This article describes a proposed protocol for the histologic diagnosis of cutaneous melanoma developed for the National Cutaneous Melanoma Registry managed by the Spanish Academy of Dermatology and Venereology (AEDV). Following a review of the literature, 36 variables relating to primary tumors, sentinel lymph nodes, and lymph node dissection were evaluated using the modified Delphi method by a panel of 8 specialists (including 7 pathologists). Consensus was reached on the 30 variables that should be included in all pathology reports for cutaneous melanoma and submitted to the Melanoma Registry. This list can also serve as a model to guide routine reporting in pathology departments.
El presente texto es una propuesta de protocolo de diagnóstico histológico para el melanoma cutáneo, realizado a instancias del Registro Nacional de Melanoma de la Academia Española de Dermatología y Venereología. Tras una búsqueda bibliográfica, un grupo de 8 panelistas (7 patólogos) decidieron entre 36 variables del tumor primario, el ganglio centinela y la linfadenectomía incluir un total de 30 variables mediante el método de Delphi modificado. Se han consensuado las variables que deberían contener un informe histológico de melanoma cutáneo para que puedan ser utilizadas en el Registro de Melanoma o servir de modelo para los distintos Servicios de Anatomía Patológica a la hora de elaborar sus propios informes de forma rutinaria.
The annual incidence of cutaneous melanoma in Spain is 8.7 cases per 100,000 population.1 This corresponds to approximately 4000 new cases every year. Access to incidence and mortality data and a system containing standardized information would facilitate melanoma research in Spain and provide valuable epidemiologic data. This information is available in provinces with cancer registries. The Spanish National Cutaneous Melanoma Registry was created in 1997 under the auspices of the Spanish Academy of Dermatology and Venereology (AEDV) and has gone through 2 organizational phases over the years.2
One of the main drawbacks of the registry is the lack of a standardized protocol to guide researchers wishing to enter data. Facilitating this task by standardizing the information to be collected, combined with the use of flexible data capture tools,3 could increase participation in the registry. In addition, a uniform reporting protocol could serve as a model to guide routine reporting of cutaneous melanoma by pathology laboratories.
Consensus is lacking on which histologic variables should be included in pathology reports for cutaneous melanoma, although certain associations, such as the College of American Pathologists, issue recommendations in the form of checklists that are updated annually.4 Previous work done in Spain includes a proposed protocol for reporting histologic data for primary cutaneous melanomas published by a group of researchers from the Community of Valencia.5
The aim of this study was to achieve consensus on which histologic variables should be recorded in pathology reports for cutaneous melanoma in Spain to facilitate their subsequent inclusion in the National Cutaneous Melanoma Registry.
Material and MethodsThis consensus statement is an initiative of the Spanish National Cutaneous Melanoma Registry’s coordinator (ATV) and the process was managed by the AEDV's Healthy Skin Foundation and the Spanish Pathology Society (SEAP). The different stages in the process are summarized in Appendix B Supplementary Fig. 1.
The Spanish Dermopathology Research Group, which is part of the AEDV, was asked to propose participants with a special interest in cutaneous melanoma. Several melanoma experts were also consulted and asked to propose additional candidates. The expert panel formed to create this consensus statement consisted of 8 experts (7 pathologists and 1 dermatologist), all with extensive experience and numerous publications on the subject.
The first phase of the process consisted of searching similar documents (mainly the most widely used guidelines in this field5–9) to identify commonly used histologic variables. Additional variables were identified by a literature search for potential prognostic factors in PubMed using the terms («Melanoma/pathology»[MAJR]) AND «Prognosis»[MeSH]) AND «Skin Neoplasms/pathology»[MAJR]).
The candidate variables were classified into 3 groups: primary tumor variables, sentinel lymph node (SLN) variables, and lymph node dissection (LND) variables.
These variables were then evaluated by the expert panel using a modified 2-round Delphi approach10 designed to achieve consensus on which variables to include in the protocol for the histologic diagnosis of cutaneous melanoma. The panelists were asked to score each variable according to 1) relevance—potential impact on decision-making or potential prognostic value—and 2) feasibility—ease with which the variable can be measured or evaluated by histology. Each variable was scored on a scale of 1 (not at all relevant/not at all feasible) to 9 (very relevant/very feasible). In the first round of the Delphi process, the panelists were able to suggest other potentially relevant or feasible variables considered not to be adequately covered by the other options. In the second round, they were given the opportunity to revise their scores from the first round. To do this, they were able to consult these scores together with the group scores presented as means, medians, modes, maximums, and minimums. At the end of the 2 rounds, the variables were classified as having achieved sufficient consensus for inclusion (median and mode scores > 7), sufficient consensus for exclusion (median and mode scores < 3), or insufficient consensus for either inclusion or excusion (median and mode scores > 3 and < 6). Variables with median and mode scores outside the above ranges were considered not to have achieved consensus.
Variables with insufficient consensus for either inclusion or exclusion were discussed by the experts at an online meeting to decide whether they should be included in the final document or not. Variables without consensus or with sufficient consensus for exclusion were discarded. Finally, the panel discussed a number of additional points following an external review.
ResultsThirty-six variables (21 primary tumor, 10 SLN, and 5 LND variables) were selected for evaluation following the review of the literature and the main protocols. Consensus for inclusion was reached for 19 variables (8 primary tumor, 6 SLN, and 5 LND variables) in the first round of the modified Delphi process (Appendix B Supplementary Table 1). An additional variable (angiotropism, relating to the primary tumor) proposed by the experts was added during this round. Consensus was reached for an additional 5 variables (all primary tumor variables) in the second round, bringing the total to 25 (Appendix B, Supplementary Table 2). In the final online meeting, consensus was reached for the inclusion of 30 variables—18 primary tumor, 7 SLN, and 5 LND variables (Appendix B supplementary table 3)—in the final version of the proposed protocol for the histologic diagnosis of cutaneous melanoma (Table 1).
