In the event of failure of maintenance therapy with biologic agents for moderate to severe plaque psoriasis, the possible approaches are to switch to another agent or escalate the dose (generally by increased dosing frequency). Knowledge of the economic impact of the 2 alternatives would be extremely useful for therapeutic decision making.
ObjectiveThe present analysis aimed to determine the moment in which the annualized additional cost of escalation exceeds a specified cost overrun.
Materials and methodsBased on the purchase cost (average wholesale price) of approved biologics for the treatment of moderate to severe psoriasis, the number of weeks of escalation of the initial biologic until the annualized cost of dose escalation ran €1000 over the cost of switching to another biologic was calculated for a typical patient weighing 80kg.
ResultsAccording to this model, switching to another biologic is always cost effective, with adalimumab followed by ustekinumab the best choices in this respect. Ustekinumab allows for a longer trial escalation period (2 to 4 injections) before the cost overrun threshold is reached, whereas the threshold is reached in a single infusion if a patient is on infliximab.
ConclusionThe study does not take into account the differential efficacy of the various biologic therapies as rescue treatment for failure of maintenance therapy given the lack of scientific evidence. The results nevertheless show substantial differences in the period during which treatment can be intensified before reaching the preset cost overrun.
En caso de fracaso secundario del tratamiento con fármacos biológicos de la psoriasis en placas moderada a grave se puede cambiar de fármaco o intensificarlo de inicio (generalmente aumentando la frecuencia de administración). Conocer el impacto económico de ambas alternativas puede resultar de gran utilidad en la toma de decisiones terapéuticas.
ObjetivoEl presente análisis pretende orientar sobre el momento en que el sobrecoste anualizado de la intensificación supera un umbral de sobrecoste predeterminado.
Material y métodosEn función de los costes de adquisición (precio de venta de laboratorio) de los agentes biológicos aprobados para el tratamiento de la psoriasis moderada a grave se ha estimado el número de semanas que podría intensificarse el biológico de inicio hasta alcanzar un sobrecoste anualizado de 1.000€ con respecto al cambio a otro biológico para un paciente tipo de 80kg de peso.
ResultadosSegún este modelo el cambio de fármaco biológico siempre es más coste efectivo, siendo adalimumab, seguido de ustekinumab, las opciones más eficientes; en caso de intensificación ustekinumab permite una mayor duración del período de prueba (entre 2 y 4 inyecciones) antes de alcanzar el umbral de sobrecoste definido, mientras que este se alcanza al cabo de una infusión en el caso de infliximab.
ConclusiónSi bien este estudio no contempla la eficacia diferencial de las diferentes terapias biológicas como tratamiento de rescate frente a fallo secundario, por existir escasa evidencia científica al respecto, los resultados muestran importantes diferencias en el periodo de tiempo durante el cual se podría intensificar el tratamiento hasta alcanzarse el umbral de sobrecoste definido.
In the event of secondary treatment failure with a biologic agent in moderate to severe plaque psoriasis, it remains unclear whether the best strategy is to switch the patient to another treatment or to escalate the current regimen (usually by reducing the dosing interval). While not mentioned in the Summary of Product Characteristics (SPC), dose escalation, a common strategy in clinical practice, has been studied in extension trials and discussed in various guidelines1 and consensus documents.2
Understanding the economic implications of the alternative strategies for dealing with secondary treatment failure may be very useful to the physician making treatment decisions. Most of the published cost-effectiveness studies do not provide information on the efficiency of dose escalation vs switching to another biologic therapy. Although not discussed in the SPC, dose escalation (an increase in the dose or a reduction in the dosing interval) has been evaluated in clinical trials and/or open-label extension studies.3–7
When determining which of the 2 options is more efficient, the cost of the new strategy must be taken into account as well as clinical criteria, such as the reason for modifying the regimen (gradual loss of response, or a flare, etc.), and the likelihood of response.
The cost of escalation will depend on its intensity and duration, while the cost of switching to a new biologic agent will include the higher cost of induction therapy and the expense of associated visits and diagnostic tests. In a preliminary analysis, the incremental cost of induction with various biologic agents was evaluated at 16 weeks using as a reference the average daily cost during maintenance therapy of the currently available biologic drugs and considering only the acquisition cost of the drugs (without taking into account any potential rebates or discounts).8 The escalation strategy was associated with a cost increase factor of between 1.2 (ustekinumab, shortening the dose interval from 12 to 10 weeks) and 2 (adalimumab and etanercept), while a switch to a different biologic agent—including the cost of induction therapy—is associated with a cost increase factor ranging from 1.18 (adalimumab) to 1.75 (etanercept) at 16 weeks. This preliminary analysis did not take into account the costs associated with a switch (direct or indirect, tangible or intangible) related to the increased number of visits and laboratory tests required during the induction phase. Moreover, in some patients, the loss of response may be transient, in which case escalation could allow them to continue treatment with the first biologic agent, thereby reducing the likelihood of exhausting currently available treatment options.
For this reason, in this new analysis we have established an arbitrary threshold of €1000 as the highest acceptable incremental cost per year for temporarily escalating biologic treatment vs the alternative strategy of switching to another biologic agent. This figure corresponds to the approximate average acquisition cost of 1 month's treatment with the currently available biologic drugs during maintenance therapy at the doses specified in the SPCs, and also the cost of induction with adalimumab, the biologic agent with the lowest induction cost (2 injections of 40 mg, €1028.29). In addition, we calculated the annualized costs of the different treatment options from the moment the clinical decision is taken either to escalate treatment or to switch to a different biologic agent when the next dose is administered.
The theoretical model used differs from actual clinical practice in various aspects that are impossible to quantify at this time owing to the lack of scientific evidence. In particular, we lack data on the likelihood of response to treatment for each of the possible strategies (measured by a decrease in the Psoriasis Area and Severity Index [PASI] or the Physician's Global assessment [PGA]). Nor do we have much information about the influence of prior treatment on the response to sequential therapy or on the possible therapeutically detrimental effect of antibodies against the p40 subunit common to interleukin (IL)-12 and IL-239 or tumor necrosis factor (TNF) antagonists (anti-TNF).10
Very little data is available on the effectiveness of either an escalation strategy or a switch to a different biologic agent. One strategy proposed in patients with primary or secondary treatment failure with adalimumab has been to increase the dosage to 40 mg weekly. This increase produced a 75% reduction in PASI (PASI 75) or made possible a return to the normal dosage (40 mg every other week) in 27% of patients at 12 weeks and 38% at 24 weeks.4 The patients most likely to respond to escalation (48%) were secondary nonresponders, patients with relatively low weight, and those with a shorter disease duration. When the dosage of adalimumab was escalated in a prospective observational cohort with poor response to treatment, a PASI 50 response was achieved by 25% of the patients at 12 weeks and 35% at 24 weeks; the corresponding percentages for combination therapy with methotrexate and no increase in the dose of the biologic were 9% and 18%, respectively.11
Current scientific evidence is insufficient to support the preferential use of any particular biologic agent when sequential treatment is being considered in patients presenting primary or secondary treatment failure with the first biologic. It has been shown that in patients with primary or secondary failure following 3 to 6 months of treatment with etanercept, a PGA of less than 2 is achieved in 49% of patients switched to adalimumab at week 16,12 and in 65% of patients treated with infliximab at week 1013; a PASI 75 response was observed after 12 weeks in 49% of the patients switched to ustekinumab 90 mg.14 Good responses (not quantified) have also been reported after a switch from infliximab to etanercept15 and from an anti-TNF agent to ustekinumab.16 It has been suggested that the response in patients previously treated with a biologic is somewhat lower when the sequential treatment is etanercept, ustekinumab, or adalimumab but that response to infliximab is not affected by prior exposure to another biologic17
For the sake of simplicity, we have assumed that the survival of the sequential treatment is 100% in all cases at 12 months after a switch or temporary escalation, and we do not consider the possibility of primary or secondary treatment failure with the second biologic. Since the aim of this study was to analyze cost minimization, it does not take differential efficacy into account and assumes that all the patients respond equally to escalation or a switch to another agent.
