We present 7 cases of postsurgical contact dermatitis due to povidone iodine. The diagnosis was based on the clinical manifestations, the history of exposure, the site of the lesions, and the results of patch tests. This type of dermatitis can develop in the area of surgery or at distant sites exposed to povidone iodine during the surgical intervention. Patch tests with 10% povidone iodine in petrolatum were positive in all patients. Based on the results of the same tests in a control group, we recommend the use of petrolatum rather than water as the vehicle for the diagnosis of this form of contact dermatitis. Repeated open application tests with a commercially available solution of povidone iodine were negative. We conclude that the presence of the solution under occlusion during surgery is necessary both for the symptoms to develop and for the diagnosis to be made. This condition may be underdiagnosed.
Presentamos 7 casos de dermatitis de contacto por povidona yodada (PVP-I) en pacientes sometidos a cirugía. El diagnóstico se basó en la clínica, la historia de exposición, la localización de las lesiones y el resultado de las pruebas epicutáneas. La dermatitis puede aparecer en el área quirúrgica, pero también en zonas distantes, aunque expuestas a la PVP-I y sometidas a oclusión durante la intervención. Las pruebas epicutáneas con PVP-I al 10% en vaselina fueron positivas en todos los pacientes. Aconsejamos utilizar este vehículo y no agua para el diagnóstico de estas dermatitis de contacto, basados en los resultados de estas pruebas en un grupo control. La prueba abierta repetida con la solución comercial de PVP-I fue negativa. Concluimos en la necesidad de que exista una oclusión, tanto para la aparición de los síntomas clínicos como para el diagnóstico de esta dermatitis de contacto, que puede estar infradiagnosticada.
Iodine-based compounds have been used for centuries as antiseptics and disinfectants. However, it is only in the past 20 years that their use has become widespread in the Spanish health service, after they became the surgical antiseptics of choice in place of the mercury derivatives, which were also known during the 20th century to be contact sensitizers. The iodine compound most widely used at the present time is povidone-iodine (polyvinylpyrrolidone-iodine [PVP-I]).
Contact dermatitis due to PVP-I has never been considered common,1 particularly in view of such widespread use. There are isolated reports of irritant2,3 and allergic4–9 contact skin reactions, but it is often difficult to discriminate between the two types of contact dermatitis because of the lack of consensus on the concentration of the iodinated compounds (PVP-I and/or iodine) and the vehicles to be used when performing diagnostic patch tests.10–14
We describe 7 patients referred to the skin allergy unit of the dermatology department for the diagnosis and treatment of contact dermatitis after undergoing surgery in various operating rooms in our hospital. In 5 of the patients the dermatitis developed in the surgical field 24 to 48 hours after the operation, but in 2 patients it appeared at distant skin sites. The antiseptic used in all cases was Betadine solution, which, in Spain, contains the following components: PVP-I, glycerol, disodium phosphate, citric acid, sodium hydroxide, nonoxynol-9, and water.
The diagnosis of contact dermatitis due to PVP-I was based on the clinical manifestations, a history of exposure, the site of the lesions, and, most relevantly, the results of the skin tests.
