Disease course in melanoma often cannot be accurately predicted by means of the prognostic factors usually considered in patients with melanoma; therefore, new factors are clearly needed. Increasingly robust scientific evidence shows that tumor lymph vessels play a key role in melanoma that metastasizes by lymphatic and hematogenous pathways. We review current knowledge and examine the implications of lymphangiogenesis in the diagnosis, treatment, and prognosis of patients with melanoma.
En pacientes con melanoma los factores pronósticos utilizados en muchas ocasiones no permiten una predicción precisa de la evolución de la enfermedad, lo que hace evidente la necesidad de búsqueda de nuevos factores pronósticos. Existe una evidencia científica cada vez más sólida de que los vasos linfáticos tumorales desempeñam un papel importante en la producción de metástasis linfáticas y también hematógenas en pacientes con melanoma. En este trabajo expondremos el estado actual del conocimiento y las implicaciones del proceso de linfangiogénesis en el diagnóstico, tratamiento y pronóstico de los pacientes con melanoma.
Melanoma accounts for less than 10% of all skin cancers, but it is responsible for more than 90% of skin-cancer–related deaths.1 The statistics from recent years are alarming, with a persistent increase in the incidence of melanoma in most countries, particularly in the younger population; such a situation obliges us to consider this tumor as a growing public health problem.2 The treatment of choice for melanoma is surgical excision, which achieves a high cure rate if performed early. However, once the tumor has spread beyond the possibility of locoregional surgical control, the prognosis worsens considerably. Fortunately, we now have drugs that have an impact on survival in patients with melanoma.3–6
The prognostic factors used in daily clinical practice in patients with melanoma are the Breslow thickness, the presence of ulceration, the mitotic index, and the state of the sentinel lymph node.7–12 However, evaluation of these parameters does not enable us to accurately predict the disease course in a substantial proportion of patients. More than 15% of patients with a Breslow thickness less than 1mm develop metastatic disease,13 while a large proportion of patients with tumors with a Breslow thickness over 4mm present a relatively long disease-free survival (58% more than 5 years).14
Angiogenesis is considered to be 1 of the key processes in the progression and spread of malignant tumors. Angiogenesis is the formation of new capillary blood vessels arising from preexisting vessels; this facilitates tumor spread and, thus, the formation of metastases. Parameters that reflect the degree of tumor angiogenesis have been investigated as prognostic factors in various types of tumors,15–18 including melanoma.19–22 However, the lack of homogeneous results from those studies means no overall conclusion can be drawn.23–27 For example, the results of the meta-analysis recently published by our group suggest that blood vessel density is very similar between patients who have developed metastases and those with no evidence of metastatic disease.28 In contrast, those 2 groups did appear to show a significant difference in the density and frequency of invasion of the lymph vessels.