We present the results of a comparative analysis of the annualized incremental cost of temporary dose escalation compared to a switch to another biologic drug at the standard dose following loss of response to the first biologic agent during maintenance therapy.
Materials and MethodsUsing Excel spreadsheets, we created a series of simulations of the annual cost of treatment from the time the dosage of the first biologic agent is escalated or the initial treatment is replaced by a different drug. For these calculations we used the standard treatment regimens for moderate to severe psoriasis with biologic agents (adalimumab, etanercept, infliximab, and ustekinumab) and the acquisition cost for each of these drugs (price charged by the manufacturer) in Spain as of June 2013.18
In the case of infliximab, the cost of treatment is directly related to the patient's weight because the dosing regimen is weight adjusted (5 mg/kg), and the common practice of optimizing vials by distributing fractions among several patients would also have to be taken into account. Thus, to simplify the simulation, all costs were calculated for a patient weighing 80 kg (4 vials). The published costs associated with intravenous infusion of infliximab in a day hospital in Spain (€247.50) were used.19
The cost of switching to another biologic agent was calculated taking into account the cost of the induction regimen specified in the SPC. In the case of etanercept, the induction dose used was 50 mg twice weekly for 12 weeks because this is the regimen most often used in clinical practice.
The annualized cost was calculated as the manufacturer's sale price plus, in the case of infliximab, the cost of the infusion; the last annual dose was prorated to the number of weeks remaining up to week 52. The annualized cost of induction and maintenance therapy for each of the biologic agents available administered in multiples of weeks is as follows: adalimumab, €13 602 (27.5 injections) vs €12 860 (26 injections); etanercept, €14 580 (64 injections) vs €11 846 (52 injections); infliximab, €17 911 (7.75 infusions of 4 vials) vs €15 022 (6.5 infusions); and ustekinumab €14 681 (5 injections) vs €12 724 (4.33 injections).
In the case of biologics for which the dosage regimen recommended in the SPC is based on multiples of 4 weeks (ustekinumab and infliximab), it has been observed that in clinical practice these are very often administered in multiples of months. This difference is significant from the point of view of cost (1 year has 12 months and 52 weeks, so monthly administration implies an annual saving of 7.7%). Likewise, adalimumab is also often administered twice monthly rather than every other week, producing a similar percentage of savings (2 injections out of 26) on the acquisition cost; however this anomaly is not reflected in the present analysis. Like dose escalation, administration at monthly intervals is not recommended in the SPC but is common in routine clinical practice. Consequently, in the present analysis, when comparing the efficiency of escalation vs switching we considered both possibilities in order to reflect the reality of routine practice.