Proposed Pathology Report for Cutaneous Melanoma.
| Cutaneous Melanoma Protocol | |
|---|---|
| I. Primary tumor biopsy | |
| 1. Location | ― …………………………………. |
| 2. Type of biopsy | Excisional Incisional Shave Punch Other:_____________ |
| 3. Macroscopic tumor diameter (mm) | ― …………………………………. |
| 4. Melanoma | In situInvasive |
| 5. Primary tumor (Breslow) thickness (mm) | ― ____ mm At least: _____ mm |
| 6. Ulceration | NoYes |
| 7. Clark level | IIIIIIIVMAt leastCannot be determined |
| 8. Mitotic rate | ― ____ mitoses/mm2 |
| 9. Histologic subtype | ―Superficial spreading melanoma―Melanoma maligna lentigo―Desmoplastic melanoma ―Spitzoid melanoma―Acral lentiginous melanoma―Mucosal melanoma―Melanoma arising in congenital nevus―Melanoma arising in blue nevus―Uveal melanoma ―Nodular melanoma |
| Tumor-infiltrating lymphocytes | DensityBriskNonbriskAbsent |
| LocationIntratumoralPeritumoralBothNeither | |
| Neurotropism | ―Absent―Present |
| Lymphovascular invasion | ―Absent―Present |
| Angiotropism | ―Absent―Present |
| Microsatellitosis | ―Absent―Present |
| Regression | ―Absent―Present ― < 75% ― > 75% |
| Association with nevus: | ―No―Congenital―Blue―Acquired―Dysplastic―Other:_________________ |
| Margin involvement | ―No―Lateral margin: distance_____ mmAt least_____ mmNot assessable ___________―Deep margin:distance_____ mmAt least_____ mmNot assessable:___________ |
| Pathologic stage | ___ pTX: Primary tumor thickness cannot be assessed (e.g., diagnosis by curettage) ___ pT0: No evidence of primary tumor (e.g., completely regressed melanoma) ___ pTis: Melanoma in situ___ pT1: Melanoma 1.0 mm or less in thickness, ulceration status unknown or unspecified ___ pT1: Melanoma < 0.8 mm in thickness, no ulceration___ pT1b: Melanoma < 0.8 mm in thickness with ulceration, or melanoma 0.8–1.0 mm in thickness with or without ulceration ___ pT2: Melanoma > 1.0–2.0 mm in thickness, ulceration status unknown or unspecified ___ pT2a: Melanoma > 1.0–2.0 mm in thickness, no ulceration ___ pT2b: Melanoma > 1.0–2.0 mm in thickness, with ulceration ___ pT3: Melanoma > 2.0–4.0 mm in thickness, ulceration status unknown or unspecified ___ pT3a: Melanoma > 2.0–4.0 mm in thickness, no ulceration___ pT3b: Melanoma > 2.0–4.0 mm in thickness, with ulceration ___ pT4: Melanoma > 4.0 mm in thickness, ulceration unknown or not specified ___ pT4a: Melanoma > 4.0 mm in thickness, no ulceration ___ pT4b: Melanoma > 4.0 mm in thickness, with ulceration |
| Sentinel lymph node biopsy | |
| Number of lymph nodes sent or found | ― nodes |
| Number of positive lymph nodes | ― nodes |
| Size of largest metastatic deposit | ― mm |
| Location of metastasis in sentinel lymph node | ―Subcapsular―Parenchymal―Combined―Multifocal ―Extensive―Cannot be determined |
| Extranodal extension | ―Present ―Absent ―Cannot be determined |
| Number of metastatic deposits | ―1―2−5―6−10―11−20―>20―Cannot be determined |
| Matted nodes | ―Absent―Present |
| Lymphadenectomy | |
| Number of lymph nodes submitted or found | ―nodes |
| Number of lymph nodes with metastatic deposits: | ―nodes |
| Lymph node ratio (number of positive nodes/total nodes examined): | ― |
| Matted nodes | ―Absent―Present |
| 5. Pathologic stage | ―pN0: No regional lymph node metastasis detected ―pN1: One tumor-involved node or microsatellites and/or satellites or in-transit metastases with no tumor-involved nodes ―pN1a: One tumor-involved node (e.g., detected by sentinel lymph node biopsy) with no microsatellites and/or satellites or in-transit metastases ―pN1b: One clinically detected tumor-involved node without satellitosis or in-transit metastases ―pN1c: Presence of microsatellites and/or satellites or in-transit metastases with no tumor-involved nodes ―pN2: Metastasis in 2 or 3 lymph nodes or microsatellites and/or satellites or in-transit metastases with just 1 tumor-involved node ―pN2a: Two or 3 clinically occult tumor-involved nodes (e.g., detected by sentinel lymph node biopsy) with no microsatellites and/or satellites or in-transit metastases ―pN2b: Two or 3 tumor-involved nodes at least 1 of which was clinically detected, with no microsatellites and/or satellites or in-transit metastases―pN2c: One clinically occult or clinically apparent tumor-involved node with microsatellites and/or satellites or in-transit metastases―pN3: Metastasis in 4 or more regional lymph nodes or microsatellites and/or satellites or in-transit metastases with 2 or more involved regional lymph nodes or any number of matted nodes―pN3a: Four or more clinically occult tumor-involved nodes (e.g., detected by sentinel node biopsy) with no microsatellites and/or satellites or in-transit metastases ―pN3b: Metastasis in 4 or more lymph nodes, at least 1 of which was clinically detected, with no microsatellites and/or satellites or in-transit metastases ―pN3c: Metastasis in 2 or more clinically occult or clinically detected nodes with microsatellite, satellite and/or in-transit metastases or any number of matted nodes with microsatellites and/or satellites or in-transit metastases |
The aim of this study was to propose a set of primary tumor, SLN, and LND histologic variables that should systematically be included in pathology reports for cutaneous melanoma.