It should be noted that, in contrast to angiogenesis, in-depth study of the process of lymphangiogenesis has only become possible relatively recently because of the lack of specific markers for the lymphatic endothelium. In order to clarify the role of lymph vessels in the progression and spread of melanoma, we believe it interesting to review the current state of knowledge, evaluating the possible implications of the process of lymphangiogenesis in the diagnosis and treatment of this type of tumor. We also consider it important to describe the more technical aspects, and we therefore provide details of the different methods used to quantify the process of lymphangiogenesis in solid tumors and outline the main characteristics of the markers of the lymphatic endothelium.
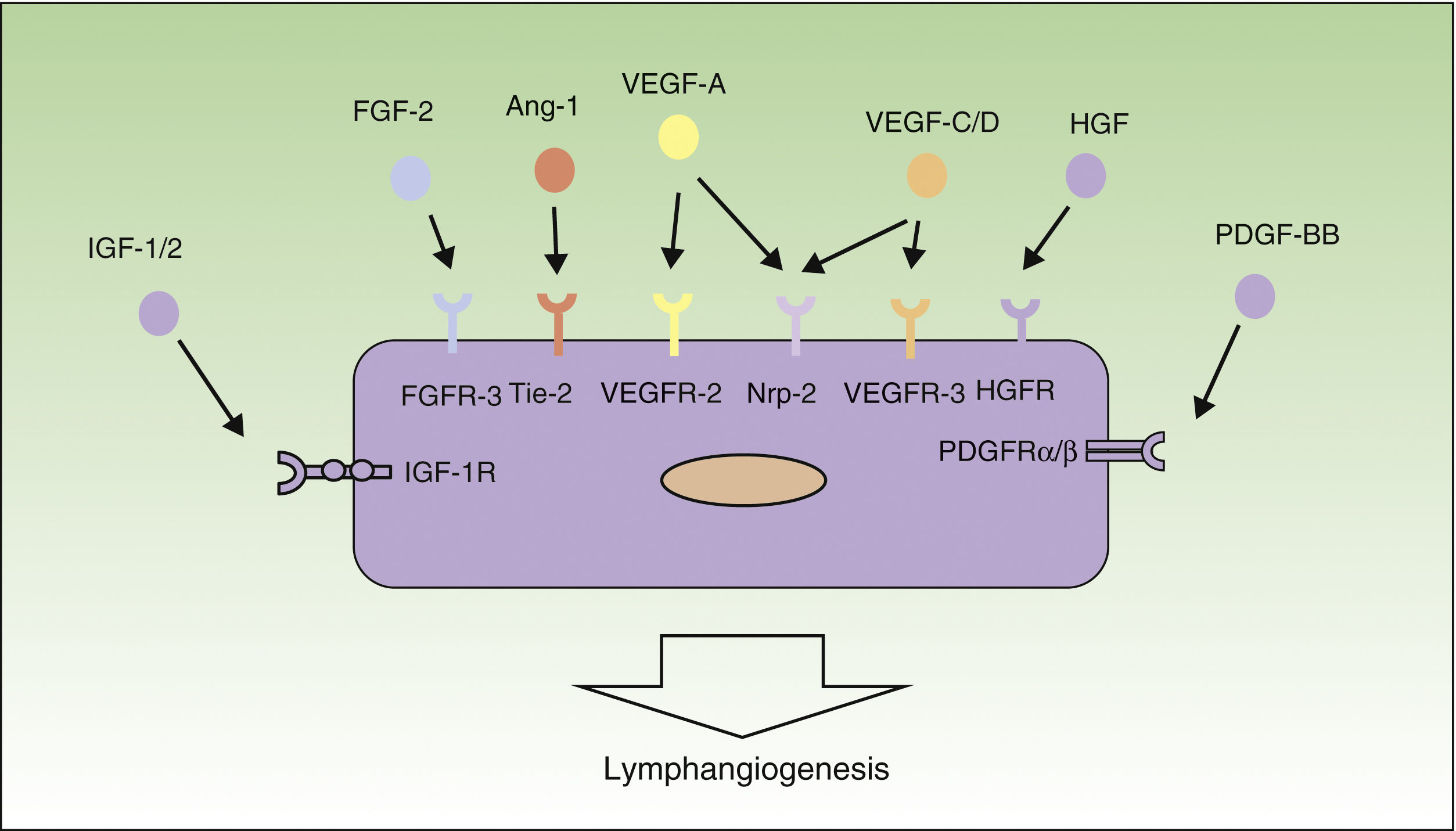
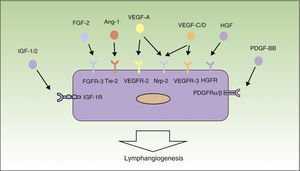
Mechanisms of LymphangiogenesisIn the last decade, research into the field of tumor lymphangiogenesis has identified many of the factors implicated in the growth, proliferation, migration, and survival of lymphatic endothelial cells. The first factors to be defined as responsible for lymphangiogenesis were vascular endothelial growth factor (VEGF)-C and -D, and their receptor, VEGF-receptor (VEGFR)-3.29–32 In addition, VEGF-A, considered to be 1 of the most important proangiogenic factors, has also been shown to be able to induce lymph vessel proliferation.33
VEGF-A, -C and -D act through specific receptors on the lymphatic endothelium (VEGFR). However, they are also able to bind to neuropilin (Nrp)-2, a semaphorin receptor whose expression was first detected in the nervous system, and subsequently in lymphatic endothelial cells. It is thought that Nrp-2 acts as a coreceptor for VEGFR-3.34 Further new inducers of the growth and proliferation of lymph vessels have recently been identified, including hepatocyte growth factor,35 fibroblast growth factor-2,36 platelet derived growth factor,37 insulin-like growth factor,38 and epidermal growth factor39 (Fig. 1). Both tumor cells and macrophages are able to secrete factors that induce lymphangiogenesis.40
Molecular control of the process of lymphangiogenesis. The diagram shows the principal growth factors involved in the process of lymphangiogenesis and their receptors on the lymphatic endothelium. Ang-1 indicates angiopoietin 1; FGF, fibroblast growth factor; FGFR, FGF receptor; HGF, hepatocyte growth factor; HGFR, HGF receptor; IGF, insulin-like growth factor; IGF-1R, IGF-1 receptor; NRP, neuropilin; PDGF, platelet derived growth factor; PDGFR, PDGF receptor; VEGF, vascular endothelial growth factor; and VEGFR, VEGF receptor.
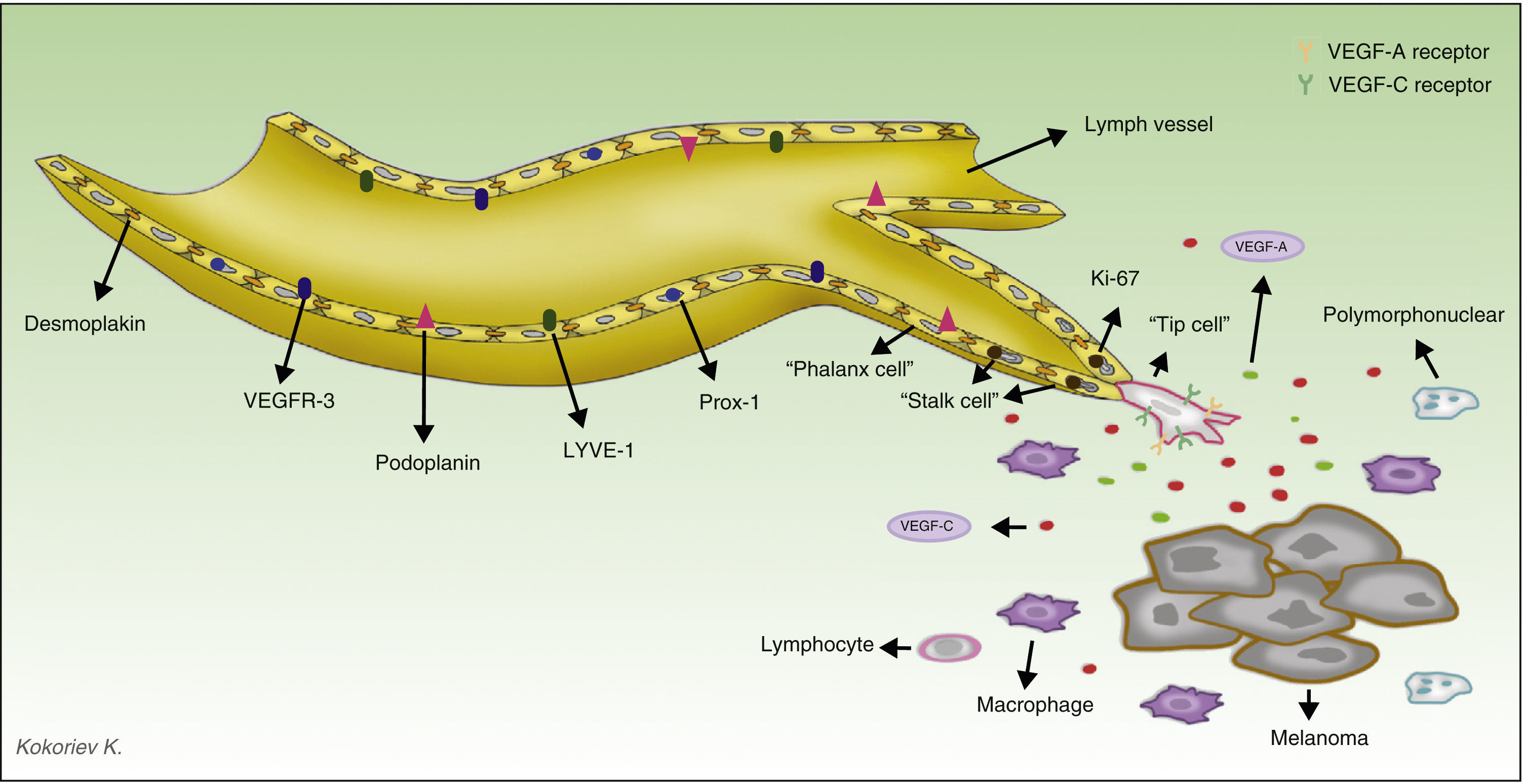
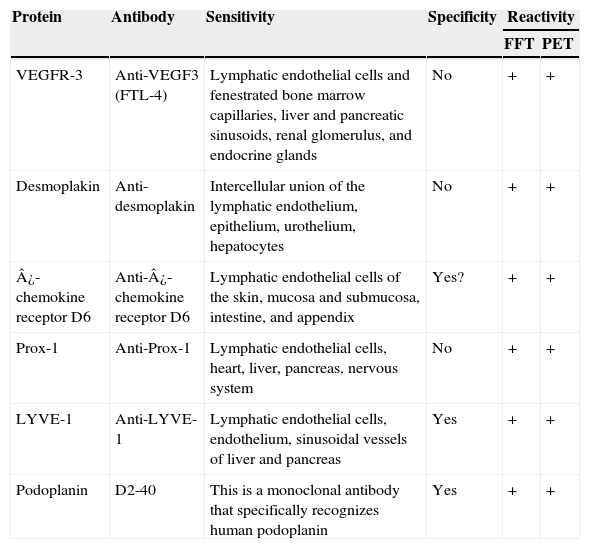
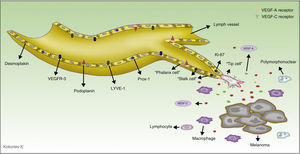