What was not studied is whether patients sustain an optimal response when they return to the normal dose following a period of dose escalation and it is not, therefore, possible to determine a standard number of weeks after which the patient would return to the dosage recommended in the SPC. Escalation of the current dosage regimen may have advantages that justify an incremental cost of around €1000 (the approximate cost of one month of maintenance therapy with any biologic drug), and in this model €1000 was set as the upper limit of an acceptable annualized incremental cost when a switch to a different biologic agent was not the desired strategy.
Dose escalation is usually more expensive than sequential therapy with another biologic: while it lasts, escalation usually entails a 100% increase in the cost of treatment in the case of adalimumab or etanercept, 33.3% for infliximab, and between 20% and 33.3% for ustekinumab. The annualized incremental cost of induction vs maintenance therapy is approximately 5.8% for adalimumab (27.5 vs 26 injections in 52 weeks), 15.4% for adalimumab (27.5 vs 26 injections), 19.2% for infliximab (7.75 vs 6.5 infusions), and 23.1% for etanercept at a dosage of 50 mg twice weekly (64 vs 52 injections in 52 weeks).
In this model we calculated the number of weeks of the escalated regimen that would be possible without exceeding a cost increment of €1000 euros compared to a switch to another biologic, assuming that from that point onwards it would be possible to return to the dosage specified in the SPC. The incremental cost of maintaining the more intense regimen can be estimated by extending the escalation lines in the figures corresponding to each biologic agent.
ResultsThe results for each biologic agent are presented in the tables and figures.
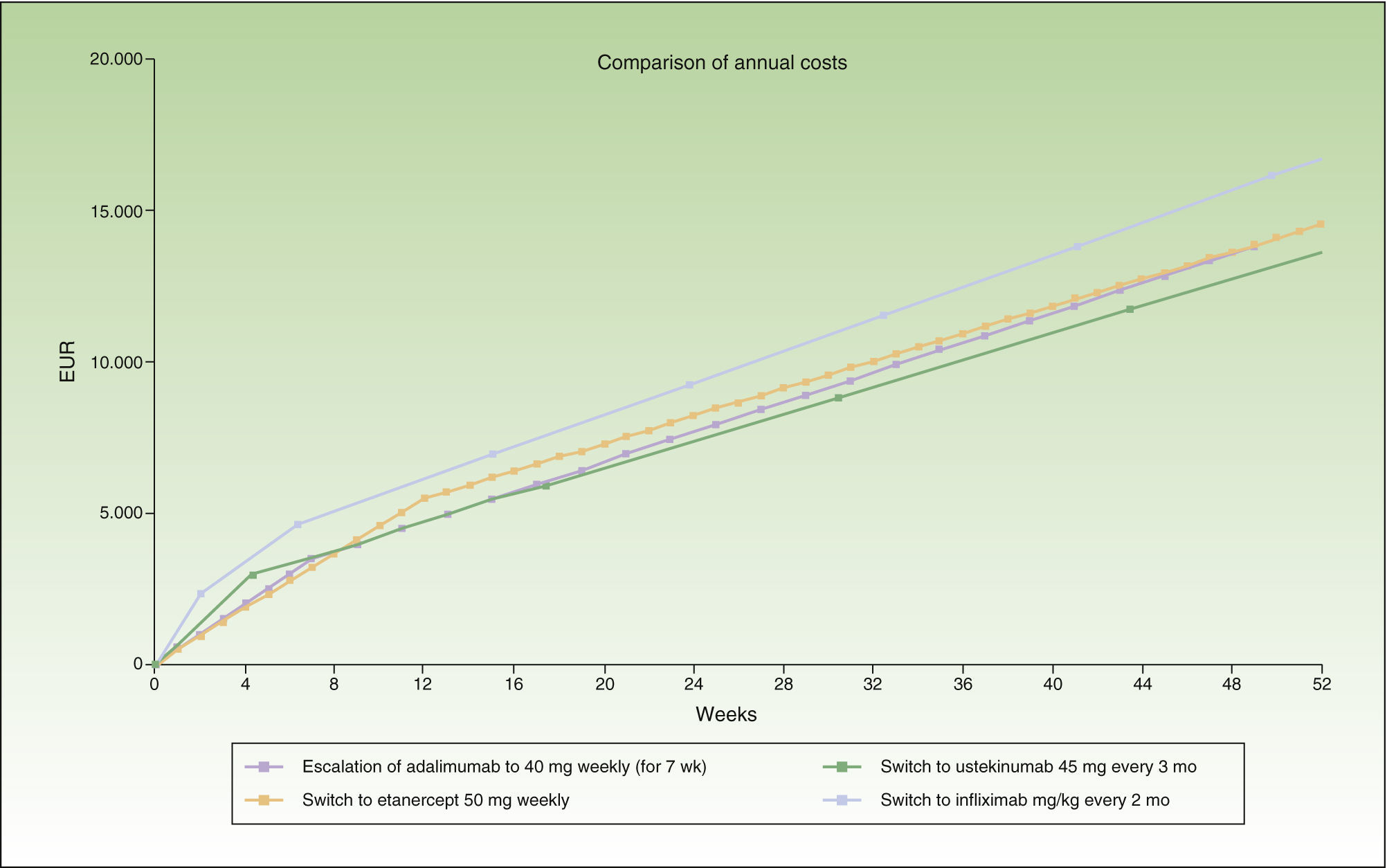
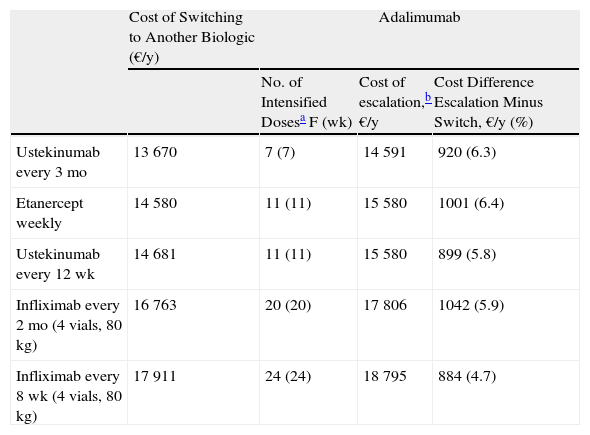
Escalation of Adalimumab Vs. a Switch to Another BiologicIn the case of loss of response to maintenance therapy with adalimumab 40 mg every other week, the options for sequential biologic therapy with an annual cost of about €1000 less than the cost of escalation would be, in descending order of efficiency (ascending annualized cost) (Table 1):
- 1.
Switch to ustekinumab every 3 months: after the eighth weekly dose of adalimumab.
- 2.
Switch to weekly etanercept or ustekinumab every 12 weeks: in both cases, after the 12th dose of adalimumab. In this case, since the difference in cost between the 2 options would be minimal (€102/y), other factors would have to be considered, such as the potentially greater likelihood of response in the case of ustekinumab because it has a different therapeutic target (IL-12 and IL-23), the convenience of the dosing interval (ustekinumab every 12 weeks vs etanercept weekly), etc.
- 3.
Switch to infliximab: after week 25 if infusions in the maintenance phase were administered every 8 weeks, or week 21 if they were administered every 2 months.