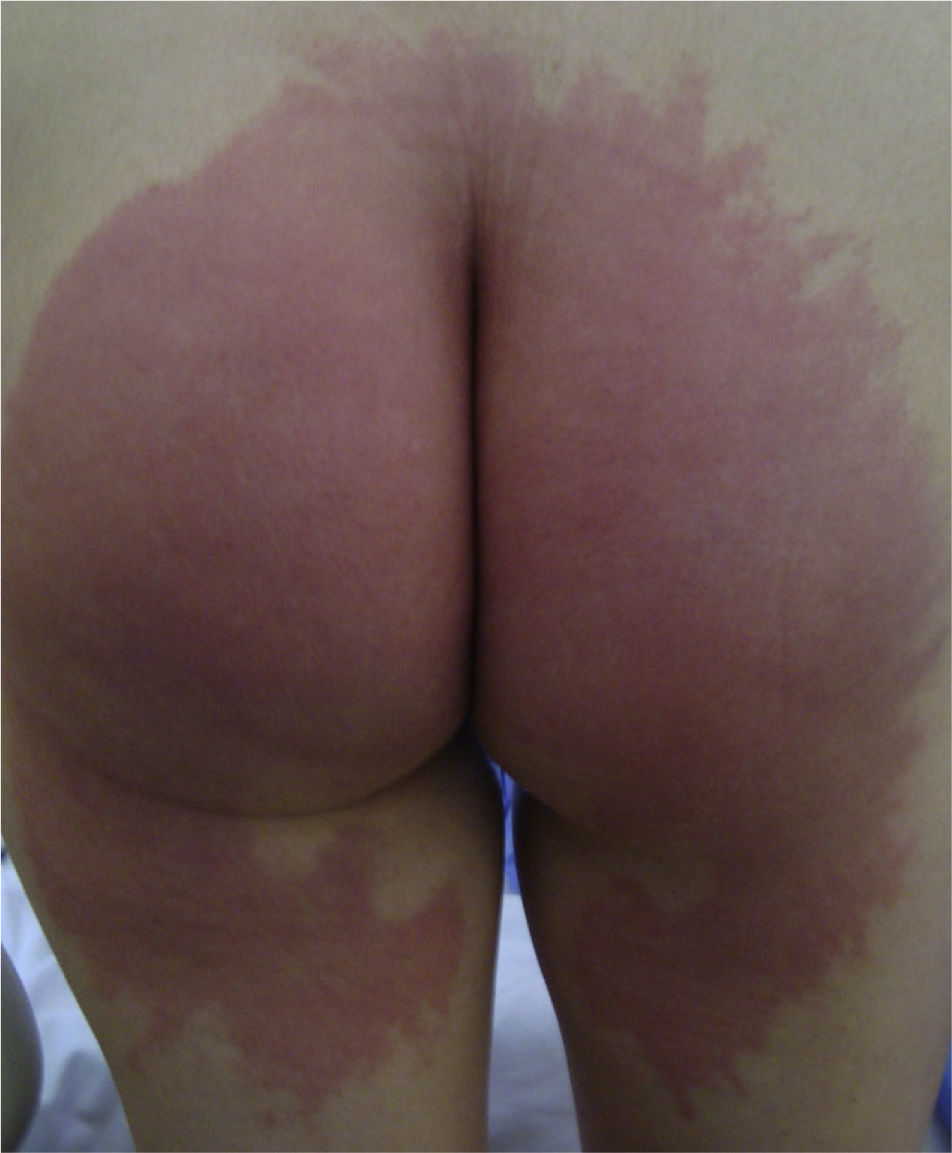
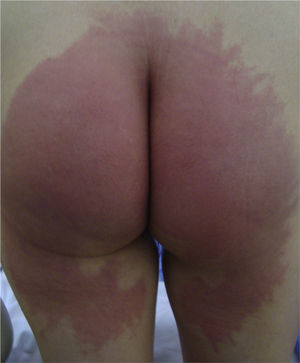
Case DescriptionsCase 1This patient was a 51-year-old woman who had undergone surgery in the gynecology operating room for uterine prolapse. Twenty-four hours after the operation she developed a subacute, erythematous dermatitis with a burning sensation, affecting the gluteal region and posterior aspect of both thighs (Fig. 1).
Case 2The patient was a 67-year-old man in whom surgery had been performed to the right hand under general anesthesia in the orthopedics operating room. Twenty-four hours after the operation he developed acute dermatitis in the area of the surgical field, affecting the right upper arm and forearm distal to the site of the pneumatic tourniquet.