The discovery and application of specific markers now makes it possible to differentiate blood vessels from lymph vessels; this represents a very important step forward in research into the field of tumor lymphangiogenesis. The most widely used markers are lymph vessel endothelial receptor (LYVE)-1, a membrane protein of unknown function expressed on lymphatic endothelial cells and on activated macrophages,41 and D2-40, an antibody that recognizes a transmembrane glycoprotein of the lymphatic endothelium called podoplanin.42,43 The main characteristics of commercially available antibodies are summarized in Table 1, and the process of lymphangiogenesis and the sites of the markers on the lymphatic endothelial cells are shown diagrammatically in Figure 2.
Summary of the Main Characteristics of the Lymphatic Endothelium Markers.
| Protein | Antibody | Sensitivity | Specificity | Reactivity | |
|---|---|---|---|---|---|
| FFT | PET | ||||
| VEGFR-3 | Anti-VEGF3 (FTL-4) | Lymphatic endothelial cells and fenestrated bone marrow capillaries, liver and pancreatic sinusoids, renal glomerulus, and endocrine glands | No | + | + |
| Desmoplakin | Anti-desmoplakin | Intercellular union of the lymphatic endothelium, epithelium, urothelium, hepatocytes | No | + | + |
| ¿-chemokine receptor D6 | Anti-¿-chemokine receptor D6 | Lymphatic endothelial cells of the skin, mucosa and submucosa, intestine, and appendix | Yes? | + | + |
| Prox-1 | Anti-Prox-1 | Lymphatic endothelial cells, heart, liver, pancreas, nervous system | No | + | + |
| LYVE-1 | Anti-LYVE-1 | Lymphatic endothelial cells, endothelium, sinusoidal vessels of liver and pancreas | Yes | + | + |
| Podoplanin | D2-40 | This is a monoclonal antibody that specifically recognizes human podoplanin | Yes | + | + |
Abbreviations: FFT, fresh frozen tissue; LYVE, lymphatic vessel endothelial receptor; PET paraffin-embedded tissue; and VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Diagram of the process of tumor lymphangiogenesis and lymph vessel markers. The melanoma cells and tumor macrophages secrete various factors capable of activating the process of lymphangiogenesis. Three types of cell are involved in lymph vessels proliferation. The tip cells express numerous prolymphangiogenic factor receptors on their surface, including vascular endothelial growth factor (VEGF)-A and VEGF-C, and through their pseudopodia they scan the environment and guide the lymphatic sprout in the direction of the highest concentration of those factors. The stalk cells, located behind the tip cells, have no pseudopodia nor do they express high concentrations of prolymphangiogenic factor receptors on their surface. These cells proliferate at a high rate (observe in the diagram that these cells are positive for Ki-67, a proliferation marker); they initiate the process of formation of the vessel lumen and participate in the elaboration of the basement membrane. During the maturation process, the stalk cells transform into phalanx cells, so-named because they arrange themselves to form a single, ordered layer of lymphatic endothelial cells, similar to the organization of soldiers in ancient Greece (phalanx). The phalanx cells share morphological characteristics with dormant endothelial cells, but unlike endothelial cells they continue to participate in basement membrane formation. LYVE indicates lymphatic vessel endothelial receptor.