Escalation of Adalimumab (Weekly) Compared to Switching to Another Biologic.
| Cost of Switching to Another Biologic (€/y) | Adalimumab | |||
| No. of Intensified Dosesa F (wk) | Cost of escalation,b €/y | Cost Difference Escalation Minus Switch, €/y (%) | ||
| Ustekinumab every 3 mo | 13 670 | 7 (7) | 14 591 | 920 (6.3) |
| Etanercept weekly | 14 580 | 11 (11) | 15 580 | 1001 (6.4) |
| Ustekinumab every 12 wk | 14 681 | 11 (11) | 15 580 | 899 (5.8) |
| Infliximab every 2 mo (4 vials, 80 kg) | 16 763 | 20 (20) | 17 806 | 1042 (5.9) |
| Infliximab every 8 wk (4 vials, 80 kg) | 17 911 | 24 (24) | 18 795 | 884 (4.7) |
No. of doses at the escalated regimen up to a difference between the cost of escalation and the cost of a switch to another biologic as close as possible to €1000/y.
Cost of escalating the initial biologic for the period corresponding to the number of doses in the third column expressed in €/y. This figure includes the cost of the weeks of more intense treatment reflected in the third column plus the cost of the maintenance regimen for the weeks required to complete 1 year of treatment.
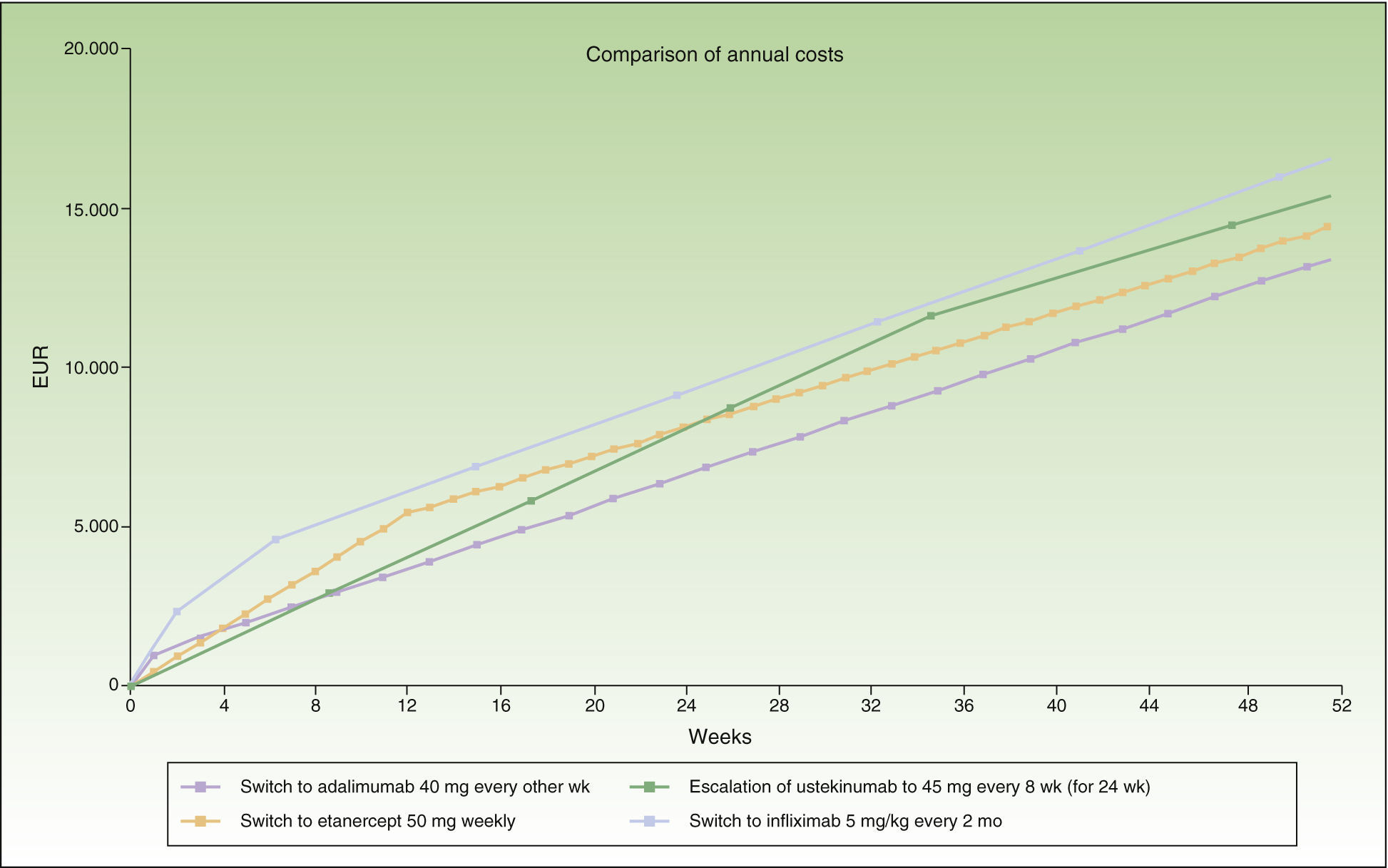
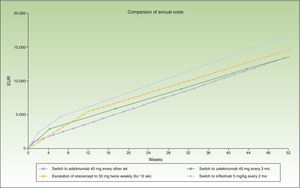
During the first 8 weeks, the cost of switching to ustekinumab is higher than the cost of escalating the dosage of adalimumab or switching to etanercept. At the end of the first year, the cost of switching to ustekinumab every 12 weeks is the same as the cost of switching to etanercept, and a switch to a regimen of ustekinumab every 3 months costs less than etanercept (Fig. 1). A switch to ustekinumab every 3 months is the most efficient alternative after 7 weeks of escalation. After that, the most efficient alternative would be a switch to ustekinumab every 12 weeks or etanercept, with a similar cost. Even assuming that there is no difference in efficacy between these 2 agents in patients with loss of response to an anti-TNF agent, etanercept has the relative disadvantage of requiring more frequent administration. Finally, switching to infliximab would be the least efficient option.
Figure 1 shows the cost of escalating adalimumab for 7 weeks compared to the cost of switching to another biologic agent, in this case infliximab or ustekinumab administered at monthly rather than 4-weekly intervals. Note that the annualized cost (at 52 weeks) is lowest for ustekinumab (€13 670), followed by etanercept (€14 580), adalimumab (€14 591), and infliximab (€16 763).
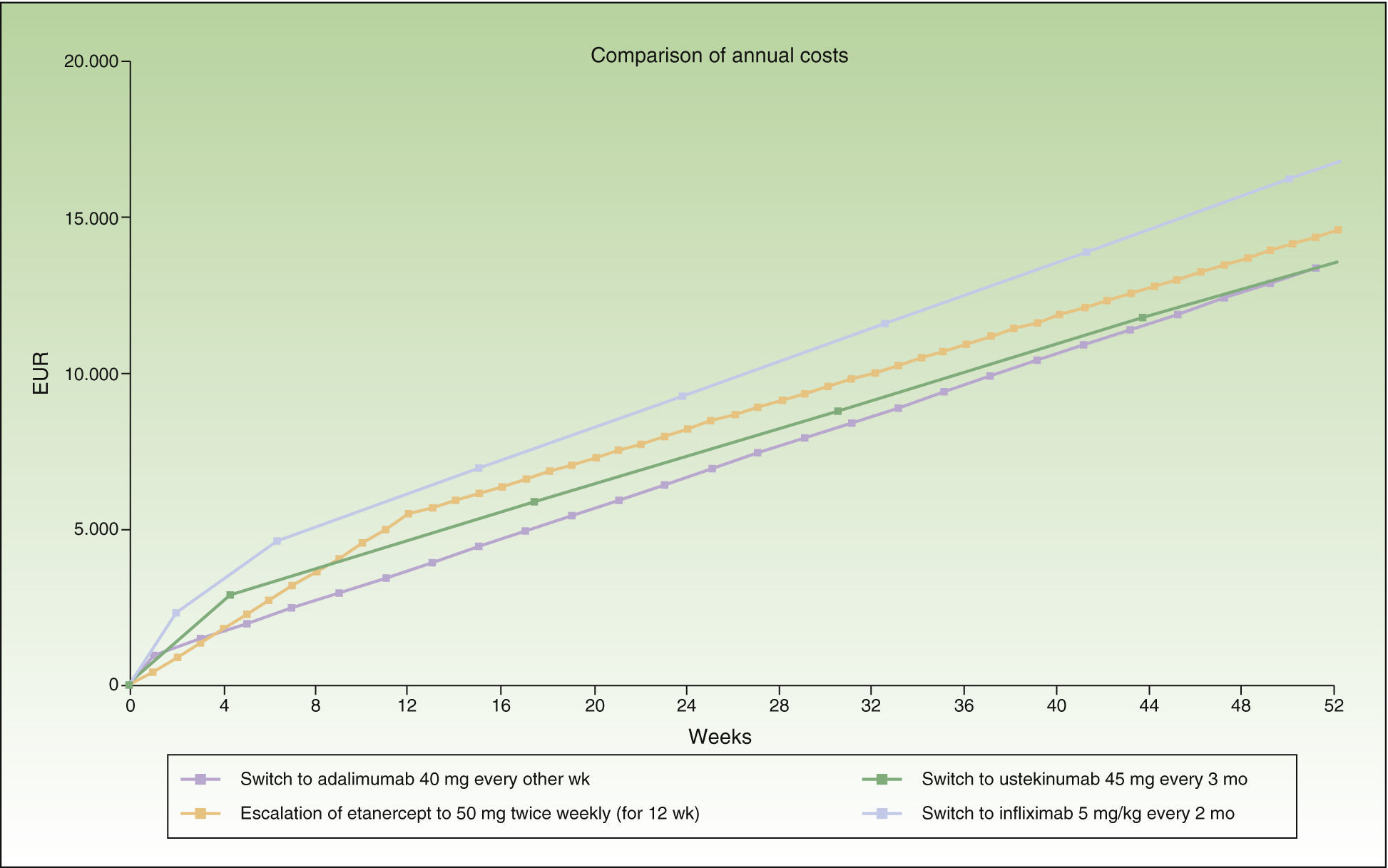
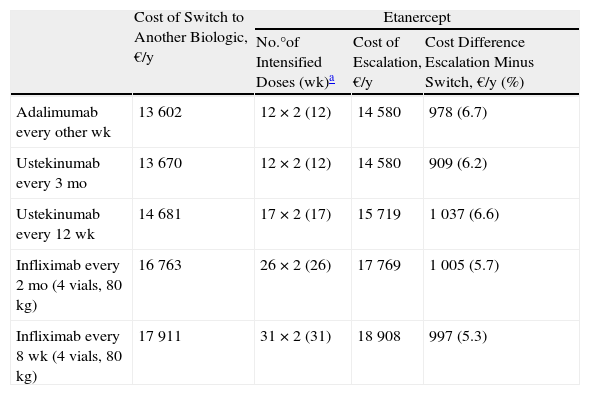
Escalation of Etanercept Compared to Switching to Another BiologicIn the case of a loss of response to maintenance therapy with etanercept 50 mg weekly, escalation to 50 mg twice weekly for up to 12 weeks (12 × 2 units administered) would be an alternative having an acceptable incremental cost (≈€1000/y) compared to the cost of a switch to another biologic (Table 2 and Fig. 2). The options for sequential biologic treatment, in decreasing order of efficiency, would be as follows:
- 1.