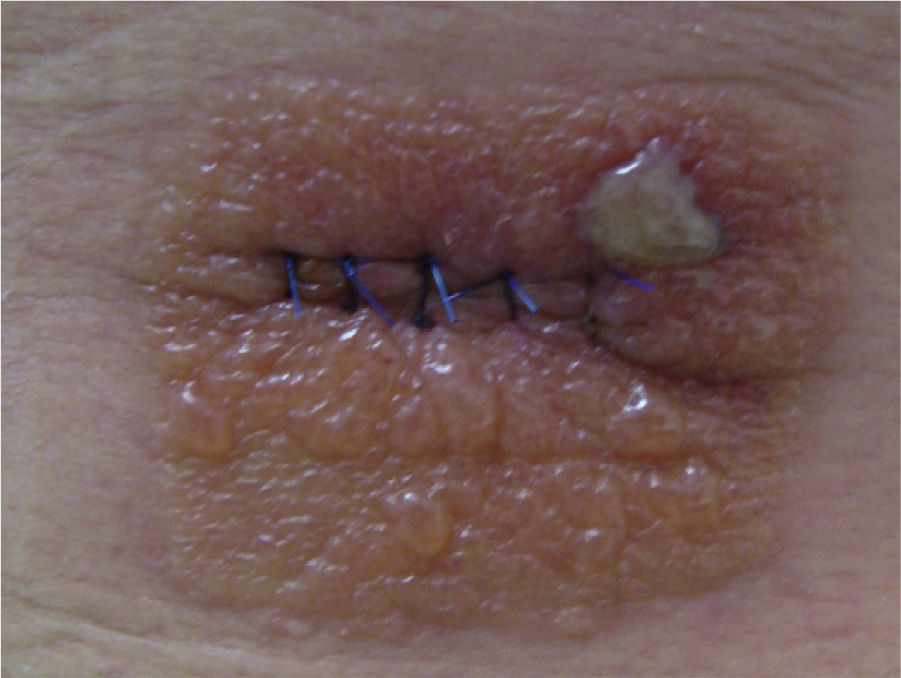
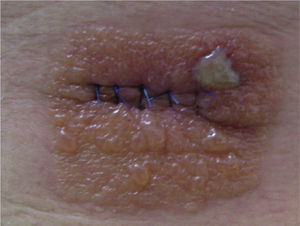
Case 3This patient was a man aged 60 years who had undergone surgery under local anesthesia in the dermatologic operating room for a melanocytic nevus on the back. After the operation, the area had been swabbed with Betadine and covered by an occlusive dressing (Hartman Cosmopore E). When the patient was seen 5 days after the operation, he presented an area of acute eczema with a morphology that mirrored the shape of the dressing (Fig. 2).
Case 4The patient was a 55-year-old man who underwent bone biopsy in the orthopedics operating room for a tumor of the right tibia. Two days after the operation he developed dermatitis affecting the surgical wound and operating field, with isolated papules and pustules. Betadine had been applied before and after the operation and the area had been covered by an occlusive dressing (Oper dres IHT).
Case 5The patient was a man aged 51 years who had undergone operation under general anesthesia in the gastrointestinal surgery operating room for colon cancer. Dermatitis with clearly defined borders had developed on the abdomen 48 hours after the operation. The surgical field had been covered by a self-adhesive dressing (Oper dres IHT).
Case 6This patient was a 61-year-old woman who underwent knee replacement surgery in the orthopedics operating room. She developed dermatitis clearly limited to the area of the surgical field. Betadine had been applied before and after the operation and the surgical field had been covered by an occlusive dressing (Tegaderm Film by 3M Health Care).