Although the main aim of creating a protocol for the histologic evaluation of cutaneous melanoma was to standardize the reporting of melanoma data in the Spanish National Cutaneous Melanoma Registry, there is no doubt that the content of the protocol can serve as a model for melanoma reports issued by pathology or dermatopathology laboratories.
It is important to note that apart from the variables required for correct melanoma staging by the American Joint Committee on Cancer (AJCC),11 the protocol contains other variables that the panel considered to be potentially relevant in terms of their prognostic value or usefulness in decision-making. Most of these variables are also used in existing protocols.7,8 Not all the variables selected for evaluation were included in the final protocol as the experts did not reach consensus on their relevance or feasibility. One example is ulceration width (part of the College of Amercian Pathologists’ protocol7), which was considered to be particularly prone to subjective interpretation. The presence of nevus cells in SLNs (featured in the European Organisation for Research and Treatment of Cancer [EORTC] protocol for the evaluation of SLNs in melanoma8) was also excluded, as it was considered that discrimination of these cells is relatively straightforward.
Other variables were excluded because it was considered that they did not add any new information not already covered (e.g., the SLN S-classification12) or because they involved particular difficulties and were not supported by sufficient evidence (e.g., SLN metastasis mitotic rate).13
Although tumor-infiltrating lymphocyte (TIL) density is also prone to subjective interpretation, it achieved sufficient consensus for inclusion, mainly because of its potential relevance in the new era of immunotherapy for melanoma.14
The variables included in the final protocol for the histologic diagnosis of melanoma are defined and briefly discussed below.
Primary Tumor VariablesLocationPathologists should be informed of the location of the melanoma and in turn specify this in the pathology report, as patients may have more than 1 tumor. In addition, prognosis varies according to location. Melanomas on the upper and lower limbs (not counting the hands and feet) are associated with a better prognosis.15,16
Type of BiopsyAn excisional biopsy is generally recommended.17 Although biopsy type (excisional, incisional, shave, or punch) has not been associated with differences in survival, it has been linked to differences in Breslow thickness and percentage of positive margins (especially in the case of shave biopsies).18
Macroscopic Tumor DiameterAlthough macroscopic tumor diameter is considered a prognostic factor in squamous cell carcinoma, this is not currently the case for melanoma. Nonetheless, several studies have reported a link between larger diameter and worse prognosis.19,20 Tumor diameter has also been used to calculate tumor volume as a prognostic factor in melanoma,21 and some authors have started to use it to calculate tumor growth rate.22
Melanoma In Situ Versus Invasive MelanomaMelanoma in situ is confined to the epidermis. As such, there is no basement membrane involvement. Although melanoma in situ is considered to be virtually curable, mortality has been described in some series.23
Maximum Tumor (Breslow) ThicknessMaximum tumor thickness must be measured using a calibrated ocular micrometer. It is measured from the upper edge of the granular layer (or stratum spinosum in the absence of this layer) to the deepest point of the tumor. If the tumor is ulcerated, the starting point for the measurement is the base of the tumor.
Breslow thickness is the most powerful prognostic factor in melanoma and is included in all AJCC staging systems.11 The measurement must be rounded to the nearest tenth of a millimeter (e.g., 0.1 mm) and not hundredth of a millimeter (e.g., 0.01 mm), as recommended in previous classifications. Accordingly, a Breslow thickness of between 0.75 and 0.84 mm must be rounded to 0.8 mm and reported as T1b, while one of between 1.01 and 1.04 mm must be reported as 1.0 mm.24
It may be difficult to measure Breslow thickness in tumors arising in a previous nevus or in variants such as nevoid melanoma with maturing nevus cells in the dermis. Periadnexal extensions can also make measurement more difficult and should not be counted in Breslow thickness measurements.25
If the deepest point is invaded by the tumor, Breslow thickness should be reported as "… at least ____ mm”.
Breslow thickness in polypoid melanomas should be measured using the same points as above (top of the granular layer to the deepest point of the tumor). Clark level is not a valid measure in polypoid tumors and should not be reported.
Breslow thickness is not a sum of measurements. In other words, the thickness of a melanoma observed on re-excision for positive margins cannot be added to the thickness calculated in the initial biopsy specimen.
It may also be difficult to measure Breslow thickness in melanomas located around hair follicles. There are 3 possible situations:
- A
A melanoma extending down from a hair follicle and then invading the perifollicular dermis
- B
A folliculotropic melanoma invading the follicle from the dermis
- C
A primary follicular melanoma extending to the dermis
In the second case (folliculotropic melanoma invading the follicle from the dermis), Breslow thickness would be measured as usual, that is, from the upper edge of the granular layer to the deepest point of the melanoma. In the other 2 cases, however, it would be more correct to measure from the innermost layer of the outer root sheath, perpendicular to the main axis of the follicle, to the furthest point of the melanoma.9
To avoid these problems, 3 thickness measurements are usually made for perifollicular melanoma:
- A
Breslow thickness: measured from the granular layer of the epidermis to the deepest point of the nonperifollicular melanoma.
- B
Follicular Breslow thickness: measured from the granular layer of the epidermis to the deepest point of the perifollicular melanoma.
- C
Follicular thickness: measured from the innermost layer of the outer root sheath, perpendicular to the main axis of the follicle, to the furthest point of the melanoma.
Breslow thickness may also be difficult to measure in acral skin when there is extensive epidermal hyperplasia. In such cases, the pathologist should add a note specifying that much of the thickness reported is due to this hyperplasia. Where possible, the thickness of the epidermal hyperplasia should also be measured and specified.
