The association of melanoma with a preexisting melanocytic nevus varies considerably between series, depending on whether the association is based on histological signs (4%-72%) or a clinically evident lesion (42%-85%). Histological association with a nevus correlates with favorable prognostic factors, whereas a clinical association correlates with unfavorable factors. In this review, we discuss the characteristics of nevus-associated melanoma from different perspectives: Whiteman's divergent pathway hypothesis for the development of cutaneous melanoma; and the factors involved in nevogenicity, including both the genetic and molecular factors involved in the development of the melanoma and its precursor lesions. Finally, a cumulative analysis of the 16 162 cases reported in the literature revealed that 29.8% of melanomas are histologically associated with a melanocytic nevus.
La asociación clínica o histológica de un melanoma con un nevo melanocítico previo varía entre las series previamente publicadas de forma prominente. Esta variación se produce tanto en función de si se tienen en cuenta los restos histológicos (4-72%) como en función de la presencia de una lesión clínicamente evidente (42-85%). La asociación histológica con un nevo se ha correlacionado con factores pronósticos favorables, mientras que la asociación clínica por el contrario lo hace con factores desfavorables. Esta revisión pretende abordar las características vinculadas con el melanoma asociado a nevo, en relación con: la teoría de las vías divergentes para el desarrollo de un melanoma cutáneo de Whiteman, los factores vinculados a nevogenicidad y la genética y biología molecular del melanoma y sus lesiones precursoras. Adicionalmente, basado en el análisis agregado de un total de 16.162 pacientes publicados en la literatura hasta la fecha, se ha calculado la proporción total de melanomas histológicamente asociados a nevo melanocítico, cifrándose en el 29,8%.
In 1967, Mishima1 predicted that melanoma would ultimately be seen as 2 distinct entities he called malignant melanocytoma, arising from lentigo senilis, and malignant nevocytoma, arising from a melanocytic nevus. Since then, great strides have been made in our understanding of the molecular biology of melanocytic neoplasms. Mishima's hypothesis, which has not yet been refuted, has contributed to highlighting the importance of potential precursors of melanoma, the most notable of which are melanocytic nevi.