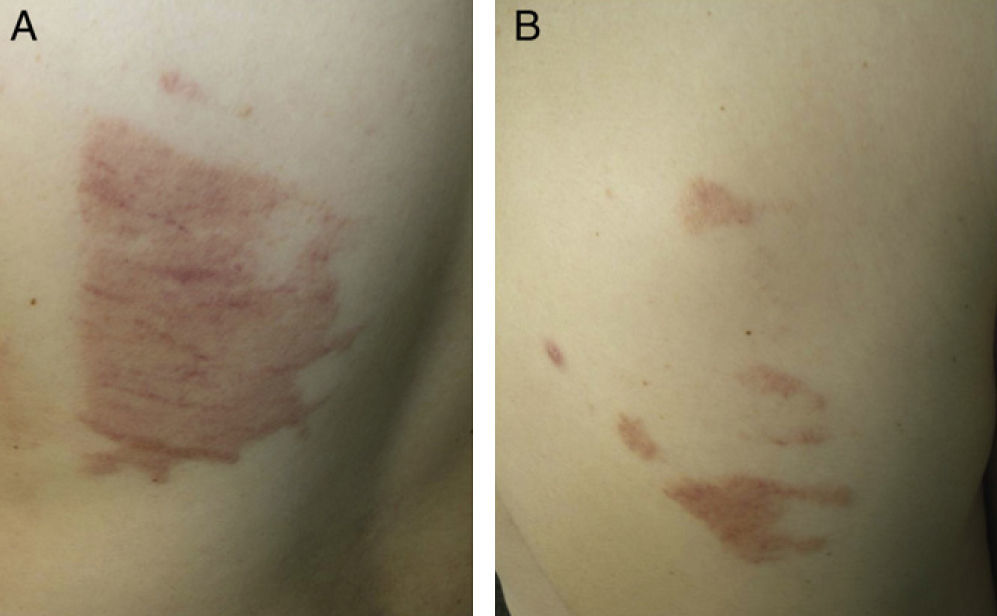
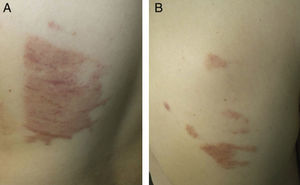
Case 7The patient was a 31-year-old man who underwent surgery in the thoracic surgery operating room for spontaneous pneumothorax. Twenty-four hours after the operation he developed dermatitis with geographic borders on the lateral chest wall and on the back (Fig. 3, A and B).
Additional TestsThe 7 patients were diagnosed with contact dermatitis, for which they received symptomatic treatment. Additional tests were performed some weeks after resolution of the dermatitis.
- 1.
Patch tests were performed using Finn-chambers aluminum patches on Scanpor held in place by Oper-Tape. The following allergens, supplied by the company Marti Tor, were used:
- a)
the standard series of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC); b) an antiseptics series (Marti Tor) that included 0.5% iodine in petrolatum and to which we added 2% nonoxynol-9 in water, as it is one of the components of Betadine; and c) povidone-iodine (Fragon Ibérica, CAS number: 25655-41-8), prepared by Marti Tor at 3 different concentrations (10%, 5%, and 1%) both in water and in petrolatum.
- a)
- 2.
The dressings used in the different operating rooms in our hospital—Hartman Cosmopore E, Oper dres IHT, and 3M Health Care Tegaderm Film—were applied to healthy skin for 48 hours.
- 3.
Repeated open application test (ROAT) with Betadine, as supplied, by open application that was repeated twice a day for 7 consecutive days to the volar surface of the forearm.
In order to determine the irritant potential of PVP-I, we studied a control group of 30 adult volunteers (9 men and 21 women) with no previous history of skin reactions to antiseptic products. These individuals underwent patch testing with PVP-I at 10%, 5%, and 1%, both in petrolatum and in water.
In both groups, application of the patch and reading of the results was performed in accordance with GEIDAC recommendations, taking 2 readings, one at 48 hours and one at 96 hours.
ResultsStudy CasesPositive results with the GEIDAC standard series were obtained with formaldehyde in 1 patient (case 1), of unknown relevance, and for the mixture of local anesthetics in another patient (case 3), known from the patient's past history.
