treatment group), the difference between the 2 treatment arms in PASI 90 response rate reached statistical significance at week 24 (42.9% of patients on the combined treatment versus 20.7% of those receiving ETN monotherapy). At week 24, the PASI 75 response rate was also higher for the combination group compared to the monotherapy group (100% versus 55.2%). The authors of that study suggested that their results could have been skewed by the small number of patients with high adherence to phototherapy (n = 7). In short, the addition of short courses of narrowband UV-B phototherapy improved clinical response in the group of patients with high adherence to narrowband UV-B phototherapy, achieving a PASI 90 response at weeks 16 and 24.
Study design.
Source: Lynde et al.34
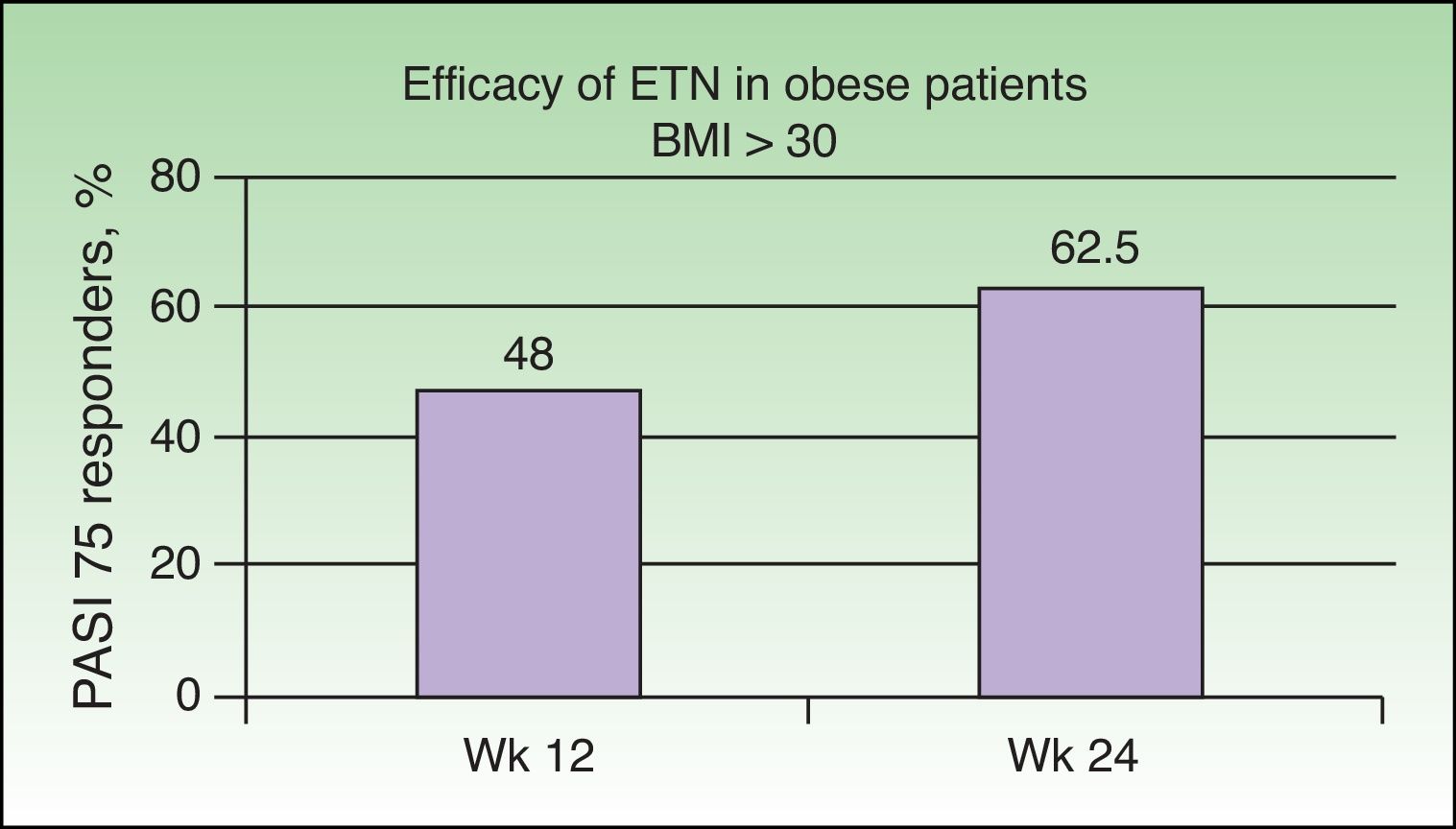
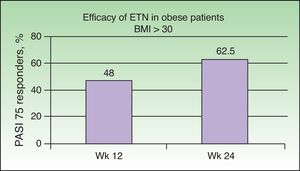
Park al.35 studied the efficacy of a regimen combining ETN and narrowband UV-B phototherapy in obese patients (body mass index [BMI] ≥ 30) with moderate to severe psoriasis, a group in which a less favorable response to ETN monotherapy has been reported than for patients with a normal BMI36,37 and in which the synergy would be particularly suitable. The aim of that study was to assess the results obtained with a combination of narrowband UV-B plus ETN (50 mg once a week) in obese patients with moderate to severe psoriasis. The proportion of patients who achieved a PASI 75 response was 48% at week 12 and 62.5% at week 24, rates similar to those achieved in pivotal studies (Figure 7).38,39 The percentage of patients achieving PASI 75 at week 24 was similar in the 2 groups (46.7% in the monotherapy group versus 53.3% in the combination group) and no significant differences were found associated with BMI. In view of these results, the authors concluded that treatment with ETN monotherapy in obese patients is effective and that this efficacy does not depend on weight when the target is a PASI 75 response, but that differences are observed when the target response is a PASI 90 or PASI 100. The addition of narrowband UV-B does not appear to provide additional benefit in this group of patients in terms of a PASI 75 response, as the study did not demonstrate any synergistic effect of the kind reported in the other studies described above.29,32,34
Overall percentage of patients with a body mass index (BMI) of 30 or higher who achieved a PASI 75 response at 12 and 24 weeks of treatment with etanercept (ETN) with or without narrowband UV-B.
Source: Park et al.35
Finally, in a recently published study, Calzavara-Pinton et al.40 describe the response to a combined regimen of ETN and narrowband UV-B phototherapy in 20 patients with plaque psoriasis who had previously failed to achieve a PASI 75 response with narrowband UV-B alone. In that study, patients initially received monotherapy with ETN (50mg twice weekly). At week 12, narrowband UV-B therapy was added to the ETN regimen in the patients who had not achieved PASI 75 (n=8). The 8 patients who required combined treatment with ETN plus narrowband UV-B achieved PASI 75 and 3 of them had a complete remission, despite having previously failed to respond adequately to monotherapy with either narrowband UV-B or ETN. All the patients tolerated the treatment well and no serious acute side effects were observed.