Switch to adalimumab every other week or ustekinumab every 3 months after the 13th week. Since the difference in cost between these 2 options would be minimal (€68/y), it would be necessary to consider other factors, such as the potentially greater likelihood of response to ustekinumab because of its different therapeutic target (IL-12 and IL-23), the differences in dose interval (ustekinumab every 3 months vs adalimumab every other week), the cost of maintenance therapy (ustekinumab every 3 months €11 745/y vs adalimumab every other week €12 860/y), etc. (Table 2).
- 2.
Switch to ustekinumab every 12 weeks: after week 18.
- 3.
Switch to infliximab: after week 32 if administered every 8 weeks, or after week 27 if administered every 2 months.
Escalation of Etanercept (50 mg Twice Weekly) Compared to Switching to Another Biologic.
| Cost of Switch to Another Biologic, €/y | Etanercept | |||
| No.°of Intensified Doses (wk)a | Cost of Escalation, €/y | Cost Difference Escalation Minus Switch, €/y (%) | ||
| Adalimumab every other wk | 13 602 | 12 × 2 (12) | 14 580 | 978 (6.7) |
| Ustekinumab every 3 mo | 13 670 | 12 × 2 (12) | 14 580 | 909 (6.2) |
| Ustekinumab every 12 wk | 14 681 | 17 × 2 (17) | 15 719 | 1 037 (6.6) |
| Infliximab every 2 mo (4 vials, 80 kg) | 16 763 | 26 × 2 (26) | 17 769 | 1 005 (5.7) |
| Infliximab every 8 wk (4 vials, 80 kg) | 17 911 | 31 × 2 (31) | 18 908 | 997 (5.3) |
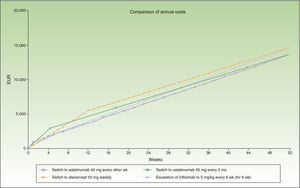
Thus, switching to adalimumab or ustekinumab administered every 3 months would be the most efficient alternatives after 12 weeks of escalation. The next most efficient alternative would be ustekinumab every 12 weeks, and finally infliximab.
Figure 2 shows the cost of escalating etanercept for 12 weeks compared to the cost of the switch to another biologic, considering infliximab and ustekinumab with a month-based maintenance dosage. The annual cost is lowest in the case of adalimumab (€13 602), followed by ustekinumab (€13 670), etanercept (€14 580), and finally infliximab (€16 763).
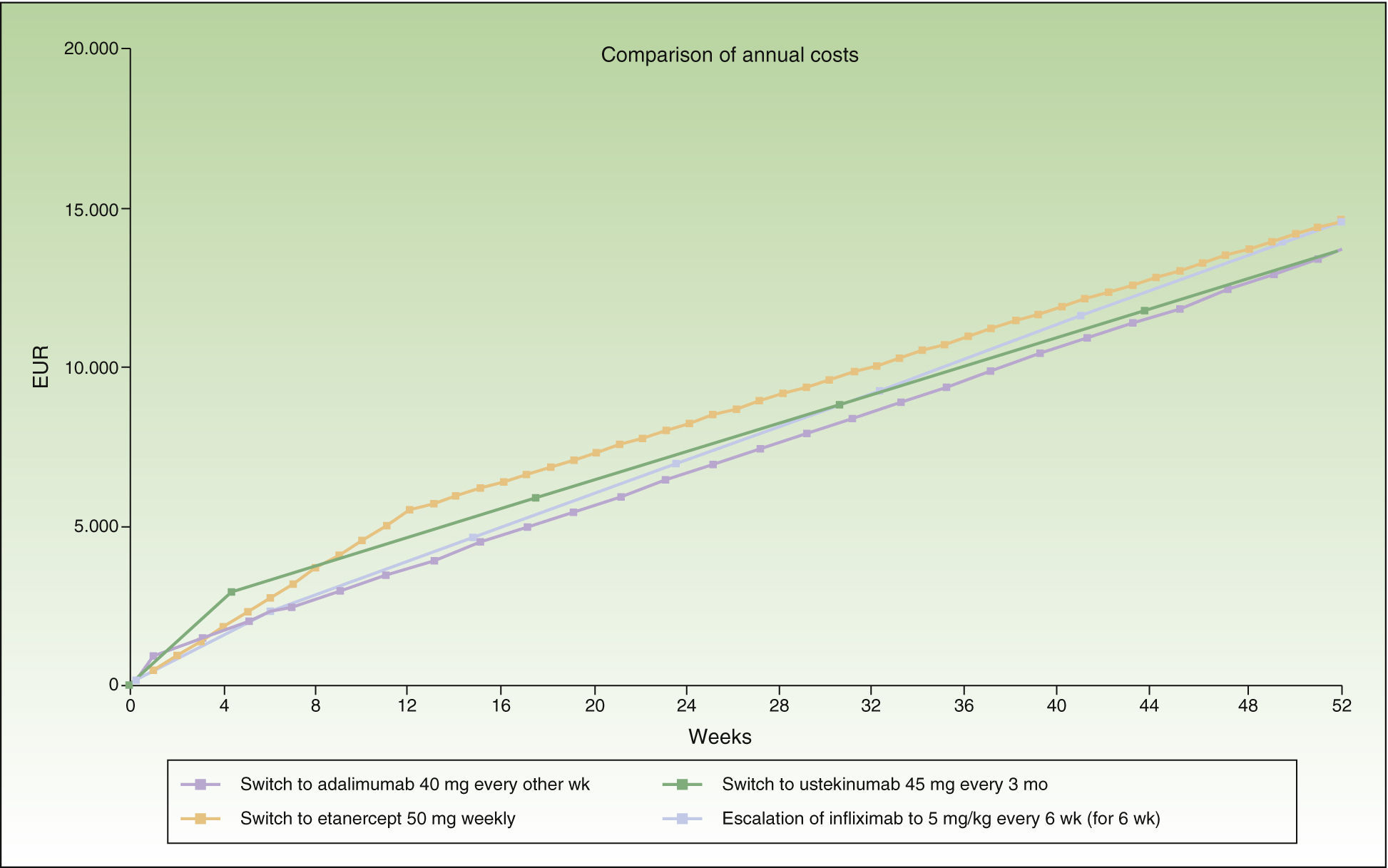
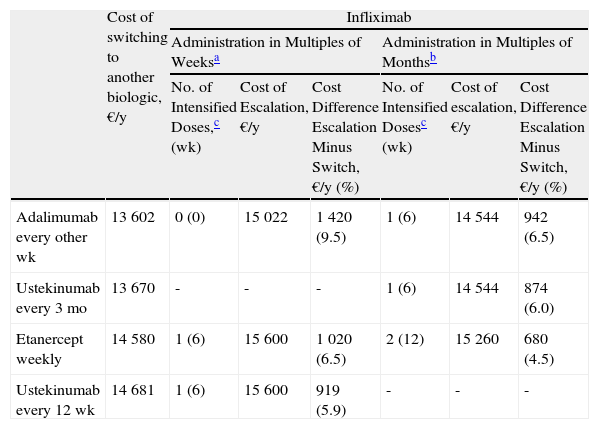
Escalation of Infliximab Compared to Switching to Another BiologicTable 3 presents the escalation of infliximab to a 6-week dose interval and incorporates data on administration in multiples of weeks or multiples of months during maintenance therapy.