One theory supported by several authors is that melanocytic nevi are benign tumors that originate from the clonal expansion of a melanocyte. This transformation would occur either spontaneously or in response to external triggers (e.g., sun exposure), giving rise to a histologically symmetric, largely uniform, proliferation of cells with homogeneous mutations affecting the majority of constituent cells.2 The findings of numerous studies support this clonal expansion of nevus cells3–5 (although conflicting findings have been reported)6,7 and have permitted the definition of melanocytic nevi as clones of melanocytes that have undergone senscence.5,8,9 Genetic mutations attributed a key role in melanoma development have also been found in certain nevi, indicating that these lesions may be the true precursors of melanoma, which would develop following the accumulation of additional mutations over time.10 In a series of 37 cases of melanoma with adjacent melanocytic lesions, Bastian et al.11 observed a gradient in the number and type of mutations present in benign, intermediate but probably benign, intermediate but probably malignant, and malignant tumors.
The above findings suggest an evolutionary trajectory where a melanocyte would undergo clonal expansion before transitioning to a melanocytic nevus and ultimately a melanoma. This proposed melanocyte–nevus–melanoma model, however, would only be applicable to certain cases, as melanomas also occur de novo, i.e., in the absence of an adjacent melanocytic lesion. This evolutionary process is particularly evident in the case of 2 histologic subtypes of melanoma: lentigo maligna melanoma and acral lentiginous melanoma. Finally, nevus-associated melanoma cannot always be histologically demonstrated in patients with a clinical history suggesting a preexisting nevus.
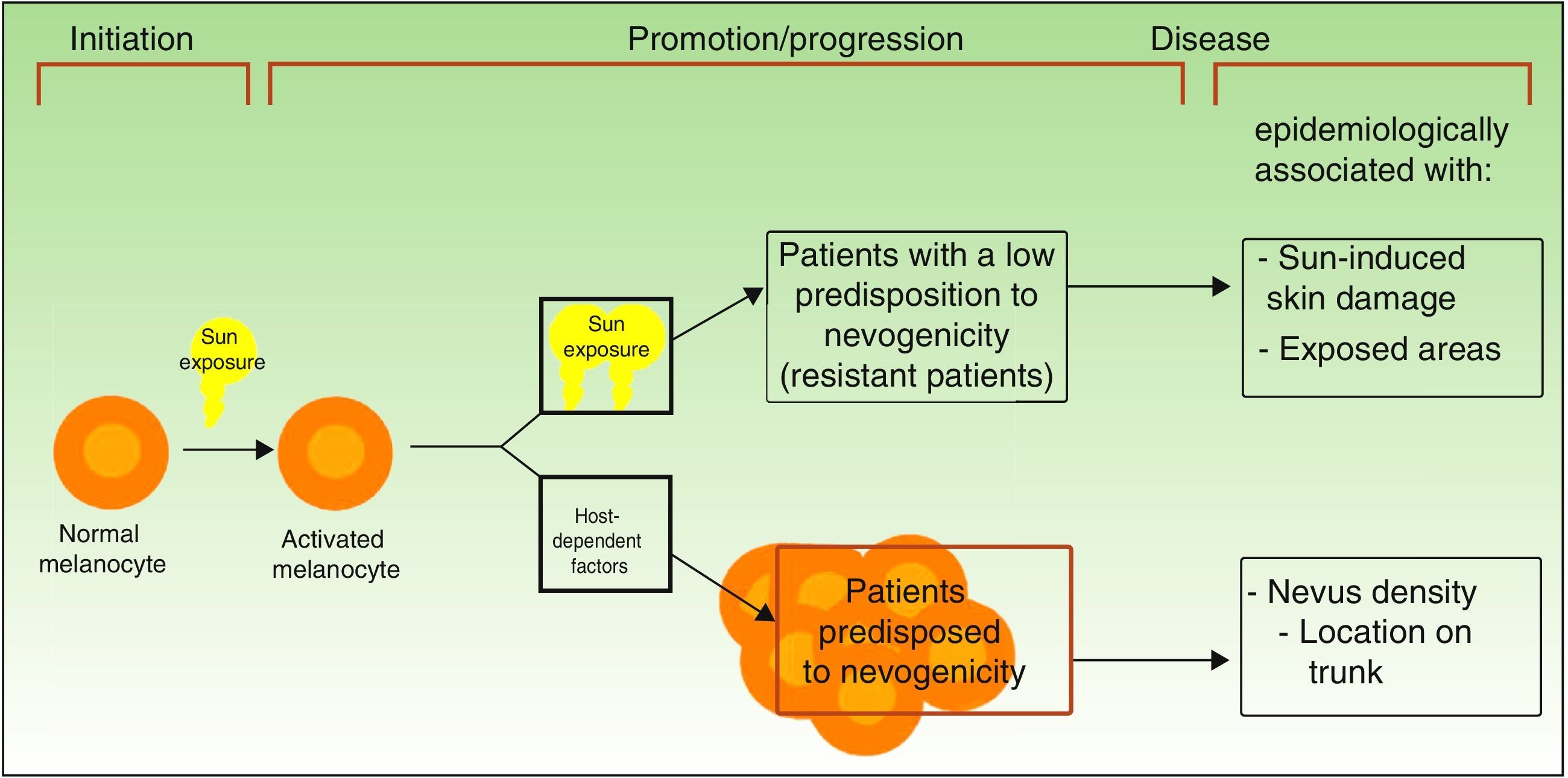
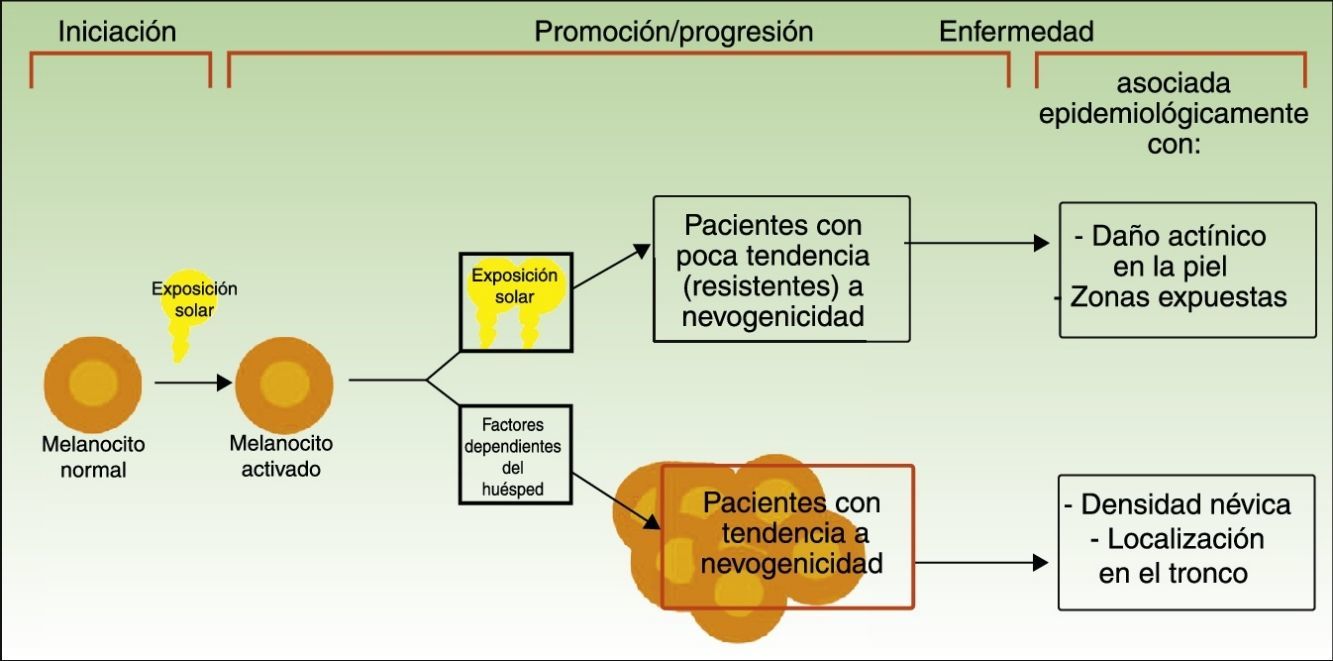
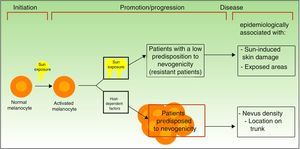
Divergent Pathways to the Development of MelanomaThe pathogenic model that assumes that melanomas arise from a melanocytic nevus following the accumulation of genetic events would appear to be consistent with the melanocyte proliferation or cell pigment instability pathway, which is one of 2 pathways proposed by Whiteman for the development of cutaneous melanoma. This double, or divergent, pathway was first proposed in 1998 but it has subsequently been confirmed in diverse studies (including a study by Whiteman's group12 and a meta-analysis13). According to this model, there are at least 2 etiologic pathways that have a key role in the development of nonacral cutaneous melanoma (Fig. 1).