As with other types of cancer, the ability to provoke lymph node metastases forms part of the clinical characteristics and natural course of melanoma. In a study by Essner et al.,44 the prevalence of lymph node metastases in a cohort of 431 patients with melanoma was 21% at the time of diagnosis. The presence of tumor cells in the sentinel lymph node is one of the most important negative prognostic factors,45 but up to 22% of patients with a negative sentinel lymph node study develop disease recurrence with a 5-year mortality of 15%.46
In 1997, de Waal et al.47 developed a technique for the selective detection of lymph vessels in order to evaluate lymphangiogenesis in melanoma. Their method was based on a double stain of histologic samples using CD31, an antibody that reacts with all types of microvessels, and PAL-E, a specific marker of blood vessel endothelium. Those authors considered that vessels that stained positive for CD31 and negative for PAL-E were lymphatic.47 However, although they were able to demonstrate a marked difference in the density of blood vessels in melanomas in a horizontal growth phase versus those with vertical growth, they detected no change in the number of lymph vessels and concluded that melanoma cells were not able to induce lymphangiogenesis.
For 5 years after the publication of that first study on lymphangiogenesis in melanoma there were no further attempts to quantify lymph vessels in this type of tumor until the appearance of commercially available specific antibodies. Using those markers, the majority of authors were able to confirm the presence of intra- and peritumoral lymph vessels in melanoma preparations. However, there has been no consensus regarding interpretation of the results based on published data. For the majority of authors, the presence of metastases in patients with melanoma correlated significantly with a lower survival, but for some, this expected hypothesis was not satisfied, giving rise to a variety of interpretations, such as the possibility that lymph vessels at the periphery of the tumor and within its parenchyma were nonfunctional.48
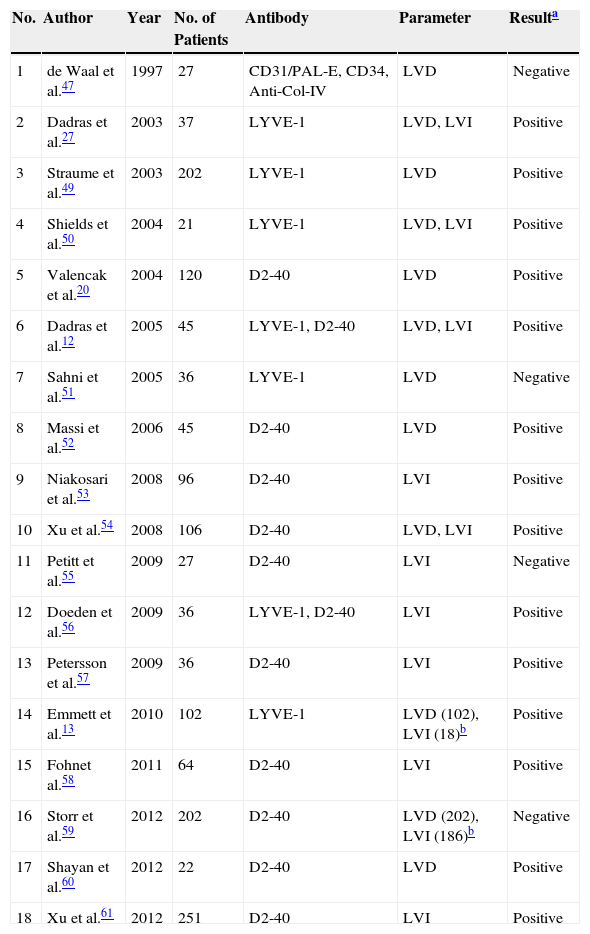
The main features of the studies that have evaluated the role of lymph vessels in patients with melanoma are summarized in Table 2. The search was performed in the PubMed database using the following MeSH keywords: melanoma and (lymphangiogenesis OR “lymphatic vessels”). This search identified 226 articles. We selected those studies that used immunohistochemistry techniques to evaluated the density or presence of invasion of lymph vessels in histological preparations of human melanomas. We also reviewed the literature references of the selected papers to identify studies not detected in the search.