Negative results were obtained for all the substances in the antiseptic series except for povidone-iodine, the reactions to which are detailed below and in the Table 1:
- 1.
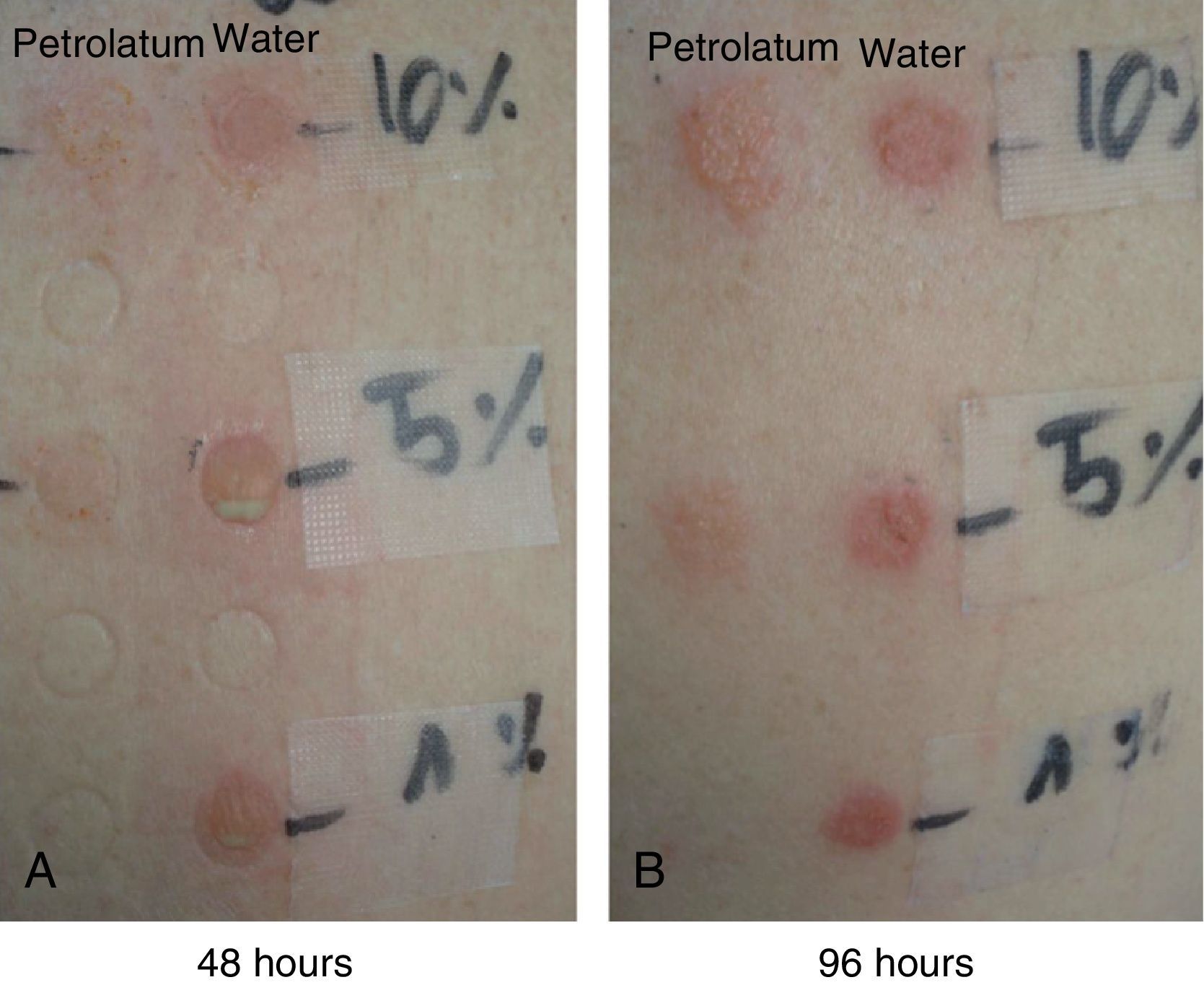
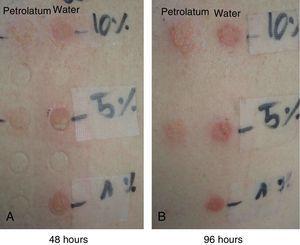
The patches with 10% PVP-I in petrolatum were positive in all 7 patients (100%) at 48 hours, and the intensity of the reaction had increased in some patients at 96 hours (Fig. 4).