Pathways to melanoma development according to sun exposure and patient predisposition. Source: modified from Whiteman et al.12
The first is the pathway of chronic sun exposure, in which the accumulation of UV radiation–induced mutations in the melanocyte would lead to the development of melanoma. It is characteristic of elderly patients with a higher constitutional sensitivity to the harmful effects of the sun (i.e., those with a low Fitzpatrick skin type) and a history of significant sun damage and nonmelanoma skin cancer. The vast majority of melanomas driven by this pathway would affect the face, neck, and lower extremities.
The second “nevogenic” or pigment cell instability pathway is characterized by a greater, genetically determined, tendency towards melanocyte proliferation. In this pathway, sun exposure in childhood, and to a lesser extent, in adulthood, would ultimately be responsible for the production of new mutations and the proliferation of genetically predisposed melanocytes to tumor cells.12,14 This pathway would affect younger patients with a genetic predisposition to melanocyte proliferation, i.e., patients with high nevus counts. Melanomas in such cases would generally affect areas of intermittent sun exposure.
From Melanocytic Nevus to Melanoma: Genetic and Molecular FactorsOur knowledge of genetic, epigenetic, and molecular factors involved in nevogenesis and melanoma development has advanced considerably in recent years, leading not only to a greater understanding of the biology and natural history of these lesions but also to the development of targeted therapies that have shown unprecedented success.
Melanocytic neoplasms feature genetic alterations that take the form of primary oncogenic events (typically, activation of protooncogenes in oncogenes) or secondary oncogenic events (generally, suppressed expression of genes involved in skin pigmentation or cellular senescence).2 The constant interaction between aggregates of melanocytes and the epithelial microenvironment would further contribute to the development of these lesions. Factors such as inflammatory phenomena,15 hypoxia,16 and paracrine exchanges, for example, have all been implicated in melanoma progression.17 This interaction between melanocytes and the microenvironment would be additionally modified by other factors, such as UV radiation.18
Following an analysis of genetic alterations in melanoma, Curtain et al.19 claimed that the mutational profile of melanocytes may be determined by sun exposure patterns. The authors proposed classifying melanomas into 4 groups based on tumor location and increasing levels of chronic sun-induced damage from the first to the fourth group. The criteria for the third and fourth groups also contemplated the presence of precursor or intermediate lesions. The 4 groups proposed are:
- 1)
Mucosal melanomas, occurring in mucosal membranes and characterized by an absence of sun exposure.
- 2)
Acral melanomas, occurring in acral locations and characterized by limited sun exposure.
- 3)
Melanomas on skin without chronic sun-induced damage (absence of histologic evidence of solar elastosis), including spitzoid melanomas. Curtain et al. considered that some of the melanomas in this group might arise from benign or intermediate precursor lesions, such as acquired nevi, dysplastic nevi, Spitz nevi, and atypical Spitz tumors.