Summary of Published Studies on the Prognostic Value of Lymph Vessel Density or Invasion in Patients With Melanoma.
| No. | Author | Year | No. of Patients | Antibody | Parameter | Resulta |
|---|---|---|---|---|---|---|
| 1 | de Waal et al.47 | 1997 | 27 | CD31/PAL-E, CD34, Anti-Col-IV | LVD | Negative |
| 2 | Dadras et al.27 | 2003 | 37 | LYVE-1 | LVD, LVI | Positive |
| 3 | Straume et al.49 | 2003 | 202 | LYVE-1 | LVD | Positive |
| 4 | Shields et al.50 | 2004 | 21 | LYVE-1 | LVD, LVI | Positive |
| 5 | Valencak et al.20 | 2004 | 120 | D2-40 | LVD | Positive |
| 6 | Dadras et al.12 | 2005 | 45 | LYVE-1, D2-40 | LVD, LVI | Positive |
| 7 | Sahni et al.51 | 2005 | 36 | LYVE-1 | LVD | Negative |
| 8 | Massi et al.52 | 2006 | 45 | D2-40 | LVD | Positive |
| 9 | Niakosari et al.53 | 2008 | 96 | D2-40 | LVI | Positive |
| 10 | Xu et al.54 | 2008 | 106 | D2-40 | LVD, LVI | Positive |
| 11 | Petitt et al.55 | 2009 | 27 | D2-40 | LVI | Negative |
| 12 | Doeden et al.56 | 2009 | 36 | LYVE-1, D2-40 | LVI | Positive |
| 13 | Petersson et al.57 | 2009 | 36 | D2-40 | LVI | Positive |
| 14 | Emmett et al.13 | 2010 | 102 | LYVE-1 | LVD (102), LVI (18)b | Positive |
| 15 | Fohnet al.58 | 2011 | 64 | D2-40 | LVI | Positive |
| 16 | Storr et al.59 | 2012 | 202 | D2-40 | LVD (202), LVI (186)b | Negative |
| 17 | Shayan et al.60 | 2012 | 22 | D2-40 | LVD | Positive |
| 18 | Xu et al.61 | 2012 | 251 | D2-40 | LVI | Positive |
Abbreviations: LVD, lymph vessels density; LVI, lymph vessel invasion; and LYVE, lymph vessel endothelial receptor.
The fact that prior to 2006 not all authors were able to demonstrate the prognostic value of lymph vessel density (LVD) and of the presence of lymph vessel invasion (LVI) in patients with melanoma, may be due partly to a lack of agreement on the methods; in 2006, the first international consensus on the methodology of lymphangiogenesis quantification in solid tumors was published.62 That paper provided a review of the most relevant aspects of the process of tumor lymph vessel quantification and provided a series of recommendations to be followed in order to achieve homogeneous results that could be compared between different studies.
A number of experimental studies in animals have confirmed the active role of tumor lymphangiogenesis, as well as of growth factors VEGF-C and VEGF-D in the spread of a tumor to the lymph nodes.63 The persistent overexpression of these factors by tumor cells leads to a marked increase in the growth of lymph vessels in the tumor parenchyma, favoring metastatic spread.64,65 In an animal model of melanoma, VEGF-C overexpression led to an increase in the number of lymph vessels within the tumor and to an increase in the diameter of the peritumoral vessels.66 Similarly, the levels of VEGF-C in melanoma samples from humans have correlated both with the density of lymph vessels within the primary tumor27 and with lymph node metastases in these patients.21,67,68
Importance of Lymphangiogenesis in the Sentinel Lymph NodeThe majority of publications on the role of lymphangiogenesis in the progression and spread of melanoma have focused on the morphology and functionality of lymph vessels associated with the primary tumor in the skin. However, the group led by Michael Detmar33 described a new concept that we consider to be very important both in clinical practice and for future research. In an animal model of skin cancer, those investigators demonstrated that the process of lymphangiogenesis was activated in the sentinel lymph node even before metastasis occurred. In other words, the findings reported by Detmar et al. suggest that the tumor cells are able to “prepare” the site to which they are going to metastasize. Those results are intriguing and change our long-standing static image of cancer, as they hint at the ability of malignant tumors to induce a number of effects at a distance from the primary tumor, before any spread of malignant cells through the body occurs.