An additional challenge in the case of verrucous (papillated) melanoma is that Breslow thickness varies enormously from the base to the apex of the papillae. The recommended strategy in such cases is to measure the thickness from a point halfway between the base and apex to the deepest point of the melanoma.26
UlcerationUlceration, that is the complete disappearance of the overlying epithelium, is associated with a worse prognosis in melanoma. It is included in the AJCC staging system.11 As ulceration is an adverse prognostic factor, its presence will result in an upstaging from “a” to “b” for any thickness (T). True ulceration is characterized by the presence of a tissue reaction to loss of epidermis with fibrin and acute inflammation.27 Although ulceration width may have prognostic implications,28 it did not achieve sufficient consensus for inclusion in this protocol.
Clark Level (Depth)Clark level reflects the depth of a melanoma from the epidermis to the subcutaneous tissue. It is measured on a scale of I to V. It was used as a staging criterion for thin melanomas in earlier AJCC classifications.29,30 The panelists considered that it may influence decision-making in certain cases, especially thin melanomas.
Dermal Mitotic RateAlthough dermal mitotic rate is not part of the AJCC staging system, it has been demonstrated to have prognostic value.31,32 The correct way to measure it is to identify the dermal hot spot (area of the dermis with the greatest mitotic activity) and then count the number of figures in an area corresponding to 1 mm2. The result should be reported as a full number. If no mitotic figures are observed, the pathologist should report the mitotic rate as 0 mitoses/mm2 and not as < 1 mitosis/mm2 or “not identified”.
According to the National Comprehensive Cancer Network (NCCN), a high dermal mitotic rate is an indication for SLN biopsy in patients with thin melanoma.17 A mitotic rate of more than 2 mitoses/mm2 has been linked to a high risk of SLN positivity in thin melanoma.33
Histologic SubtypeHistologic subtype is based on the 2018 World Health Organization classification of melanomas, which takes into account UV radiation exposure, cellular origin, and genetic characteristics or evolutionary pathways.34
Tumor-Infiltrating LymphocytesTILs are regarded as a host response to the tumor. Their density has been linked to prognosis.35
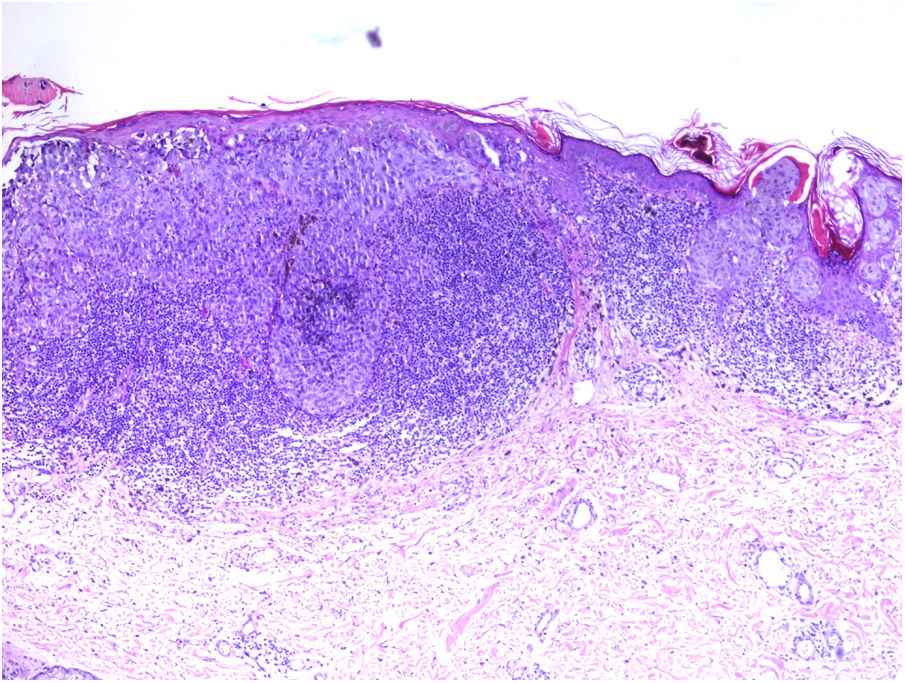
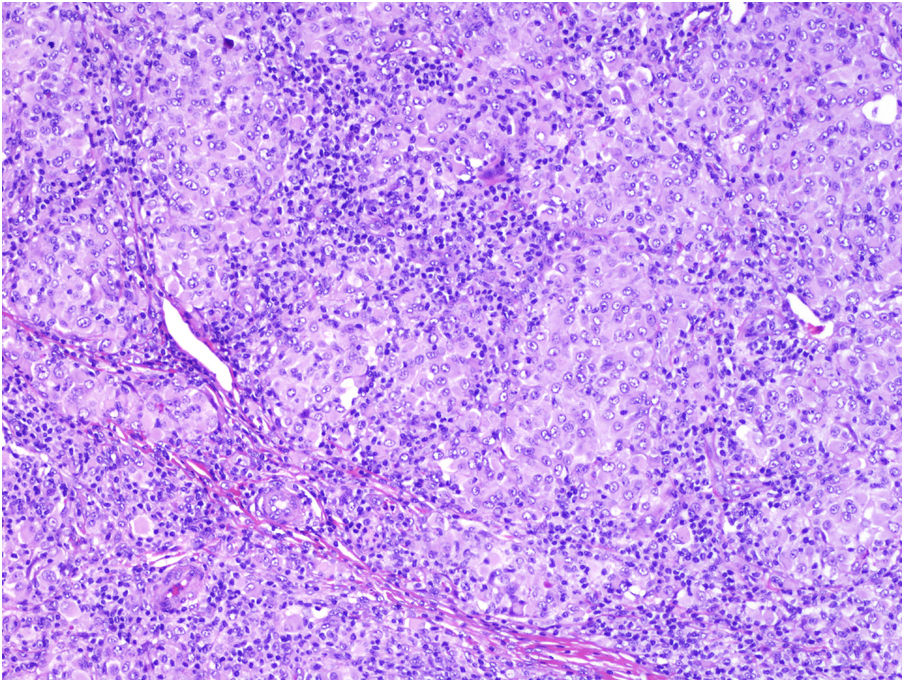
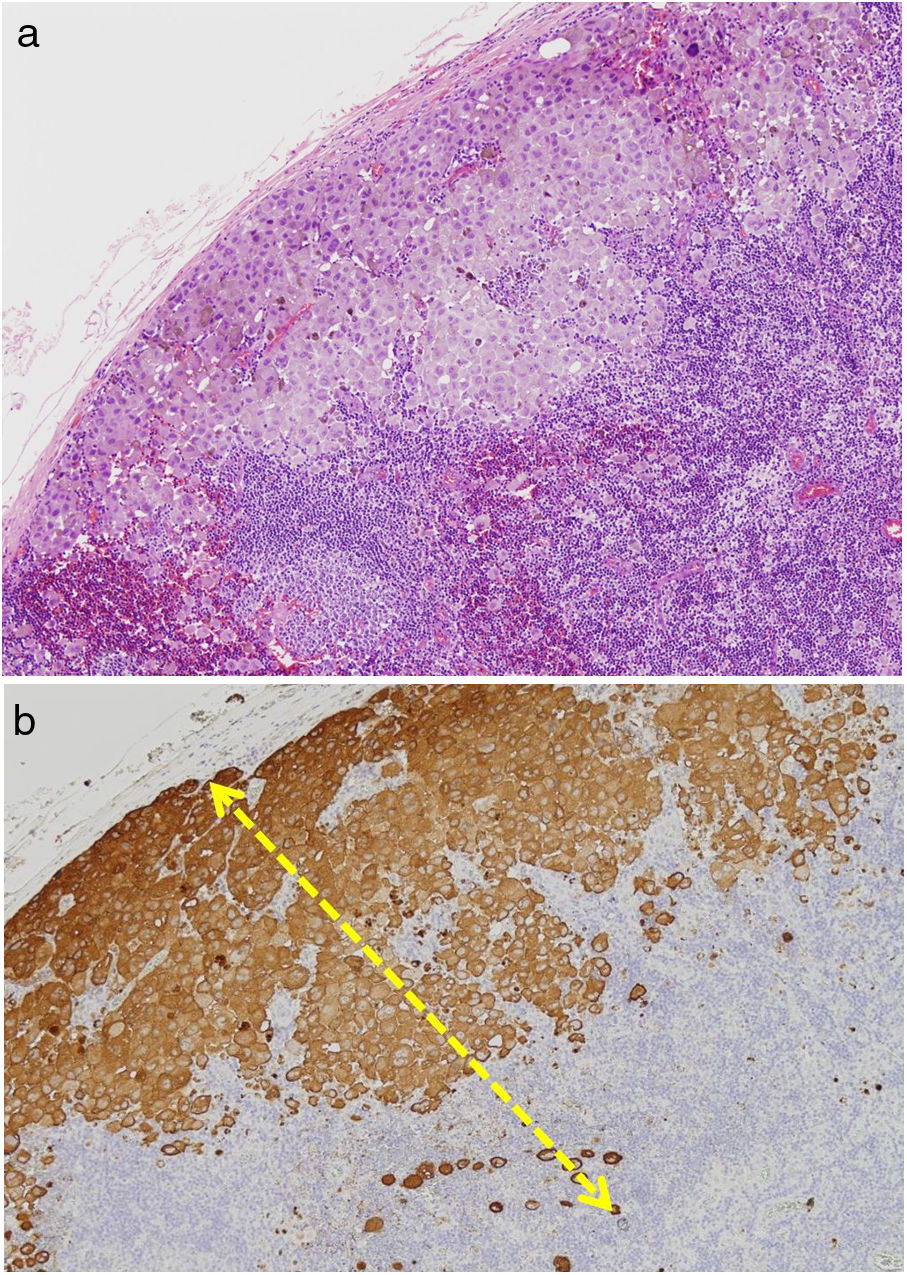
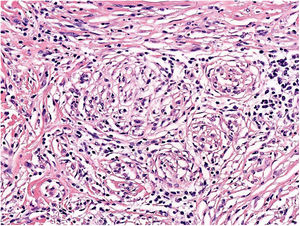
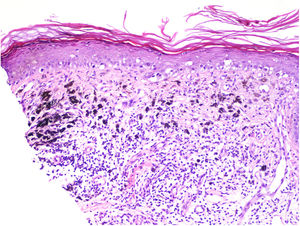
TILs should be classified as absent (not identified or identified but not in contact with the tumor), brisk (infiltration of the entire base of the tumor [Fig. 1) or diffuse infiltration of the tumor) (Fig. 2), or nonbrisk (focal infiltration or infiltration of part of the base of the tumor).
The pathology report should also specify whether the infiltrate is intratumoral, peritumoral, or both.
