Metastatic or locally advanced unresectable melanoma carries a high morbidity and mortality. However, notable advances have been made in recent years in the systemic treatment of this disease, with the appearance of targeted therapy using tyrosine kinase inhibitors that block the mitogen activated protein kinase pathway, and of modern immunotherapy with immune-modulating monoclonal antibodies. In this paper, we provide an update of available data on new immune therapies and we review the clinical development that led to their approval for use in routine clinical practice.
El melanoma irresecable localmente avanzado y metastásico es una situación clínica asociada a una elevada tasa de morbimortalidad. En los últimos años, sin embargo, han acontecido notables avances en el ámbito del tratamiento sistémico de esta enfermedad, con la irrupción de la terapia dirigida con inhibidores tirosincinasa que actúan bloqueando la vía de las MAPKinasas, y de la moderna inmunoterapia con anticuerpos monoclonales inmunomoduladores. En la presente revisión se realiza una actualización sobre los datos disponibles con los nuevos inmunoterápicos, así como un repaso del desarrollo clínico que ha permitido la aprobación para su uso en la práctica clínica habitual.
Melanoma is the type of skin cancer with the highest mortality rate1 because, even though most tumors are diagnosed in their early stages, a significant proportion (19%-25%) recur and/or are detected with advanced locoregional and/or distant disease.
Until recently, systemic treatment of metastatic melanoma was mainly chemotherapy-based, although outcomes were in general disappointing, with mean survival ranging from 6 to 9 months.2 However, in recent years, a revolution has occurred in systemic melanoma therapy, driven by targeted therapies and modern immunotherapy.
The increased understanding of the molecular biology of skin tumors and the successful development of molecules targeting specific molecular aberrations are based on molecular biology studies indicating that activation of the RAS-RAF kinase signaling pathway is implicated in the development of certain melanomas. It has been shown that RAF kinases are components of the mitogen-activated protein (MAP) kinase pathway, which is its only substrate. Between 40% and 60% of melanomas have a mutation in the gene that codes for BRAF kinase, conferring on it constitutive activation independently of external stimulae.3,4 Between 70% and 80% of these mutations correspond to substitution of glutamic acid with valine at amino acid 600 (V600E); other mutations at the V600 site correspond to substitutions of alternative amino acids (V600K, V600D, V600R). BRAF inhibitors (vemurafenib, dabrafenib) have shown a clear impact on response rate, progression-free survival, and overall survival in patients with advanced melanoma with a V600 mutation compared with standard chemotherapy.5,6
In addition, in the MAP kinase pathway, inhibition of MEK, the only substrate phosphorylated by BRAF, has been considered an effective target for treatment of melanomas with a BRAF or NRAS mutation. Mature data from phase iii studies show that combination treatment with a BRAF inhibitor and a MEK inhibitor such as dabrafenib in combination with trametinib or vemurafenib and cobimetinib in patients with the V600 mutation of the BRAF gene have improved survival compared with any of the agents used in monotherapy.7,8
In parallel with the development of targeted therapy in melanoma, advances in new immunotherapy have been made and clinical development has blossomed, with a large number of potential immunomodulatory monoclonal antibodies. These antibodies are directed against proteins that control immune response (immune checkpoints) present in the virtual space denominated immune synapse established between T lymphocyte effectors and antigen presenting cells and/or tumor cells. Currently, solid scientific evidence attests to clinical benefit; there is an impact on response rates, disease control, and survival with this immunotherapy approach compared with conventional therapies for melanoma.9,10
This aim of this article is to provide an update on the main clinical data obtained with immunomodulatory antibodies in advanced melanoma.
Costimulatory Molecules and CoinhibitorsThe interaction between antigen-presenting cells, tumor cells, and T lymphocytes, as well as between T lymphocytes and other cells in the organism occurs through a series of costimulatory molecules and coinhibitors. These molecules regulate T lymphocyte activation. Immune response is a finely tuned dynamic process that is often governed by inducible molecules with a tendency to maintain what is known as immune homeostasis. Cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and programmed death-1 (PD1) are 2 of the main inhibitory molecules. Activation of these molecules dampens T lymphocyte activity, thereby decreasing immune response to tumor cells.
The first of the immunotherapeutic drugs to be approved by the FDA in metastatic melanoma was ipilimumab (anti-CTLA-4). The molecules currently approved in Spain and reimbursed by the Spanish National Health System are ipilimumab, nivolumab, and pembrolizumab (the latter 2 agents are anti-PD1 agents).
Anti-CTLA-4 AntibodiesT-lymphocyte-associated protein-4 belongs to the immunoglobulin superfamily of coinhibitory receptors. It is expressed both on the surface of T cells (CD4+, CD8+) and on the surface of regulatory T cells (CD25+, FOXP3+).11 In the first phase of immune response, antigen-presenting cells expose their tumor antigens to T lymphocytes. The T lymphocyte receptors, CTLA-4 (inhibitory molecule) and CD28 (stimulatory molecule) compete to bind with B7-1 (CD80) and B7-2 (CD86) molecules of the antigen-presenting cells. Stimulation of CTLA-4, on binding to its B7 ligand, triggers an inhibitory signal that blocks T lymphocyte response.