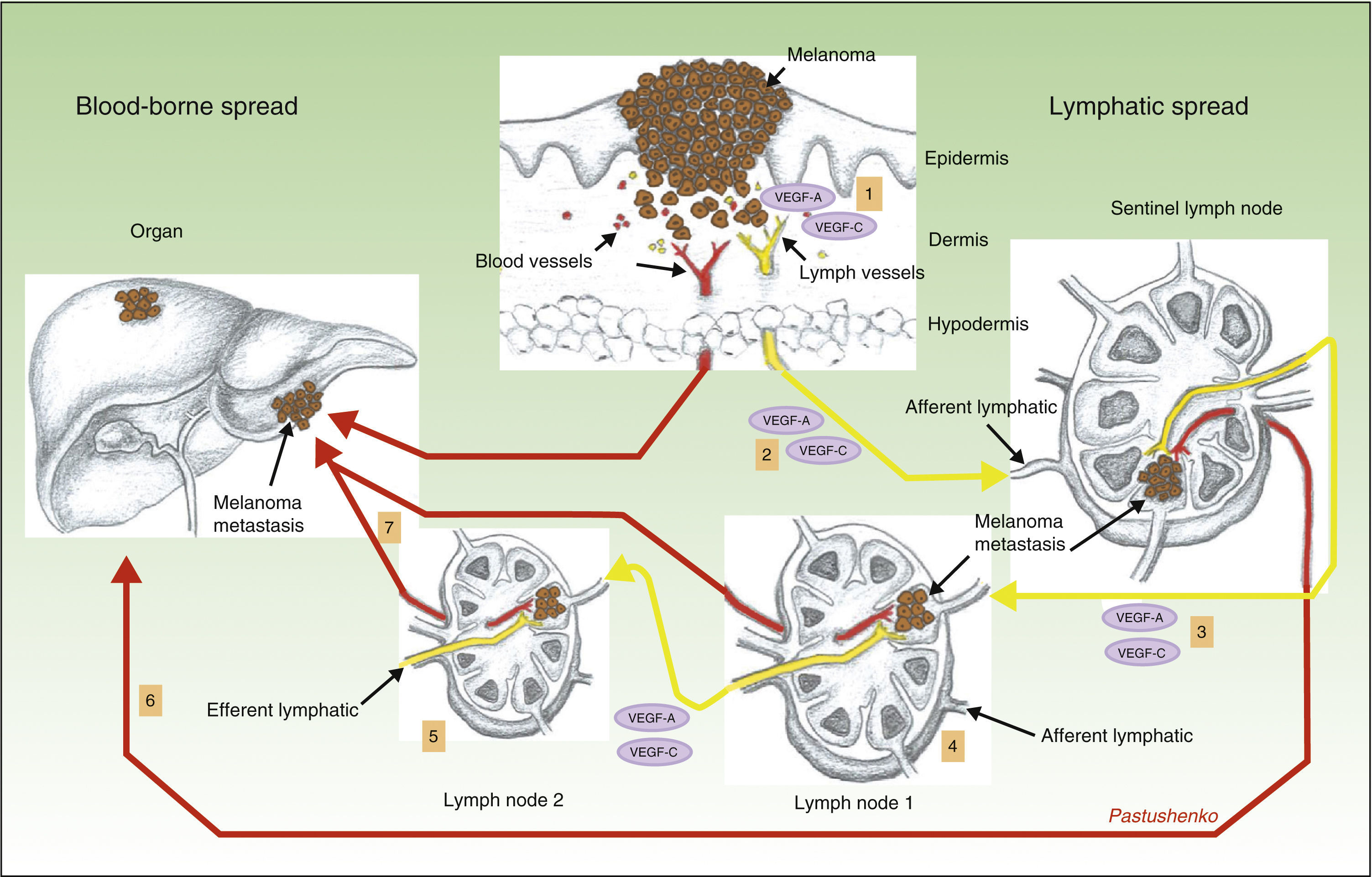
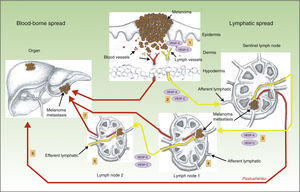
Another surprising finding by the same group was that lymphangiogenesis in the sentinel lymph node was associated with a higher frequency of metastases in distant lymph nodes, as well as a higher incidence of blood-borne metastases.69 This again is a finding of great importance, as it shows that hematogenous and lymphatic spread are not 2 independent processes, but rather that they are very closely related. The mechanism thought to be responsible for this phenomenon is shown diagrammatically in Figure 3.
Role of lymphangiogenesis in the distant spread of neoplastic cells. Diagram of the role of lymphangiogenesis in the development of lymphatic and blood-borne metastases. Tumor cells release vascular endothelial growth factor (VEGF)-A and VEGF-C, inducing lymph vessels proliferation in the peritumoral connective tissue (1). Simultaneously, VEGF-A and -C are transported to the sentinel lymph node (2), where they induce blood and lymph vessel proliferation (premetastatic niche). When the tumor cells reach the first node, the levels of VEGF-A and -C increase, and these factors are then transported to distant nodes (3), facilitating the appearance of metastases in those nodes (4 and 5), as well as distant blood-borne metastases via the thoracic duct or systemic blood vessels (6 and 7).
The interpretive hypothesis would allow us to argue that tumor cells apparently release VEGF-A33 and VEGF-C,69 which are transported to the sentinel lymph node where they induce lymphangiogenesis, creating a premetastatic niche for the tumor cells. When the tumor cells arrive at the first lymph node, the concentrations of VEGF-A and VEGF-C increase and, once again, these factors are transported through the lymph vessels to distant nodes, leading to an expansion of their lymph vessel network; these findings have also been demonstrated in melanoma.70 It must be remembered that VEGF-A is a potent angiogenesis-inducing factor, which may explain the increase in the incidence of distant metastases observed in tumors with activation of lymphangiogenesis in the sentinel lymph node.
Methods for the Quantification of Lymph Vessels in Solid TumorsLymphangiogenesis quantification was held back for years by a lack of specific markers for the lymphatic endothelium. Fortunately, in the last decade a number of markers that selectively bind lymphatic endothelial cells have been identified and made available on the market, enabling lymph vessels to be differentiated from blood vessels in samples of tumor tissue.
Quantification of the process of lymphangiogenesis, as occurs with angiogenesis, is complex because vessel formation is a dynamic process. By analogy with angiogenesis, the majority of studies that have evaluated the prognostic value of lymphangiogenesis in patients with different types of cancer have focused on the final result of lymphangiogenesis, the LVD. A number of methods have been developed for the quantification of tumor vessels; these methods were initially described for the quantification of blood vessels, but could subsequently also be applied to lymphangiogenesis. Below we describe the most important methods.