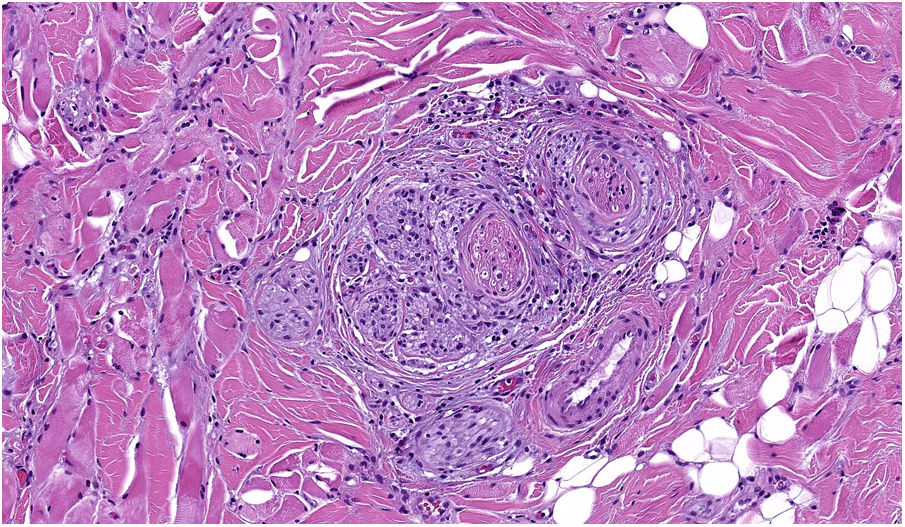
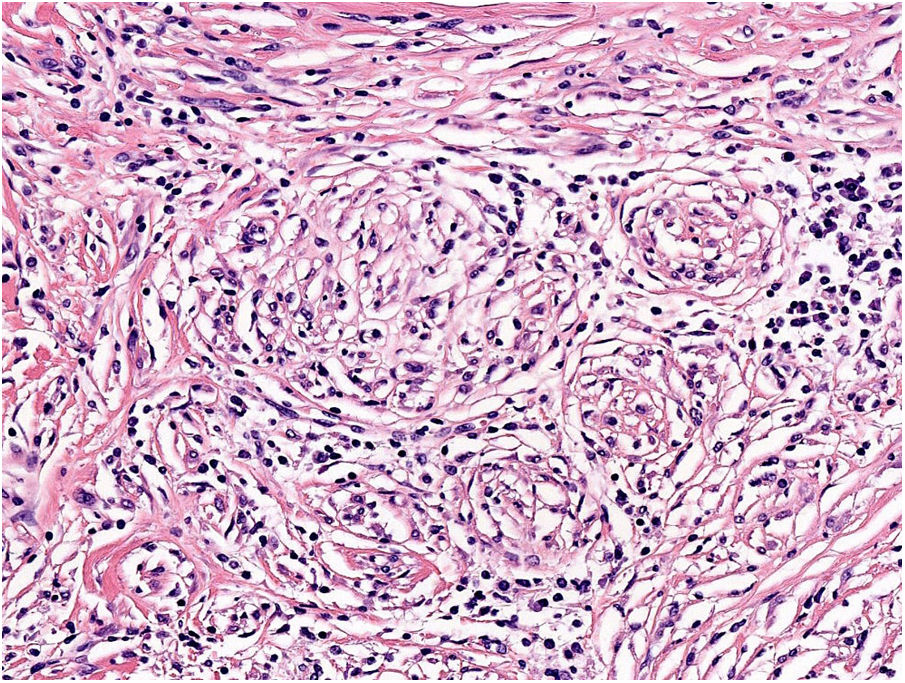
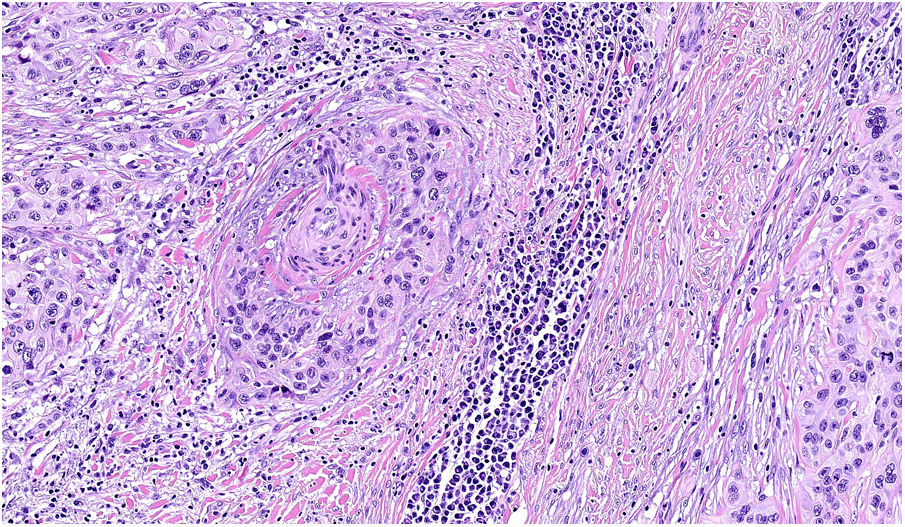
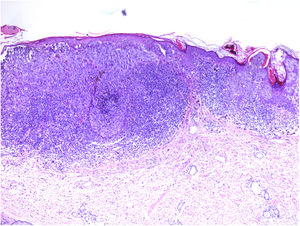
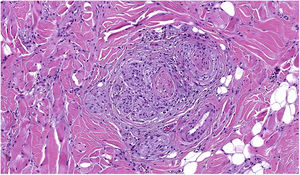
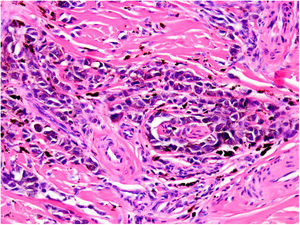
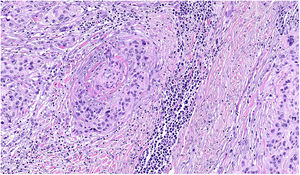
NeurotropismNeurotropism is defined as the presence of melanoma cells either adjacent to nerve sheaths, usually circumferentially (perineural invasion) (Fig. 3), or within a nerve (intraneural invasion) (Fig. 4). It is more commonly seen at the periphery of the tumor. Nerve entrapment due to an expanding tumor should not be regarded as neurotropism. Neurotropism is often observed in desmoplastic melanomas. It can sometimes extend beyond the primary tumor and is therefore associated with a higher risk of local recurrence.36 Neural differentiation in melanomas, generally desmoplastic, is also considered to be a form of neurotropism (Fig. 5).10
Lymphovascular invasion is defined as the unequivocal presence of endothelium-attached tumor cells within the lumina of lymphatic or blood vessels. Immunohistochemical staining with D2-40 and CD31 together with melanocytic cell markers is sometimes used to aid visualization. Lymphovascular invasion is associated with a worse prognosis.37,38
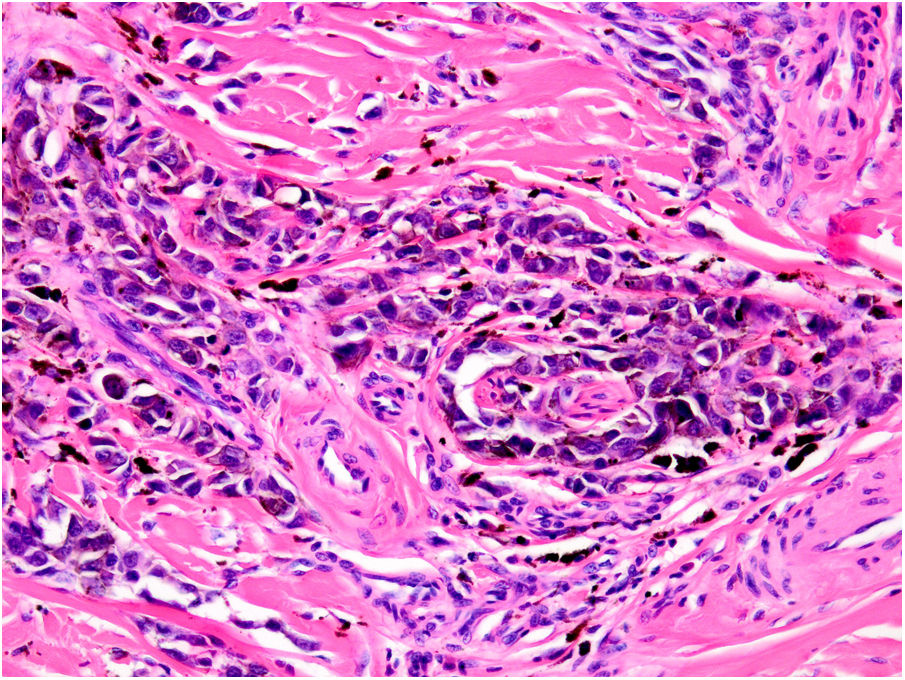
AngiotropismAngiotropism is defined as the presence of melanoma cells in perivascular spaces, similar to perineural invasion; the cells are considered to act in a pericytic manner (pericytic mimicry) without intravasation (Fig. 6). Angiotropism has been associated with an increased risk of metastasis.39
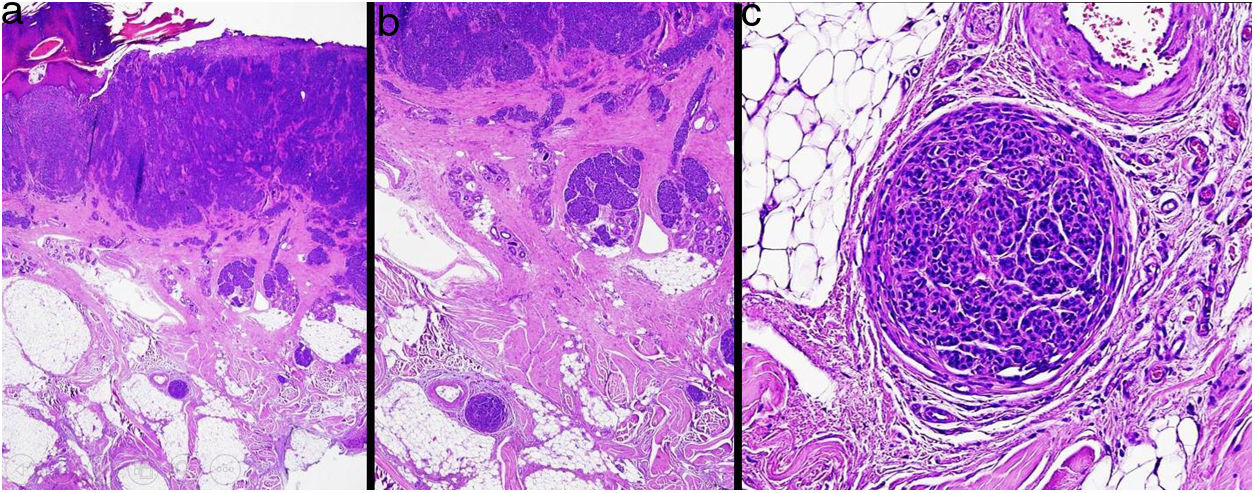
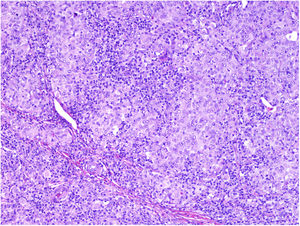
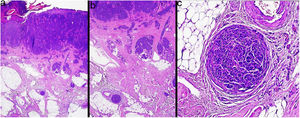
MicrosatellitosisMicrosatellitosis is defined as the presence of micrometastases adjacent or deep to the primary tumor. It is visualized as a discontinuous nest of metastatic cells separated from the primary tumor by normal skin (Fig. 7A-C). The minimum size and distance requirements specified in the 7th edition of the AJCC Cancer Staging Manual no longer apply. It is advisable to check other tissue sections to ensure that the cells truly correspond to microsatellitosis and are not a continuation of the tumor or an extension of eccrine sweat glands.24