- 2.
The patches with 5% PVP-I in petrolatum were positive in 4 patients (57%).
- 3.
The patches with 1% PVP-I in petrolatum were negative in all patients.
- 4.
The patches with 10% and 5% PVP-I in water were positive in all 7 patients (100%) at 48 hours, and the intensity of the reactions had decreased at 96 hours (Fig. 4).
- 5.
The patches with a 1% aqueous solution of PVP-I were only positive in 1 patient (pustular reaction) (Fig. 4).
- 6.
The ROAT with Betadine as supplied was negative in all 7 patients.
- 7.
The test on healthy skin of the application of the dressings used in the different operating rooms was negative.
Patch Test Results in the Study Cases.
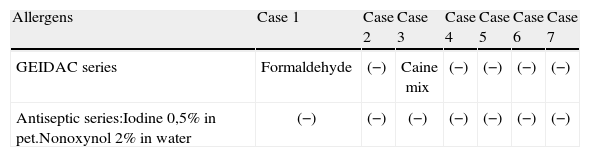
| Allergens | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
| GEIDAC series | Formaldehyde | (−) | Caine mix | (−) | (−) | (−) | (−) |
| Antiseptic series:Iodine 0,5% in pet.Nonoxynol 2% in water | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| 48h | 96h | 48h | 96h | 48h | 96h | 48h | 96h | 48h | 96h | 48h | 96h | 48h | 96h | |
| PVP-I 10% in pet. | + | ++ | +? | +++ | ++ | ++ | ++ | +++ | + | + | + | + | + | ++ |
| PVP-I 5% in pet. | (−) | (−) | (−) | ++ | + | + | ++ | +++ | (−) | (−) | (−) | (−) | + | + |
| PVP-I 1% in pet. | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| PVP-I 10% in water | ++ | + | +++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | + | ++ | + |
| PVP-I 5% in water | ++ | + | ++++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | + | ++ | + |
| PVP-I 1% in water | (−) | (−) | ++++ | + | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| Dressings, as supplied | NP | NP | (−) | (−) | (−) | (−) | NP | |||||||
Abbreviations: NP, not performed; pet, petrolatum.
In the control group of 30 individuals, patch testing with PVP-I at 10%, 5%, and 1% in petrolatum was negative in all volunteers.
Patch tests with 10% PVP-I in water were positive (+? and +) in 9 controls at 48 hours, but became negative in 3 of these 9 patients at 96 hours. Patches with 5% PVP-I in water were also positive in 4 of these 9 cases.
The patches with 1% PVP-I in water were negative in all 30 controls.
DiscussionContact dermatitis caused by solutions containing PVP-I is a more common adverse reaction after surgery than previously thought and may be underdiagnosed. It should be suspected when dermatitis develops around the surgical wound or in the operating field, although only patients with more intense manifestations tend to be referred to a dermatologist. This dermatitis is typically limited to the area occluded by the surgical dressing or covered by the drapes used during the operation, and is usually more intense and with more clearly defined borders where there is greater pressure and occlusion; it does not necessarily or commonly affect the surgical wound itself. Dermatitis arising on other areas of the skin, at a distance from the operating field, can cause diagnostic doubt, even for the specialist. In these cases, the dermatitis may be aggravated by occlusion for hours against absorbent pads, sheets, or the surface of the operating room stretcher or table, or under the return electrode or cables of the electric scalpel, drainage tubes, etc., all of which can become impregnated or covered with PVP-I solution when it is applied generously before the operation.