Escalation of Infliximab (Every 6 Weeks) Compared to Switching to Another Biologic.
| Cost of switching to another biologic, €/y | Infliximab | ||||||
| Administration in Multiples of Weeksa | Administration in Multiples of Monthsb | ||||||
| No. of Intensified Doses,c (wk) | Cost of Escalation, €/y | Cost Difference Escalation Minus Switch, €/y (%) | No. of Intensified Dosesc (wk) | Cost of escalation, €/y | Cost Difference Escalation Minus Switch, €/y (%) | ||
| Adalimumab every other wk | 13 602 | 0 (0) | 15 022 | 1 420 (9.5) | 1 (6) | 14 544 | 942 (6.5) |
| Ustekinumab every 3 mo | 13 670 | - | - | - | 1 (6) | 14 544 | 874 (6.0) |
| Etanercept weekly | 14 580 | 1 (6) | 15 600 | 1 020 (6.5) | 2 (12) | 15 260 | 680 (4.5) |
| Ustekinumab every 12 wk | 14 681 | 1 (6) | 15 600 | 919 (5.9) | - | - | - |
The maintenance regimen for infliximab was every 8 weeks before and after escalation. The maintenance regimen for ustekimumab was every 12 weeks.
In the case of loss of response to maintenance therapy with infliximab every 8 weeks (Table 3 and Fig. 3), the alternatives would be, in order of efficiency:
- 1.
Switch to adalimumab every other week: switching before escalation is recommended because the difference in cost of €1000/year is reached from the outset.
- 2.
Switch to weekly etanercept or ustekinumab every 12 weeks: after administration of the second dose. Since the difference in cost between these 2 options would be minimal (€101/y), other factors would have to be considered.
If the administration interval is in multiples of months (infliximab administered every 2 months and ustekinumab every 3 months in the maintenance phase), the period of escalation to infliximab every 6 weeks would be 6 weeks (1 dose), and after this the most efficient options for switching would be, in decreasing order of efficiency:
- 1.
Switch to adalimumab every other week or ustekinumab every 3 months: after administration of the second dose. Since the difference in cost between these 2 options would be minimal (€68/y), other factors should be considered, such as the convenience of the dose interval, differences in the cost of maintenance therapy, etc.
- 2.
Switch to weekly etanercept: after administration of the third dose.
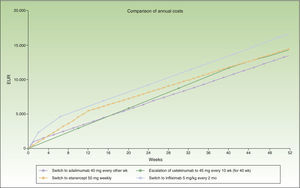
The switch to adalimumab would be more efficient than escalating infliximab to a 6-week dose interval. It is also the most efficient alternative for sequential biologic therapy, followed by ustekinumab, and finally etanercept. If administration is in multiples of months, switching to adalimumab or ustekinumab every 3 months would be the most efficient alternative, although the annualized incremental cost of escalating the regimen to infliximab every 6 weeks does not reach €1000.
Figure 3 shows the cost of escalating the infliximab regimen for 6 weeks vs the cost of switching to another biologic, considering a maintenance dosage in multiples of months for infliximab and ustekinumab. The annualized cost is lowest for adalimumab (€13 602), followed by ustekinumab (€13 670), infliximab (€14 544), and finally etanercept (€14 580).
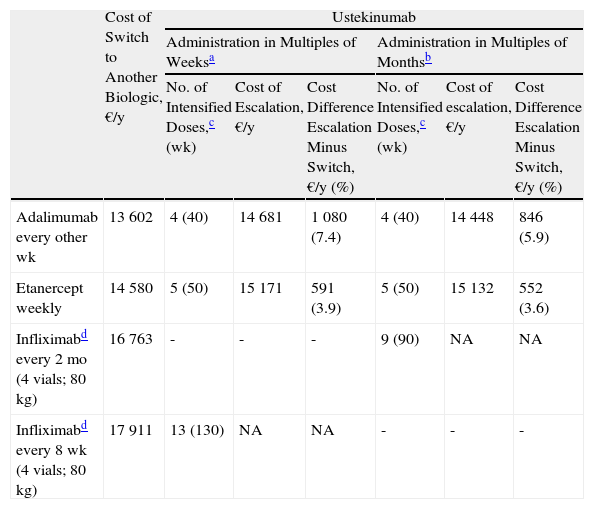
Escalation of Ustekinumab Compared to Switching to Another BiologicTables 4 and 5 present two possible scenarios: ustekinumab every 10 weeks and ustekinumab every 8 weeks. In both cases, two sets of results are presented: administration in multiples of weeks (as per the SPC) and administration in multiples of months. The latter regimen was included because it has become increasingly common in routine clinical practice.
Escalation of Ustekinumab (Administered Every 10 Weeks) Compared to Switching to Another Biologic Agent.
| Cost of Switch to Another Biologic, €/y | Ustekinumab | ||||||
| Administration in Multiples of Weeksa | Administration in Multiples of Monthsb | ||||||
| No. of Intensified Doses,c (wk) | Cost of Escalation, €/y | Cost Difference Escalation Minus Switch, €/y (%) | No. of Intensified Doses,c (wk) | Cost of escalation, €/y | Cost Difference Escalation Minus Switch, €/y (%) | ||
| Adalimumab every other wk | 13 602 | 4 (40) | 14 681 | 1 080 (7.4) | 4 (40) | 14 448 | 846 (5.9) |
| Etanercept weekly | 14 580 | 5 (50) | 15 171 | 591 (3.9) | 5 (50) | 15 132 | 552 (3.6) |
| Infliximabd every 2 mo (4 vials; 80 kg) | 16 763 | - | - | - | 9 (90) | NA | NA |
| Infliximabd every 8 wk (4 vials; 80 kg) | 17 911 | 13 (130) | NA | NA | - | - | - |
The maintenance regimen for ustekimumab was every 12 weeks before and after escalation. Infliximab maintenance therapy was every 8 weeks.
Ustekimumab maintenance regimen was every 3 months before and after escalation. In the case of infliximab, the maintenance regimen considered was every 2 months.