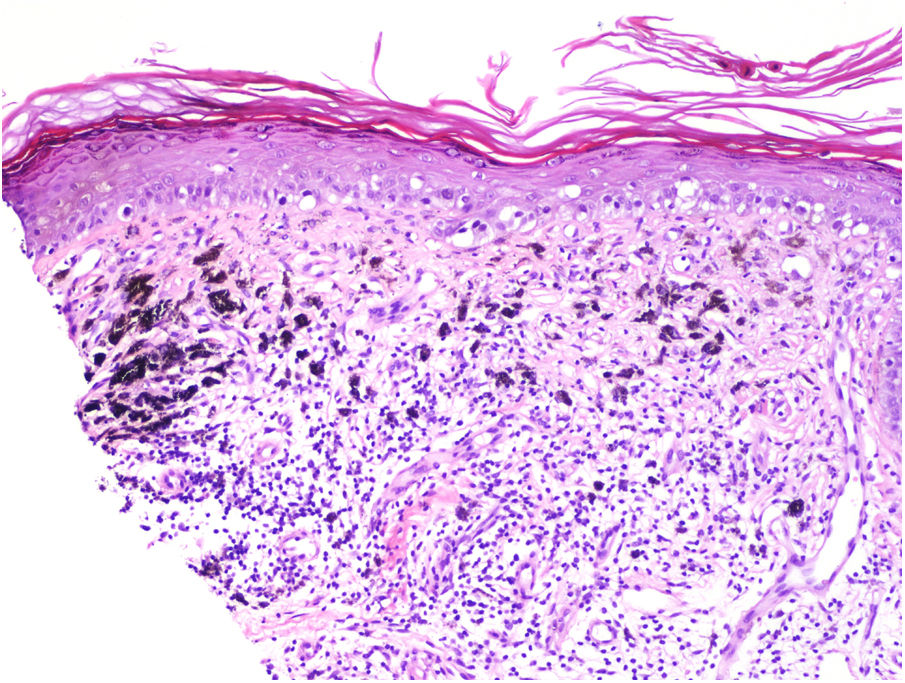
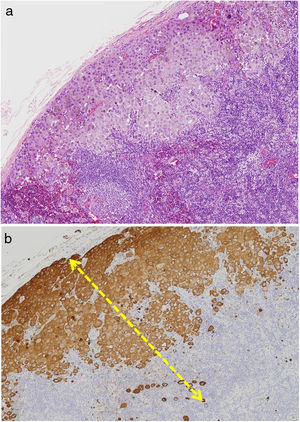
Tumor RegressionRegression in melanoma is regarded as a host response to the presence of the tumor. Characteristic features include replacement of tumor cells by lymphocytic inflammation, attenuation of the epidermis, and nonlaminated dermal fibrosis with inflammatory cells, melanophagocytosis, and increased microvascular density (Fig. 8). The panelists recommend calculating the percentage of regression on the horizontal surface of the tumor and specifying this as > 75% or < 75% in the pathology report.40,41
Excision Margins and Distance to Lateral and Deep MarginsIt is essential to know margin status and distance from the melanoma to the deep and lateral margins, as based on current recommendations, the surgical margins used (0.5–2 cm) should be proportional to the thickness of the tumor.17 Clinical and histologic margins are not closely correlated in melanoma. It has also been demonstrated that tumors with a larger diameter will need wider margins to achieve clearance.17,42,43
Regional Lymph Node Metastasis VariablesNumber of SLNs AnalyzedNumber of SLNs removed has been associated with prognosis and false-negative rate.44
Number of Positive SLNsNumber of positive SLNs is a prognostic factor in the 8th version of the AJCC staging manual.11
Size of Largest Metastatic Deposit in SLNWhile not currently considered a staging criterion by the AJCC, size of the largest metastatic deposit in SLNs should be specified in pathology reports as it has been correlated with survival (Fig. 9).24,45
Location of SLN MetastasesThe Dewar criteria should be used to report the location of micrometastases (subcapsular, parenchymal, combined, multifocal, or diffuse).46,47
Extranodal ExtensionAlthough extranodal extension is rare in melanoma, its presence in SLNs is predictive of a worse prognosis.48
Number of Metastatic DepositsAlthough the number of metastatic deposits identified in the SLN may vary according to the tissue section analyzed, as recommended by the EORTC this figure should be reported as 1, 2−5, 6–10, 11–20, or > 20.8