- 4)
Melanomas on skin with chronic sun-induced damage (solar elastosis), including desmoplastic melanomas possibly arising in unstable solar lentigines.19,20
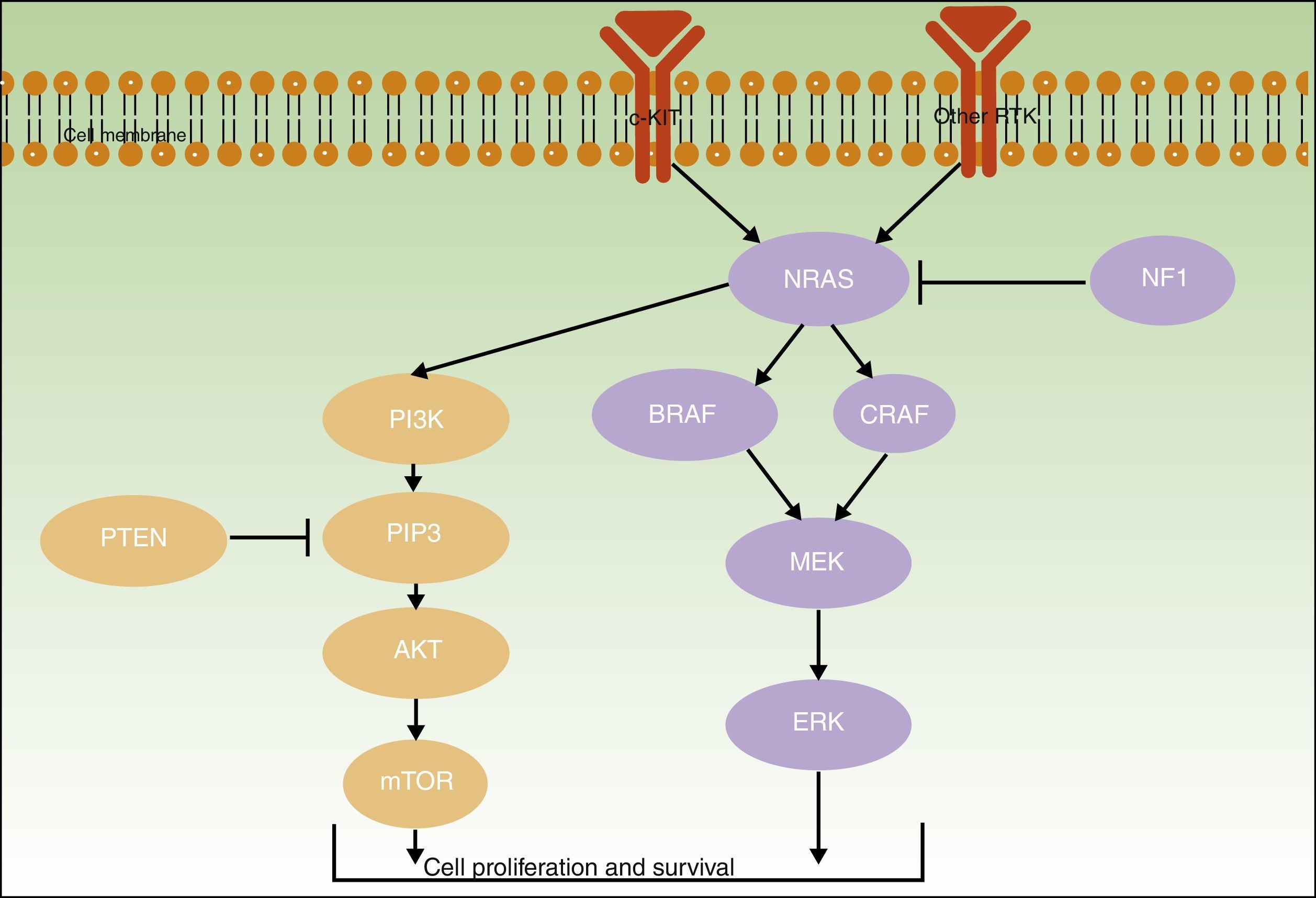
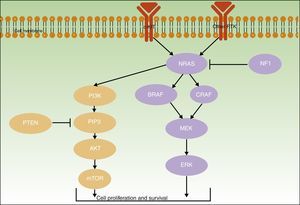
Initiating or driver mutations have been described for all of the above melanomas. The 2 main pathways involved—the mitogen-activated protein kinase (MAPK) pathway and the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway–are shown in Figure 2.
The B-Raf proto-oncogene, serine/threonine kinase gene (BRAF) and the NRAS proto-oncogene, GTPase gene (NRAS) encode activator proteins at different levels of the MAPK pathway. The proteins regulate signal transduction from the surface of melanocytes for the transcription of factors that regulate cell proliferation, growth, survival, and apoptosis.19,23 The proportion of somatic activating BRAF and NRAS mutations reported for melanocytic lesions varies considerably from series to series.
BRAF mutations are the most common mutations associated with the pigment cell instability pathway24 and have been found in over 80% of common melanocytic nevi.10,25–27 They have also been identified in approximately 40% to 50% of primary melanomas and are more common in nevus-associated melanomas and superficial spreading melanomas in areas of intermittent sun exposure.27,28
NRAS mutations are less common and have been observed in up to 56% of congenital melanocytic nevi27 and in less than 5% of acquired nevi.27,29 Nevertheless, findings to date may have been confounded by the difficulty of distinguishing between acquired common nevi and small congenital nevi.30NRAS mutations have been observed in 20% to 30% of melanomas and are particularly common in nodular melanomas located in areas of chronic sun exposure.28,31
BRAF and NRAS mutations may have a role in initiating the proliferation of the constituent melanocytes of pigmented lesions8 and in the early stages of melanoma development.10 Additional mutations, however, would be necessary to drive malignant transformation.10,25–27
NF1The neurofibromin 1 tumor suppressor gene (NF1) has a regulatory function in the MAPK pathway. The presence of somatic inactivating NF1 mutations in different types of cancer generates RAS resistance through negative regulation, triggering the secondary activation of ERK. NF1 mutations have been implicated in initiating melanoma development and are also believed to exert a negative regulatory function that results in RAF inhibitor resistance.32,33
TERTThe telomerase reverse transcriptase gene (TERT) is essential for preventing shortening of telomeres in somatic and germinal tissues and also has a role in senescence and carcinogenesis. Telomerase expression is inhibited in most cells after birth but cancer cells display increased telomerase activity. Somatic TERT promoter mutations typically have the characteristic signature of UV radiation–induced mutations (generation of pyrimidine dimers).34,35 They have been identified in approximately 40% of melanomas, most of which occur in sun-exposed areas.36TERT mutations are associated with a worse prognosis, as they have more aggressive characteristics that determine shorter disease-free and melanoma-free survival.37 They have also been linked to faster growth, particularly when accompanied by BRAF or NRAS mutations.38