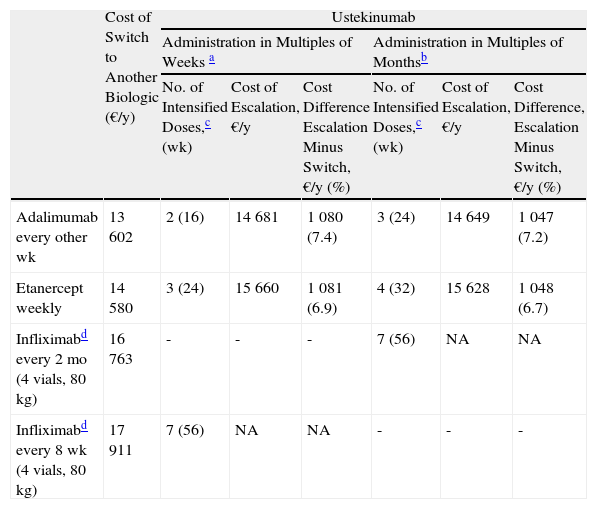
Escalation of Ustekinumab (Every 8 Weeks) vs Switching to Another Biologic Agent.
| Cost of Switch to Another Biologic (€/y) | Ustekinumab | ||||||
| Administration in Multiples of Weeks a | Administration in Multiples of Monthsb | ||||||
| No. of Intensified Doses,c (wk) | Cost of Escalation, €/y | Cost Difference Escalation Minus Switch, €/y (%) | No. of Intensified Doses,c (wk) | Cost of Escalation, €/y | Cost Difference, Escalation Minus Switch, €/y (%) | ||
| Adalimumab every other wk | 13 602 | 2 (16) | 14 681 | 1 080 (7.4) | 3 (24) | 14 649 | 1 047 (7.2) |
| Etanercept weekly | 14 580 | 3 (24) | 15 660 | 1 081 (6.9) | 4 (32) | 15 628 | 1 048 (6.7) |
| Infliximabd every 2 mo (4 vials, 80 kg) | 16 763 | - | - | - | 7 (56) | NA | NA |
| Infliximabd every 8 wk (4 vials, 80 kg) | 17 911 | 7 (56) | NA | NA | - | - | - |
The maintenance regimen of ustekinumab was every 12 weeks before and after escalation. In the case of infliximab, the maintenance regimen considered was every 8 weeks.
Ustekinumab every 3 months for maintenance before and after the escalation and ustekinumab every 2 months during escalation. In the case of infliximab the maintenance regimen considered was every 2 months.
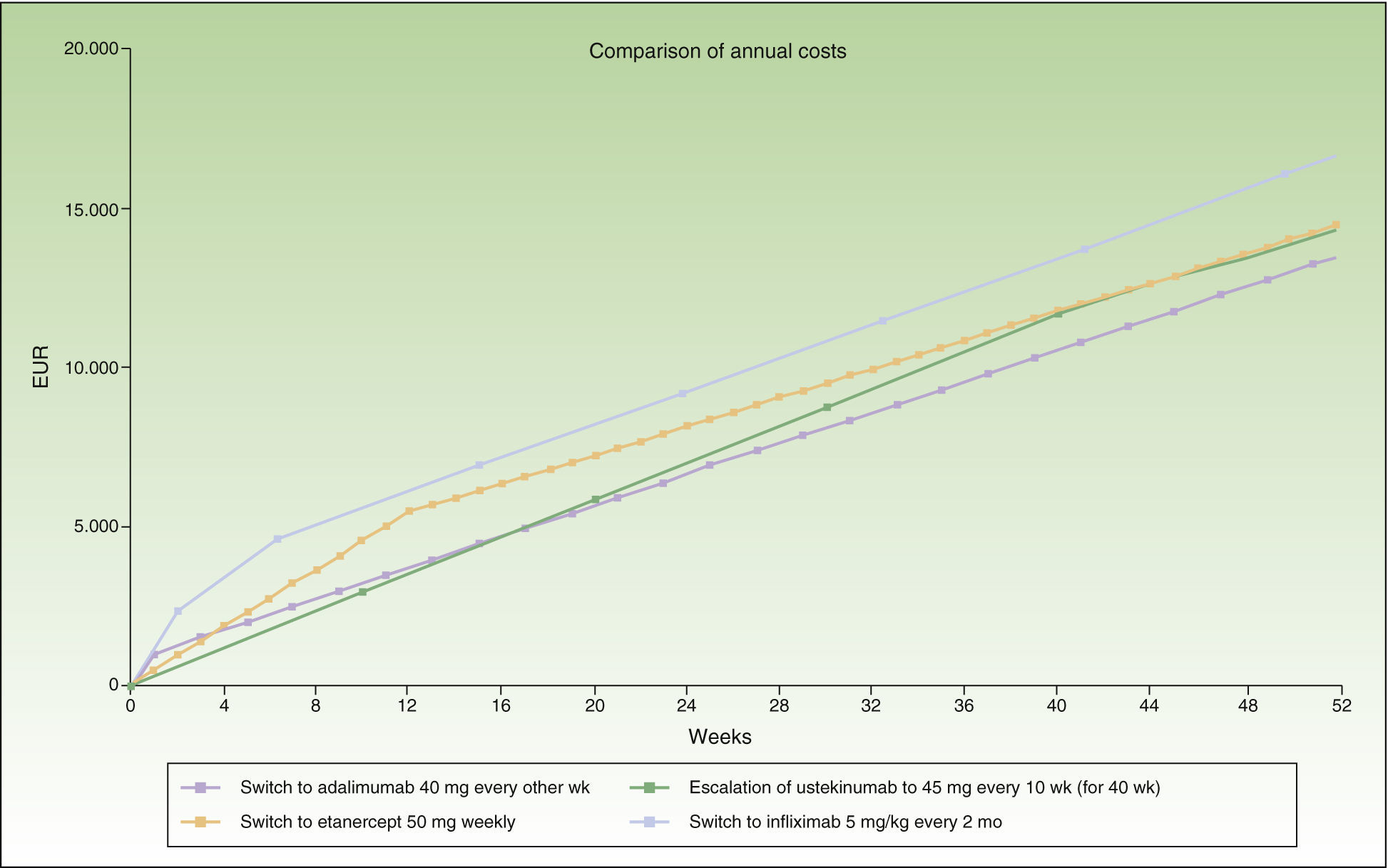
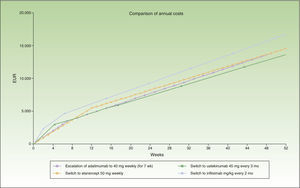
In the case of a loss of response to maintenance therapy with ustekinumab, escalation to ustekinumab every 10 weeks for 40 weeks (4 doses) would be an alternative with a similar incremental cost (a difference of about €1000/y) to switching to another biologic agent (Table 4 and Fig. 4). The alternatives for sequential biologic treatment would be, in order of efficiency:
- 1.
Switch to adalimumab every other week: after administration of the fifth dose.
- 2.
Switch to weekly etanercept: after administration of the sixth dose.
- 3.
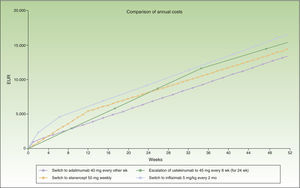
Switch to infliximab: escalation to a regimen of ustekinumab every 10 weeks could be prolonged for more than a year without exceeding the maximum incremental cost of €1000 a year compared to a switch.
Figure 4 shows the cost of escalating ustekinumab for 10 weeks compared to the cost of switching to another biologic agent, considering a maintenance regimen for infliximab and ustekinumab in multiples of months. The annualized cost is lowest for adalimumab (€13 602), followed by ustekinumab (€14 448), etanercept (€14 580), and infliximab (€16 763).