The Weidner TechniqueThe Weidner technique consists of quantifying the lymph vessels at “hot spots”, defined as areas of increased vessel density.71 It is thought that the formation of these hot spots is due to local changes in oxygen levels (hypoxia) that induce the focal release of prolymphangiogenic factors.62 Hot spots are considered to be areas of biological importance, as they arise from tumor cells with a high angiogenic potential, and these cells have a greater ability to enter the blood or lymphatic circulation and thus to produce metastases via these routes.72
Based on Weidner's description (1991), histological preparations of the tumors are first examined at low power (×10) to identify hot spots. Magnification is then increased (×40) in order to quantify the number of microvessels in those hot spots, considering any clearly separated antibody-stained cell or group of cells as a microvessel. Identification of a vascular lumen is not required to establish the presence of a microvessel. In several studies, a significant association has been found between the number of lymph vessels in the primary melanoma and a poor prognosis.19
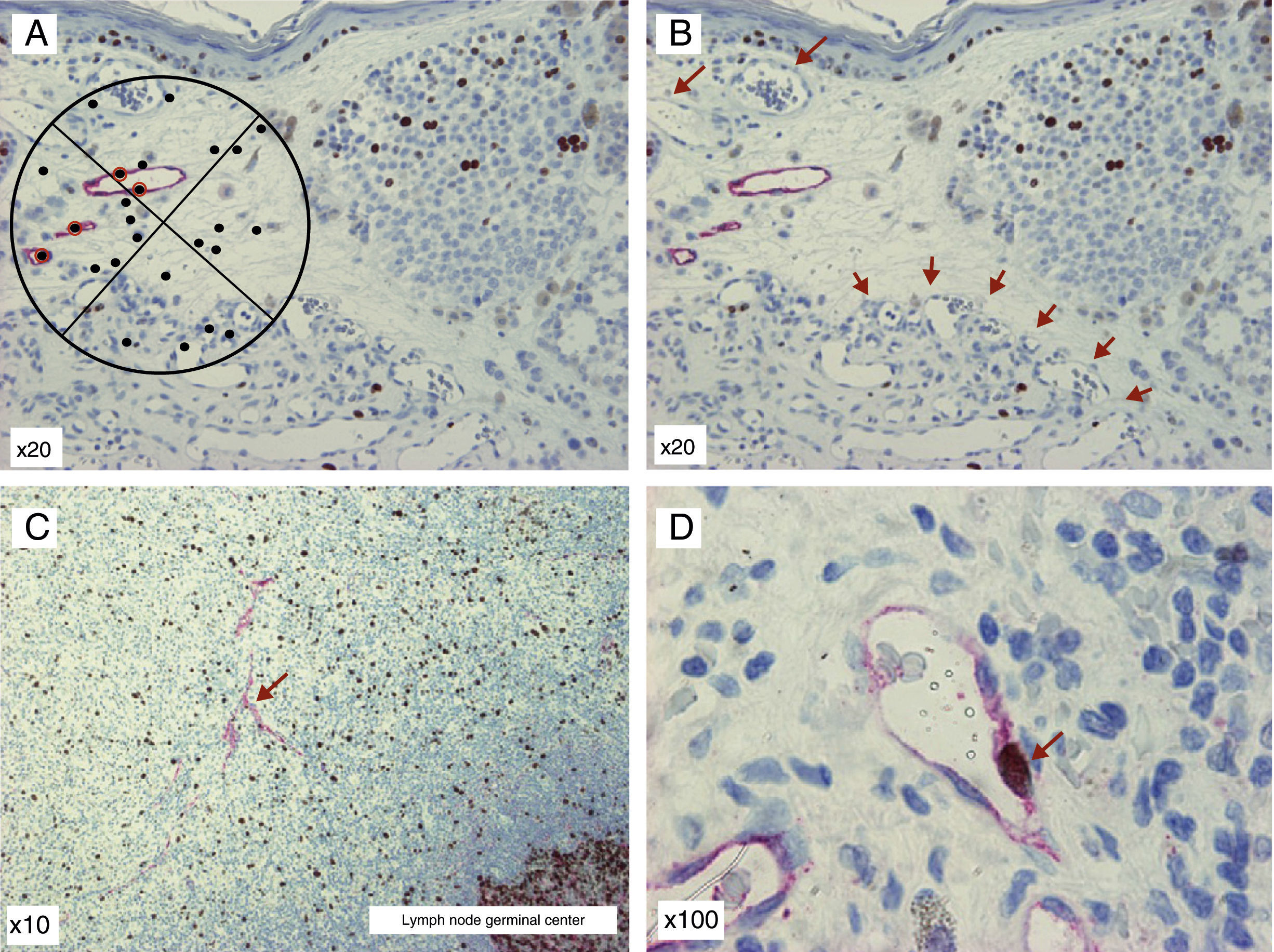
The Chalkley MethodThe Chalkley method is based on the use of a Chalkley eyepiece graticule with 25 randomly distributed points.73 After selecting the hot spots (in a similar way to the Weidner method), the graticule is introduced into the microscope eyepiece and is rotated until the largest possible number of points are positioned over the antibody-stained cells or over the lumens of the vessels whose walls are clearly stained with the endothelial marker (Fig. 4A). Instead of quantifying the number of vessels, as Weidner did, this method counts the number of points of the graticule overlying the vascular structures described. The score obtained is an indicator of the relative area occupied by lymph vessels.62 This method is considered more objective and reproducible, as it avoids one of the most subjective steps in the vessel count, which is how to decide if 2 adjacent structures should be counted as 2 independent microvessels or if they are part of the same vascular structure.72
D2-40/Ki-67 double stain of melanoma preparations and of a lymph node metastasis. A, Chalkley graticule. The points marked with red circles are considered positive. B, D2-40–positive lymph vessels in the primary tumor and numerous D2-40–negative blood vessels occurring in a hot spot in the lower part of the image (arrows). C, Melanoma sentinel lymph node metastasis with D2-40–positive lymph vessels (arrow). D, Melanoma sentinel lymph node metastasis at higher magnification. Proliferating lymph vessels can be seen. The walls of the vessels are clearly positive for D2-40 (cytoplasmic pattern, fast red chromogen, Dako) and the nucleus is positive for Ki-67 (nuclear pattern, DAB chromogen, Dako [arrow]).