The first anti-CTLA-4 agent approved by the FDA in 2011 in metastatic melanoma was the humanized IgG1 monoclonal antibody known as ipilimumab. On the basis of results obtained in early-phase dose-finding studies,12,13 2 phase III studies were initiated in first- and second-line locally advanced, unresectable and/or metastatic melanoma.
The first trial included 676 patients with unresectable stage iii or stage iv melanoma who had progressed after treatment for metastatic disease. Patients were randomized 3:1:1 to receive 4 doses of ipilimumab 3mg/kg every 3 weeks along with the gp100 peptide vaccine (403 patients); ipilimumab as monotherapy at the same dose (137 patients); and the gp100 vaccine as monotherapy (136 patients). This trial found a median overall survival of 10.1 months for the ipilimumab arm in monotherapy, 10 months for the ipilimumab/gp100 arm, and 6.4 months for the arm who received gp100 alone (HR, 0.8; P<.001).14
The second trial included 502 patients with advanced melanoma who had not received prior treatment for metastatic disease. Patients were randomized 1:1 to receive ipilimumab 10mg/kg plus dacarbazine 850mg/m2 every 3 weeks or placebo plus dacarbazine at the same dose. The trial found a median overall survival of 11.2 months for the ipilimumab plus dacarbazine arm compared with 9.1 months for the dacarbazine arm. Likewise, a higher survival rate was observed in the combination group: 47.3% vs 36.3% at 1 year, 28.5% vs 17.9% at 2 years, and 20.8% vs 12.2% at 3 years (HR, 0.72; P<.001). The median duration of response was 19.3 months in the combination therapy arm and 8.1 months in the dacarbazine arm.15 Recently reported data after minimum follow-up of 5 years showed 5-year survival with dacarbazine plus ipilimumab of 18.2% versus 8.8% in the dacarbazine monotherapy arm. The survival curves start to flatten and enter a plateau from the third year onwards, such that overall survival in the arm that includes ipilimumab is 21.3% at 3 years and and 18.2% at 5 years.16
Further support for the survival data of the phase 3 trials came from the meta-analysis by Schadendorf et al.17 That analysis included 1861 patients from the phase 2 and 3 trials with ipilimumab (at different dose levels and lines of treatment) as well as 4486 patients from the expanded use program. Overall survival at 3 years was 22% and 21%, respectively, once again showing flattening of the overall survival curve from the third year on.
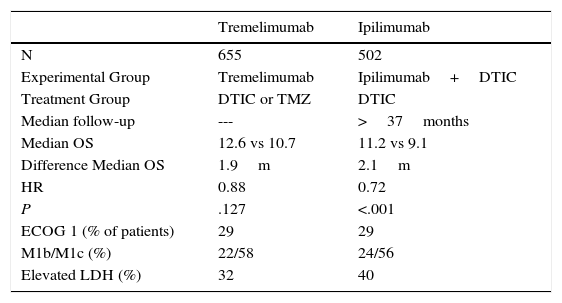
Another anti-CTLA-4 compound investigated in melanoma is the humanized IgG2 monoclonal antibody known as tremelimumab. The efficacy of this drug was assessed as first-line treatment in a phase ii clinical trial that included 655 patients with unresectable stage iiic or stage iv melanoma. The study was negative as no statistically significant differences were detected in overall survival, although the median duration of response in those patients who responded was 35.8 months with tremelimumab and 13.7 months in the chemotherapy arm (Table 1).18
Key Data on Phase 3 Clinical Trials of First-Line Ipilimumab and Tremelimumab in Metastatic Melanoma.
| Tremelimumab | Ipilimumab | |
|---|---|---|
| N | 655 | 502 |
| Experimental Group | Tremelimumab | Ipilimumab+DTIC |
| Treatment Group | DTIC or TMZ | DTIC |
| Median follow-up | --- | >37months |
| Median OS | 12.6 vs 10.7 | 11.2 vs 9.1 |
| Difference Median OS | 1.9m | 2.1m |
| HR | 0.88 | 0.72 |
| P | .127 | <.001 |
| ECOG 1 (% of patients) | 29 | 29 |
| M1b/M1c (%) | 22/58 | 24/56 |
| Elevated LDH (%) | 32 | 40 |
Abbreviations: DTIC, dacarbazine; HR, hazard ratio; LDH, lactate dehydrogenase, OS, overall survival; TMZ, temozolomide.