KIT and PTENThe KIT proto-oncogene receptor tyrosine kinase gene (KIT), which has a role in the growth and proliferation of embryonic melanoblasts,39 is involved in the PI3KT/AKT and MAPK pathways40 (Fig. 2). PI3K generates phosphatidylinositol-3,4,5-triphosphate, a phospholipid that activates both AKT proteins (which have a regulatory role in the cell cycle, proliferation, survival, and neoplastic transformation) and mTOR proteins (which intervene in tumorigenesis).41 Within the PI3K/AKT/mTOR pathway, special mention should be made of the phosphatase and tensin homolog tumor suppressor gene, PTEN, whose main function is to inhibit the activation of AKT by degrading phosphatidylinositol-3,4,5-triphosphate through its phosphatase activity.42
KIT and PTEN mutations in the PI3K/AKT/mTOR pathway are heterogeneous, affect different genes, and may coexist with BRAF (17%) or NRAS mutations (9%).43 Somatic KIT mutations are found in 2% of melanomas. They appear to be particularly common in acral melanomas (40% of acral lentiginous melanomas have KIT mutations) and mucosal melanomas (23%).41 Finally, somatic PTEN mutations have been detected in up to 22% of melanomas.43
CDKN2AThe cyclin-dependent kinase Inhibitor 2A gene (CDKN2A) is located on the short arm of chromosome 9 and encodes 2 tumor suppressor proteins that have a key role in cell proliferation and senescence. The first is p14-ARF, which regulates cell proliferation through p53 stabilization, inducing the expression of the cyclin-dependent kinase inhibitor p21. The second, p16-INK4A, exerts the same role by inhibiting the association between cyclin-dependent kinases (CDK4/6) and cyclin D1 (CCND1).44–46CDKN2A is the best-known familial melanoma locus. Germline CDKN2A mutations that alter the function of 1 or 2 of the proteins encoded by CDKN2A have been identified. In our setting, CDKN2A mutations are found in approximately 15% of familial melanomas.47,48 Somatic CDKN2A alterations resulting in impaired or lost function (homozygous deletions, mutations, or DNA methylation–induced epigenetic silencing) have been described in almost 90% of melanomas49 and have been linked to invasive capacity.10,33 Somatic mutations are not found in common melanocytic nevi, but have been identified in 10% of dysplastic nevi.49
One whole-exome sequencing study of dysplastic melanocytic nevi and melanoma showed that melanocytic nevi have relatively stable genomes and that progression to melanoma seems to require the presence of mutations in key tumor suppressor genes.50 Likewise, a model for lesions at certain sites proposed the existence of a genetic–molecular continuum of melanocytic neoplasms on which benign lesions, following the accumulation of mutations in genes encoding subunits SWI/SNF and p53 (e.g., BRAF, NRAS, TERT, CDKN2A), would transition to intermediate lesions and ultimately to melanoma in situ or invasive or metastatic melanoma.11
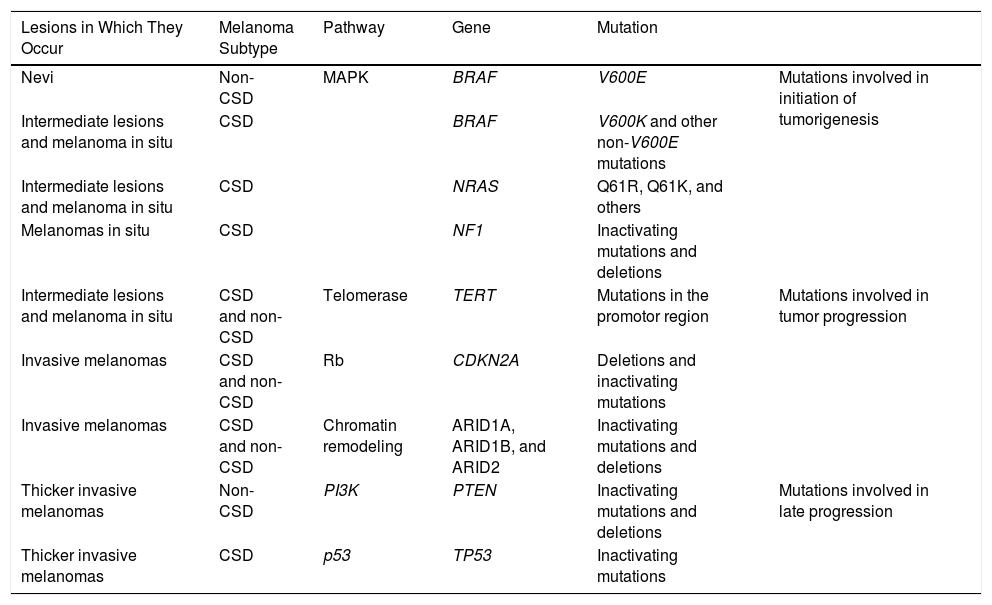
Melanocytic nevi and melanomas would therefore share genetic alterations that would accumulate along this continuum from precursor lesions (benign or intermediate malignancy) to neoplasms with metastatic potential. The main mutations currently considered to be involved in this process are summarized in Table 1.33
Common Genetic Mutations in Melanocytic Neoplasms and Their Role in the Transition to Melanoma.