ConclusionsAlthough no regimen involving the combination of a systemic and a biologic drug has been approved for the management of moderate to severe psoriasis, the results of several relevant studies the usefulness of what has been happening in routine clinical practice for several years.41 Nonetheless, published data are scarce and as yet no adequate monitoring guidelines have been established.
In light of the accumulated experience, it would appear that it is possible to achieve increased efficacy with a combined regimen compared to that obtained by either the systemic drug or the biologic agent in monotherapy. This effect is attributable to the synergistic effect derived from combining drugs with different mechanisms of action.1–5 These combination regimens could offer a potential benefit when monotherapy with either type of therapy has proved inadequate. It is also possible to decrease the toxicity associated with a drug in monotherapy through the dose reduction made possible by the synergistic effect of the combination. Furthermore, such dose reductions will improve the efficiency of biologic therapy in psoriasis. In other cases, the combination achieves a more rapid response to treatment than is obtained with either therapy alone. Other situations in which combination regimens could be recommended include the treatment of psoriasis refractory to monotherapy, during the transition period when switching therapies, and to prevent loss of efficacy due to anti-drug antibodies through the concomitant use of MTX. Finally, the addition of a systemic drug to the regimen is one of the options that can be considered in the case of secondary failure of a biologic, that is, loss of response to a drug that initially achieved good results but loses efficacy over time. A combination regimen offers a way to avoid having to increase the dose or decrease the frequency of administration of the biologic agent, both options that are always more expensive. In this way, combination therapy can help to prolong the efficacy and survival of biologic drugs. The role of regimens combining biologic and systemic agents is still evolving, but every day these combinations are playing a more central role in the management of moderate to severe psoriasis.
Conflicts of InterestMariano Ara has served as a consultant for and received speaking fees from AbbVie, MSD, Pfizer and Janssen.
The remaining authors declare no conflicts of interest.
This review was funded by Pfizer Spain. However, the company had no influence on the content, which was written freely by the authors.
Please cite this article as: Ara M, Gracia T, Pastushenko E. Tratamiento combinado con etanercept y fármacos sistémicos/fototerapia en psoriasis. Actas Dermosifiliogr. 2015;106:180–188.