Lymph Node Ratio After LNDLymph node ratio, defined as the number of positive lymph nodes out of the total number of lymph nodes excised after LND, has been proposed as a prognostic factor in melanoma.49
ConclusionsThe strength of this study lies in its design, as the Delphi technique is a standardized, validated method for preparing guidelines and quality indicators50 in medicine.
It should be noted, however, that the outcomes of our study may have been biased to some degree by the choice of participants or the lack of evidence on some of the variables excluded from the final protocol. There was significant disagreement among the experts in some cases, meaning that certain variables were not included because of insufficient consensus. They could be reassessed in future updates. One example is the recommendation to report Breslow thickness to a precision of 2 decimal places. Although the AJCC recommends reporting thickness to a single decimal place, there are cases when 2 decimal points are recommended if this is practical or feasible.51 Other discrepancies were related to the presence or absence of solar elastosis as a sign of more or less sun exposure, which forms the basis of the latest World Health Organization classification of cutaneous tumors,52 and the quantification of metastatic deposits in SLNs due to the inherent difficulties and lack of evidence.
In conclusion, we believe that an improved prognostic classification of cutaneous melanoma will ultimately lead to better patient management. It is therefore essential to standardize the reporting of melanoma data to facilitate analysis and subsequent interpretation. We believe that this proposed protocol for the histologic diagnosis of cutaneous melanoma will facilitate and increase participation in the Spanish National Register of Cutaneous Melanoma.
FundingThis study was promoted and funded by the AEDV’s Healthy Skin Foundation. No other entity has funded this project or participated in its design or execution.
Please cite this article as: Tejera-Vaquerizo A, Fernández-Figueras MT, Santos-Briz Á, Ríos-Martín JJ, Monteagudo C, Fernández-Flores Á et al. Protocolo de diagnóstico histológico para muestras de pacientes con melanoma cutáneo. Documento de consenso de la SEAP y la AEDV para el Registro Nacional de Melanoma. Serie de casos. Actas Dermosifiliogr. 2021;112:32–43.