| Lesions in Which They Occur | Melanoma Subtype | Pathway | Gene | Mutation | |
|---|---|---|---|---|---|
| Nevi | Non-CSD | MAPK | BRAF | V600E | Mutations involved in initiation of tumorigenesis |
| Intermediate lesions and melanoma in situ | CSD | BRAF | V600K and other non-V600E mutations | ||
| Intermediate lesions and melanoma in situ | CSD | NRAS | Q61R, Q61K, and others | ||
| Melanomas in situ | CSD | NF1 | Inactivating mutations and deletions | ||
| Intermediate lesions and melanoma in situ | CSD and non-CSD | Telomerase | TERT | Mutations in the promotor region | Mutations involved in tumor progression |
| Invasive melanomas | CSD and non-CSD | Rb | CDKN2A | Deletions and inactivating mutations | |
| Invasive melanomas | CSD and non-CSD | Chromatin remodeling | ARID1A, ARID1B, and ARID2 | Inactivating mutations and deletions | |
| Thicker invasive melanomas | Non-CSD | PI3K | PTEN | Inactivating mutations and deletions | Mutations involved in late progression |
| Thicker invasive melanomas | CSD | p53 | TP53 | Inactivating mutations |
Despite the increasing recognition that the dynamic evolutionary model of melanocytic neoplasms may explain the development of some melanomas, we cannot ignore the fact that most melanomas are de novo tumors and that only a variable proportion of melanomas contain histologic remnants of a melanocytic nevus (nevus-associated melanoma).
Proportion of Nevus-Associated MelanomasThe risk of a nevus transitioning to a melanoma is generally considered to be low. The cumulative risk of transformation of any individual nevus to a melanoma over the course of 80 years has been estimated at 0.03% (1 in every 3164 nevi) in men and 0.009% (1 in every 10 800 nevi) in women.52
The estimated prevalence of cutaneous melanomas clinically or histologically associated with a preexisting melanocytic nevus ranges from 4% to 85%, depending on the series. The rates are generally lower when only melanomas featuring histologic remnants of a previous nevus are taken into account (4%-72%)53–82 and higher when patients report a history of a clinically evident nevus (42-85%).83,84 Our discussion of nevus-associated melanoma in the next section is based only on cases with objective histologic evidence of a previous nevus.
In 1988, Ackerman claimed that de novo melanomas were more common than nevus-associated melanomas. This claim was based on an analysis of over 75 000 melanomas in white patients, only 20% of which were associated with a previous nevus.85 The findings, however, were not applicable to black or Asian patients, most of whom had de novo tumors affecting the palms or soles or the nail apparatus.85 A wide variety of studies conducted since then have largely shown that nevus-associated melanomas are probably more common than originally believed and do not only affect white patients.86 The variations in estimates can be partly explained by advances in our understanding of the biology of melanocytic nevi and improvements to diagnostic criteria, particularly in the case of borderline lesions. Methodologic differences may, of course, also have led to classification biases. Given the low interobserver agreement observed for the histopathologic diagnosis of clinically suspicious pigmented lesions87 and melanomas in situ, some dermatopathologists recommend that only invasive lesions should be considered when investigating nevus-associated melanomas.88
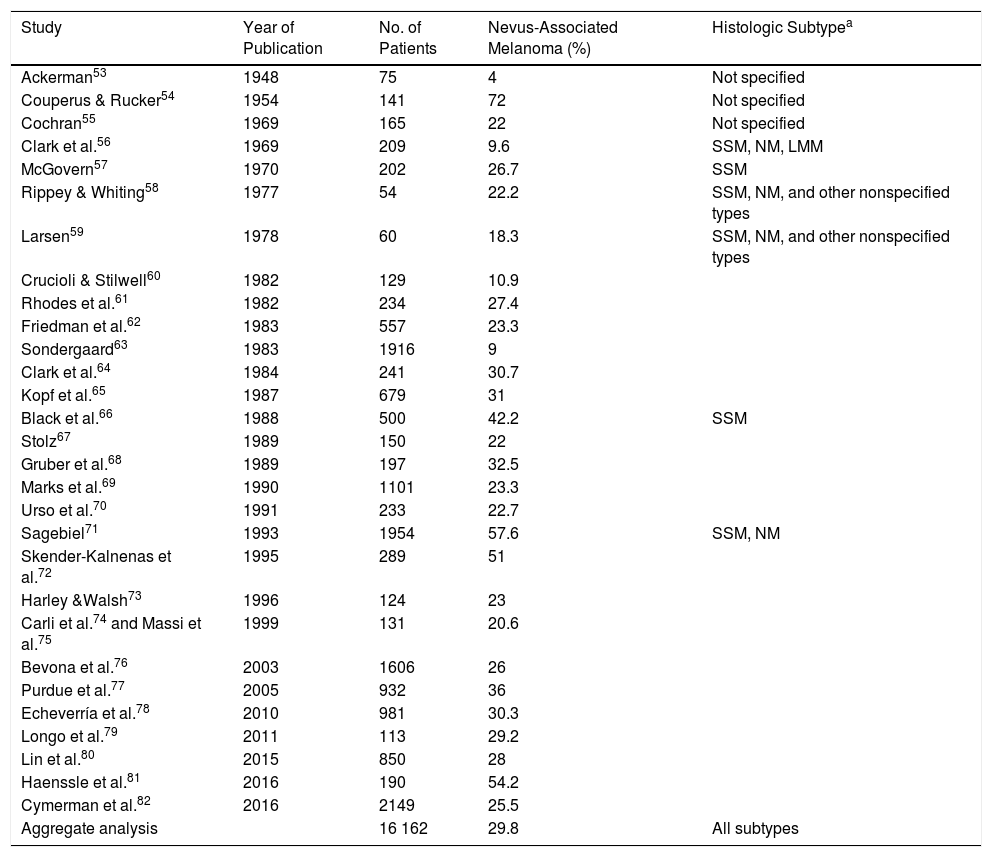