Automated analysis using computer systems obviously offers a more objective and reproducible evaluation of the tumor vascular network. In addition, the majority of programs designed for this purpose provide important additional information, such as the area and the circumference of the vessel lumens. However, the more widespread use of these systems is hindered by the need for costly specialized equipment. A further limitation of the technique is the observation that some antibodies nonspecifically stain other structures, easily distinguished from vessels by an experienced pathologist but which can confound automated systems that detect the luminic signals, without taking into account structure and morphology.62
Calculation of the Fraction of Lymphatic Endothelial Cells in ProliferationCalculation of the fraction of lymphatic endothelial cells in proliferation is performed by double stain of the tumor tissue with antibodies against lymphatic endothelium (anti-podoplanin and anti-LYVE-1) and a marker of cells in proliferation (Ki-67 or anti-PCNA). Lymph vessels with proliferating nuclei have been found in melanoma,27,49 which would suggest that active tumor lymphangiogenesis occurs in this type of tumor. Some images of the D2-40/Ki-67 double stain are shown in Figure 4B and 4C.
Future ProspectsThere is ever stronger and more specific scientific evidence of the importance of lymphangiogenesis in the progression and spread of malignant tumors in general, and of cutaneous melanoma in particular. The main reason why it has not been possible to include parameters related to tumor lymphangiogenesis in the routine analysis of melanoma preparations has probably been the lack of consensus in the methodology employed in the studies published to date, and it is therefore very important for future research to follow the recommendations of international experts.62
A hypothetical issue of great relevance for future research in this area is whether serum markers can be used to measure lymphangiogenesis in patients with this neoplastic disease. For example, the serum levels of VEGF-C could be monitored. A recently published study appears to suggest that this is possible as lower levels of VEGF-C were found in the serum of patients with satellitosis or subcutaneous metastases close to the primary melanoma lesion than in patients with distant metastases.68 This hypothetical research option is difficult to evaluate, as the circulating levels of VEGF-C differ widely between individuals,74 and thus there are still no conclusive data that enable VEGF-C or other serum prolymphangiogenic factors to be used as prognostic indicators with any degree of reliability in patients with melanoma.
These hypotheses for future research almost inevitably lead to the question of whether inhibition of VEGF-C, VEGF-D or their receptor VEGFR-3 could achieve a therapeutic effect in patients with melanoma. Experimental studies in different types of tumor (gastric carcinoma, breast carcinoma) appear to suggest that neutralizing antibodies against VEGF-D75 and VEGFR-376–78 are able to inhibit tumor lymphangiogenesis and metastasis in animal models. The process of tumor lymphangiogenesis thus warrants consideration both as a prognostic marker and as a possible future therapeutic target.
Finally, the importance of tumor lymphangiogenesis in the sentinel lymph node, even prior to the appearance of metastases, would suggest that its evaluation using imaging techniques in patients who have undergone excision of a melanoma could be of value for the early diagnosis of metastases or even for their prevention.79
In this paper, based on a review of data in the literature and on personal experience of the use of certain parameters, we have described a series of hypotheses and reasonable doubts concerning the current state of understanding of tumor lymphangiogenesis as it relates to melanoma. We hope that, in the short to medium term, this will enable us to achieve results of wide interest in the field of dermatology.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors of this review would like to thank K. Kokoriev and Y. Pastushenko for their invaluable help in the preparation of the diagrams.
Please cite this article as: Pastushenko I, Conejero C, Carapeto FJ. La linfangiogénesis. Sus implicaciones en el diagnóstico, tratamiento y pronóstico del melanoma. Actas Dermosifiliogr. 2015;106:7–16.


![D2-40/Ki-67 double stain of melanoma preparations and of a lymph node metastasis. A, Chalkley graticule. The points marked with red circles are considered positive. B, D2-40–positive lymph vessels in the primary tumor and numerous D2-40–negative blood vessels occurring in a hot spot in the lower part of the image (arrows). C, Melanoma sentinel lymph node metastasis with D2-40–positive lymph vessels (arrow). D, Melanoma sentinel lymph node metastasis at higher magnification. Proliferating lymph vessels can be seen. The walls of the vessels are clearly positive for D2-40 (cytoplasmic pattern, fast red chromogen, Dako) and the nucleus is positive for Ki-67 (nuclear pattern, DAB chromogen, Dako [arrow]). D2-40/Ki-67 double stain of melanoma preparations and of a lymph node metastasis. A, Chalkley graticule. The points marked with red circles are considered positive. B, D2-40–positive lymph vessels in the primary tumor and numerous D2-40–negative blood vessels occurring in a hot spot in the lower part of the image (arrows). C, Melanoma sentinel lymph node metastasis with D2-40–positive lymph vessels (arrow). D, Melanoma sentinel lymph node metastasis at higher magnification. Proliferating lymph vessels can be seen. The walls of the vessels are clearly positive for D2-40 (cytoplasmic pattern, fast red chromogen, Dako) and the nucleus is positive for Ki-67 (nuclear pattern, DAB chromogen, Dako [arrow]).](https://static.elsevier.es/multimedia/15782190/0000010600000001/v2_201502060417/S1578219014002996/v2_201502060417/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)