Our 7 patients were initially diagnosed with irritant contact dermatitis due to PVP-I, as the open test (ROAT) with Betadine solution on healthy skin was negative. The clinical appearance in some patients, with well-defined borders of the dermatitis, also suggested this diagnosis. However, we were interested by the type of individual susceptibility or idiosyncrasy of the skin of these patients that could cause them to develop such intense irritant reactions, as, under the same conditions, the majority of surgical patients in our hospital tolerate PVP-I well.
This observation led us to consider the possibility that contact dermatitis caused by solutions of PVP-I after surgery involved not only an irritant but also an allergic mechanism, and that PVP-I becomes a complete antigen only after it becomes associated with transport proteins in the skin of these patients, and this requires occlusion to be maintained for a certain length of time.
Although in general we agree with Lachapelle14 that ROAT on healthy skin is the most suitable tool to confirm (or exclude) allergic sensitization to a given product, this is not always the case. Experience has shown us that there may be exceptions and that ROAT is not necessarily positive, for example, in patients sensitized to volatile allergens, such as ethyl alcohol.15 We believe that the same may occur with the aqueous PVP-I solution, though for a different reason. This compound may require a sealed environment (which occurs with patch testing but not with ROAT) to trigger contact dermatitis in sensitized patients.
A number of studies have given rise to controversy regarding the solvents that should be used and the concentrations of PVP-I and iodine that should be analyzed in the patch tests. Whilst Lee et al.10considered that a 10% concentration in water should be used, Lachapelle14 considered that 10% PVP-I in water is still irritant. Kouzuka12 recommended patch testing with 10% PVP-I in water, but allowing the solution to dry before applying the patch. Based on the results obtained in our controls with the patches of 10% and 5% PVP-I in aqueous solution, we consider that this solvent should be excluded. We agree that PVP-I dissolved in water is irritant, but not PVP-I dissolved in petrolatum.
The combination of irritation and allergy in the case of patches with PVP-I in water would explain the greater intensity of the positive patch tests at 48 hours in our patients (with pustular blisters in one of them) compared with the positive tests with PVP-I in petrolatum. At 96 hours, the reactions of PVP-I in water were less intense, whereas the reactions to PVP-I in petrolatum were more intense, as usually occurs only in allergic reactions.
In conclusion, we consider that humidity and occlusion are necessary for contact dermatitis to Betadine to develop after surgical interventions. This therefore leaves many specialists with diagnostic, clinical, and pathogenic doubts, as, in our opinion, this reaction is due to a dermatitis that is both irritant and allergic.
The open test (ROAT) with this antiseptic, which appears to lose its irritant and allergenic properties as it dries on the skin, is usually negative in these patients.
When using patch tests to diagnose this type of contact dermatitis, we suggest using 10% PVP-I in petrolatum, as the results obtained with PVP-I in aqueous solution in patients and in some of the controls lead us to exclude this solvent due to the demonstrated irritability.
Ethical DisclosuresProtection of human and animal subjects.The authors declare that the procedures followed adhere to the ethical guidelines of the responsible committee on human experimentation and comply with the Declaration of Helsinki of the World Medical Association.
Confidentiality of dataThe authors declare that they followed their hospital's regulations regarding the publication of patient information and that written informed consent for voluntary participation was obtained for all patients.
Right to privacy and informed consentThe authors obtained informed consent from the patients and/or subjects referred to in this article. This document is held by the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: de la Cuadra-Oyanguren J, Zaragozá-Ninet V, Sierra-Talamantes C, Alegre de Miquel V. Dermatitis de contacto por povidona yodada tras cirugía: un dilema diagnóstico. Actas Dermosifiliogr. 2014 105:300–304.