A meta-analysis published in 2015 reported an estimated prevalence of 36% for nevus-associated melanoma in 10 102 patients, but this estimate fell to 32% when studies that only considered superficial spreading melanomas were excluded.80 The meta-analysis did, however, have some limitations. It included, for example, studies that did not clearly specify the number of patients with melanoma or the proportion of patients with nevus-associated melanoma.85,89,90 In addition, while it logically did not take into account some of the more recent studies conducted, it also missed out on some relevant earlier publications. In an attempt to provide a more accurate estimate of the proportion of melanomas histologically associated with nevi, we conducted an exhaustive literature review that included studies not analyzed in the 2015 meta-analysis. We followed a similar methodology,80 which consisted of searching the PubMed database using the terms melanoma, nevi, precursor, and pathology. The search retrieved 169 studies published up to February 7, 2017. The abstracts of all the articles retrieved were screened. Review articles and editorials that did not present new data were excluded, but their reference lists were reviewed to identify original articles mentioning nevus-associated melanoma in the title or abstract. The estimated prevalence of nevus-associated melanoma was calculated using the data from 29 studies reporting on 16 162 patients (Table 2).
Proportion of Melanomas Histologically Associated With a Nevus: Estimated Rates According to Published Studies.
| Study | Year of Publication | No. of Patients | Nevus-Associated Melanoma (%) | Histologic Subtypea |
|---|---|---|---|---|
| Ackerman53 | 1948 | 75 | 4 | Not specified |
| Couperus & Rucker54 | 1954 | 141 | 72 | Not specified |
| Cochran55 | 1969 | 165 | 22 | Not specified |
| Clark et al.56 | 1969 | 209 | 9.6 | SSM, NM, LMM |
| McGovern57 | 1970 | 202 | 26.7 | SSM |
| Rippey & Whiting58 | 1977 | 54 | 22.2 | SSM, NM, and other nonspecified types |
| Larsen59 | 1978 | 60 | 18.3 | SSM, NM, and other nonspecified types |
| Crucioli & Stilwell60 | 1982 | 129 | 10.9 | |
| Rhodes et al.61 | 1982 | 234 | 27.4 | |
| Friedman et al.62 | 1983 | 557 | 23.3 | |
| Sondergaard63 | 1983 | 1916 | 9 | |
| Clark et al.64 | 1984 | 241 | 30.7 | |
| Kopf et al.65 | 1987 | 679 | 31 | |
| Black et al.66 | 1988 | 500 | 42.2 | SSM |
| Stolz67 | 1989 | 150 | 22 | |
| Gruber et al.68 | 1989 | 197 | 32.5 | |
| Marks et al.69 | 1990 | 1101 | 23.3 | |
| Urso et al.70 | 1991 | 233 | 22.7 | |
| Sagebiel71 | 1993 | 1954 | 57.6 | SSM, NM |
| Skender-Kalnenas et al.72 | 1995 | 289 | 51 | |
| Harley &Walsh73 | 1996 | 124 | 23 | |
| Carli et al.74 and Massi et al.75 | 1999 | 131 | 20.6 | |
| Bevona et al.76 | 2003 | 1606 | 26 | |
| Purdue et al.77 | 2005 | 932 | 36 | |
| Echeverría et al.78 | 2010 | 981 | 30.3 | |
| Longo et al.79 | 2011 | 113 | 29.2 | |
| Lin et al.80 | 2015 | 850 | 28 | |
| Haenssle et al.81 | 2016 | 190 | 54.2 | |
| Cymerman et al.82 | 2016 | 2149 | 25.5 | |
| Aggregate analysis | 16 162 | 29.8 | All subtypes |
Abbreviations: LMM, lentigo maligna melanoma; NM, nodular melanoma; SSM, superficial spreading melanoma.
Current evidence indicates that nevus-associated melanomas, regardless of the type of benign melanocytic lesion in which they arose, share certain characteristic features (Table 3).
Characteristics Associated With Nevus-Associated Melanoma.