Escalation to Ustekinumab Every 8 WeeksIn the case of a loss of response to maintenance therapy with ustekinumab every 12 weeks, escalation to ustekinumab every 8 weeks for 16 weeks (2 doses) would reach the preset limit of €1000 in incremental cost over a switch to another biologic agent (Table 4). If the dose interval for maintenance therapy with ustekinumab were every 3 months, this period would be 24 weeks (3 doses) (Fig. 5).The alternatives for switching, in descending order of efficiency, would be:
- 1.
Switch to adalimumab every other week: after administration of the third dose (the fourth if the dosage interval for ustekinumab in the maintenance regimen was every 3 months).
- 2.
Switch to weekly etanercept: after administration of the fourth dose (the fifth if the dosage interval for ustekinumab in the maintenance regimen was every 3 months).
- 3.
Switch to infliximab: the more intense regimen of ustekinumab every 8 weeks could be prolonged for more than a year without exceeding the incremental cost limit of €1000 a year.
In conclusion, in the case of treatment failure with ustekinumab, a switch to adalimumab after 4 doses on a more intense regimen (administration every 10 weeks) is the option that comes closest to the upper limit for incremental cost of €1000 a year. If the dosage is escalated to every 8 weeks or 2 months, the switch to adalimumab would be more efficient after 2 or 3 doses, depending on whether these regimens are based on multiples of weeks or months. The next most efficient option would be etanercept, and finally infliximab.
DiscussionThe aim of the present analysis was to provide information on the point at which the different escalation regimens reach an annualized incremental cost of €1000 compared to a switch to another biologic agent for a typical patient weighing 80 kg and taking into account only the acquisition cost of the drug (and the cost of administering the infusions in the case of infliximab). Therefore, it provides information about the efficiency of the different options based on the assumption that the likelihood of response to treatment after the switch to another biologic agent is independent of the treatment prescribed.
It is important to stress the limitation of assuming that all patients who experience secondary failure to the first biologic will respond to an escalated regimen or a switch to another biologic agent. However, owing to the lack of scientific information regarding the efficacy of biologic therapies following a switch and/or dose escalation, the efficiency analysis in this study is based on the assumption that all patients respond similarly to all the sequential options.
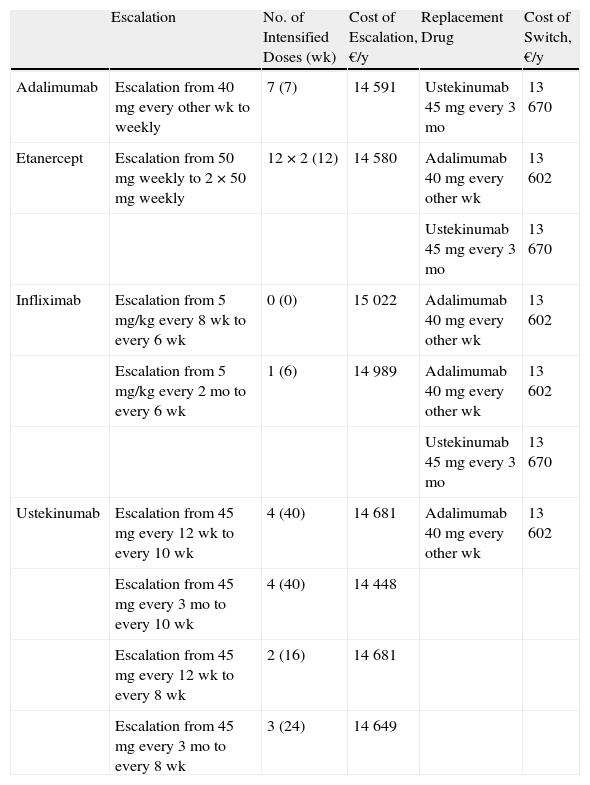
Table 6 shows the maximum number of weeks during which escalation may be efficient (up to a predefined upper limit for incremental cost of about €1000/y) and the most efficient option for sequential biologic therapy.
Summary of Escalation Compared to a Switch to Another Biologic Agent.
| Escalation | No. of Intensified Doses (wk) | Cost of Escalation, €/y | Replacement Drug | Cost of Switch, €/y | |
| Adalimumab | Escalation from 40 mg every other wk to weekly | 7 (7) | 14 591 | Ustekinumab 45 mg every 3 mo | 13 670 |
| Etanercept | Escalation from 50 mg weekly to 2 × 50 mg weekly | 12 × 2 (12) | 14 580 | Adalimumab 40 mg every other wk | 13 602 |
| Ustekinumab 45 mg every 3 mo | 13 670 | ||||
| Infliximab | Escalation from 5 mg/kg every 8 wk to every 6 wk | 0 (0) | 15 022 | Adalimumab 40 mg every other wk | 13 602 |
| Escalation from 5 mg/kg every 2 mo to every 6 wk | 1 (6) | 14 989 | Adalimumab 40 mg every other wk | 13 602 | |
| Ustekinumab 45 mg every 3 mo | 13 670 | ||||
| Ustekinumab | Escalation from 45 mg every 12 wk to every 10 wk | 4 (40) | 14 681 | Adalimumab 40 mg every other wk | 13 602 |
| Escalation from 45 mg every 3 mo to every 10 wk | 4 (40) | 14 448 | |||
| Escalation from 45 mg every 12 wk to every 8 wk | 2 (16) | 14 681 | |||
| Escalation from 45 mg every 3 mo to every 8 wk | 3 (24) | 14 649 |
Owing to the diverse dose intervals used, there are important differences between biologic agents with respect to the possibilities for escalation and the number of weeks during which the regimen can be intensified before reaching the threshold for switching (an incremental cost of €1000/y). In this analysis, the response to treatment escalation is assumed to be acceptable in all cases and similar to the response achieved following a switch. Before the cost overrun threshold is reached the dosage of ustekinumab can be escalated for 40 weeks, that of etanercept for 12 weeks, and that of adalimumab for 7 weeks. Infliximab is the drug least suitable for escalation because the threshold of €1000 a year is reached between weeks 0 and 6.
In general, when considering a switch to another biologic, the alternatives with the lowest annualized incremental cost are adalimumab and ustekinumab (depending on the agent being replaced).
Additional studies are needed to assess the differential effectiveness of all the biologic agents as rescue therapy in the case of primary or secondary failure with the initial biologic. Other factors that must be taken into account include the role of antidrug antibodies, which are drug-specific, and pharmacokinetic considerations.1 In this regard, it is important to note that the model used did not take into account the incremental cost associated with weight-adjusted dosing of infliximab and ustekinumab or the differences in the effectiveness of some biologic agents associated with the patient's weight.20
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their hospitals concerning the publication of patient data and that all patients included in this study were appropriately informed and gave their written informed consent.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestLluis Puig has participated in clinical trials sponsored by Abbvie, Amgen, Lilly, Merck, Novartis, Pfizer España, and VBL and has received honoraria for acting as a consultant and as a speaker at events sponsored by Abbvie, Merck, Janssen, Merck, Novartis, and Pfizer.
Please cite this article as: Puig L. Tratamiento de la psoriasis en placas moderada a grave con fármacos biológicos: análisis del sobrecoste de la intensificación temporal frente a cambio a otro biológico en caso de fracaso secundario. Actas Dermosifiliogr. 2014;105:401–412.