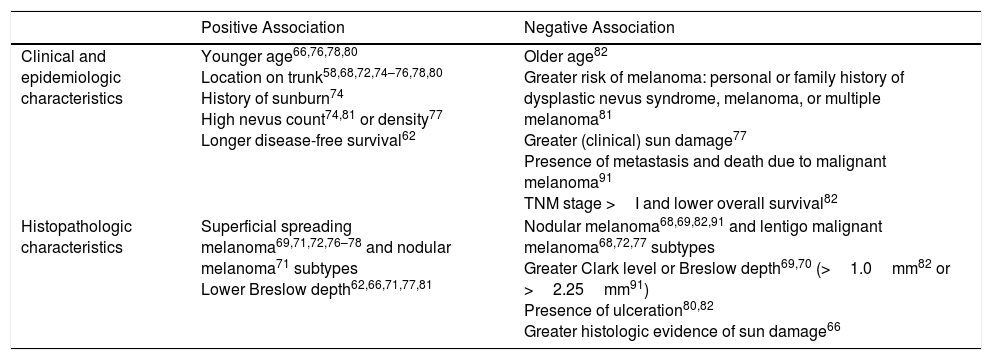
| Positive Association | Negative Association | |
|---|---|---|
| Clinical and epidemiologic characteristics | Younger age66,76,78,80 Location on trunk58,68,72,74–76,78,80 History of sunburn74 High nevus count74,81 or density77 Longer disease-free survival62 | Older age82 Greater risk of melanoma: personal or family history of dysplastic nevus syndrome, melanoma, or multiple melanoma81 Greater (clinical) sun damage77 Presence of metastasis and death due to malignant melanoma91 TNM stage >I and lower overall survival82 |
| Histopathologic characteristics | Superficial spreading melanoma69,71,72,76–78 and nodular melanoma71 subtypes Lower Breslow depth62,66,71,77,81 | Nodular melanoma68,69,82,91 and lentigo malignant melanoma68,72,77 subtypes Greater Clark level or Breslow depth69,70 (>1.0mm82 or >2.25mm91) Presence of ulceration80,82 Greater histologic evidence of sun damage66 |
Melanomas containing histologic remnants of a preexisting nevus are associated with a younger age at diagnosis,66,76,78,80 a high nevus count74,81 (or density),77 and a history of sunburn.74 They are mainly located on the trunk and in areas of intermittent solar exposure58,68,72,74–76,78,80 (which would explain why they have fewer clinical77 and histologic66 signs of chronic sun damage). Nevus-associated melanomas are mostly superficial spreading melanomas69,71,72,76–78 and have histologic characteristics associated with a better prognosis (lower Breslow depth62,66,71,77,81 and absence of ulceration).80,82
The above characteristics contrast with those typically observed in melanomas with clinical rather than histologic evidence of a preexisting nevus. In a study of 377 patients with melanoma, Breslow depth was greater in a subgroup of patients with a clinical history of a previous pigmented lesion (42%), although it was not associated with worse survival.84 This link between tumor thickness and melanoma that is clinically associated with a previous nevus, however, was not corroborated by a later Spanish study that using a similar methodology analyzed 165 melanoma patients, 49% of whom reported a previous nevus.83
Melanomas with histologic evidence of a preexisting nevus appear thus to have more favorable clinical and histopathologic features. Although most publications that have specifically studied nevus-associated melanomas have not found significant differences in survival rates with respect to de novo melanomas, findings suggest that these more favorable prognostic features might be linked to longer survival. One study from 1983, for example, that analyzed 557 patients with melanoma found greater disease-free survival in a subgroup of 130 patients with nevus-associated melanoma.62 A later study, from 2016, of 2 cohorts of patients with melanoma from 2 different time periods (1024 and 1125 patients, of whom 198 [19.3%] and 349 [31.0%] had nevus-associated melanoma) found that patients with histologic evidence of a previous nevus had better overall survival, even after adjusting for classic prognostic factors.82 Finally, a recent multicenter study of 2184 patients with melanoma (that did not specifically study nevus-associated melanoma) found that patients with multiple nevi appeared to have better melanoma-specific survival.92
ConclusionsBased on our pooled analysis of 16 162 patients from 29 different studies, we can conclude that close to 30% of melanomas are histologically associated with a melanocytic nevus. While nevus-associated melanomas appear to have more favorable prognostic features, most of the articles found no significant differences in survival following adjustment for the usual prognostic factors.
As discussed above, the estimated proportion of nevus-associated melanomas varies considerably from series to series, and in addition, there is low agreement between histologic and clinical diagnosis in these cases. These discrepancies could have several explanations:
- -
The probable existence of slow-growing melanomas with a benign clinical appearance.93
- -
The possibility of all remnants of a nevus being completely obliterated by a growing melanoma. In line with previously suggestions,84 we believe that this might particularly be the case with larger tumors, implying that series of thinner melanomas would have higher rates of nevus-associated melanoma.
- -
The consideration of atypical melanocytic proliferation at the margins of melanomas in early publications, which would explain the relative increase in the proportion of nevus-associated melanomas in more recent series.
- -
The possibility of missing histologic remnants of a preexisting nevus as it is not standard practise to analyze 100% of biopsy samples. Orientation of samples during processing could also lead to underdiagnosis.
Whatever the situation, current evidence suggests that patients with nevus-associated melanomas are younger and have lesions that predominantly affect the trunk, a history of sunburn, and a higher nevus count or density. It would therefore seem reasonable to recommend paying special attention to any changes in nevi in patients with multiple nevi, as is standard practice in pigmented lesion units in our setting.
Many questions remain about the biologic behavior of melanoma and its precursors and on how this influences survival. Although most studies suggest that nevus-associated melanomas are less aggressive than their de novo counterparts, it is not entirely clear whether this is due to their seemingly more favorable histologic features or to their biologic characteristics. New, more specific, studies addressing these issues are needed to guide the monitoring and treatment of patients with melanoma.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that no private patient data appear in this article.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Martín-Gorgojo A, Nagore E. Melanoma asociado a nevo melanocítico. Actas Dermosifiliogr. 2018;109:123–132.