Between 40% and 50% of patients with advanced melanoma have the BRAF V600 mutation. In this subgroup of patients, treatment with an anti-CTLA-4 agent has been shown to have a similar efficacy to that obtained in patients with the wild-type BRAF mutation.19
Anti-PD1 AntibodiesAnti-PD1 antibodies are directed against PD1 (CD279), which are proteins located on the membrane of T and B lymphocytes and monocytes. Their ligands, PDL1 and PDL2 are expressed on the membrane of antigen-presenting cells, in tumor cells, and in other cells in the organism.20
These molecules therefore appear to act in the second phase of immune response (effector phase) when the activated T lymphocyte (PD1) binds to its ligand (PDL1) expressed on tumor cells, leading to T lymphocyte deactivation. Different types of tumors overexpress PDL1 on their surface and on cells of the tumor microenvironment in an attempt to evade antitumor immune response.
NivolumabOne of the first anti-PD1 antibodies assessed in the clinic in advanced melanoma was the humanized IgG4 antibody known as nivolumab. In different phase I studies, nivolumab was able to demonstrate promising efficacy (objective response rate of 30% to 40%) with a favorable safety profile,21 encouraging pivotal phase 3 studies for approval.
One of the phase 3 studies included patients with metastatic melanoma refractory to ipilimumab or targeted anti-BRAF therapy. An objective response rate of 32% was reported in the nivolumab arm compared with 11% in the chemotherapy arm,22 with lower toxicity in the experimental arm compared with the chemotherapy one.
Efficacy data of first-line use of nivolumab are also available thanks to the results of a phase 3 study of 418 patients (unmutated BRAF) who had not received prior treatment for metastatic melanoma. Patients were randomized 1:1 to receive ipilimumab 3mg/kg every 2 weeks or dacarbazine 1000mg/m2 every 3 weeks. After 1 year of follow-up, overall survival was 72.9% (95% CI, 65.5%-78.9%) in the nivolumab arm and 42.1% (95% CI, 33%-50.9%) with dacarbazine (HR, 0.42; 95% CI, 0.25-0.73; P<.001). The median progression-free survival was 5.1 months in nivolumab arm and 2.2 months in the dacarbazine arm (HR, 0.43; 95% CI, 0.34-0.56; P<.001). The objective response rate was 40% (95% CI, 33.3%-47%) in the nivolumab arm and 13.9% (95% CI, 9.5%-19.4%) with dacarbazine (odds ratio, 4.06; P<.001). It was also concluded that the favorable nivolumab response rates were independent of PDL1 expression.23 In addition, in a clinical trial performed subsequently, patients with the BRAF V600 mutation were also found to benefit from anti-PD1 treatment.24
PembrolizumabPembrolizumab is another human monoclonal antibody of the IgG4 type (HuMab) that also binds to the negative regulator of lymphocyte activity (PD-1), thus blocking its interaction with PD-L1 and PD-L2.
A phase 2 clinical trial included 540 patients with metastatic melanoma refractory to ipilimumab. These patients were randomized 1:1:1 to receive pembrolizumab 2mg/kg every 3 weeks, 10mg/kg every 3 weeks, or chemotherapy chosen by the investigator (carboplatin+paclitaxel, carboplatin, paclitaxel, dacarbazine, or temozolomide). After 6 months of follow-up, the progression-free survival rate was 34% (95% CI, 27% to 41%) and 38% (95% CI, 31% to 45%) for pembrolizumab at 2mg/kg and 10mg/kg, respectively, compared with 16% (95% CI, 10% to 22%) for the chemotherapy arm. The objective response rate was 21% at doses of 2mg/kg; 25% at doses of 10mg/kg; and 4% in the chemotherapy arm.25
After this phase 2 study, pembrolizumab was compared with the anti-CTLA-4 antibody ipilimumab in the KEYNOTE-006 phase 3 trial (analyzed in the following section).
Anti-CTLA-4 Antibodies versus Anti-PD1 AntibodiesAs explained above, CTLA-4 and PD1 are expressed and act at different immune checkpoints. Clinical trial results are now available that compare the efficacy of monoclonal antibodies specifically targeting these molecules.24,26
The KEYNOTE-006 trial compared the efficacy of pembrolizumab (anti-PD1) and ipilimumab (anti-CTLA-4).24 This trial included 834 patients with advanced melanoma, the majority of whom had not received prior treatment for metastatic disease. Patients were randomized 1:1:1 to receive pembrolizumab 10mg/kg every 2 or 3 weeks, or 4 doses of ipilimumab 3mg/kg every 3 weeks. The progression-free survival rate estimated at 6 months was 47.3% for pembrolizumab every 2 weeks, 46.4% for pembrolizumab every 3 weeks, and 26.5% for ipilimumab (HR, 0.58; P<.001 for both pembrolizumab regimens versus ipilimumab; 95% CI, 0.46-0.72 and 0.47-0.72, respectively). The estimated survival rate at 12 months in each case was 74.1%, 68.4%, and 58.2%, respectively (for pembrolizumab every 2 weeks HR, 0.63; 95% CI, 0.47-0.83; P=.0005; for pembrolizumab every 3 weeks HR, 0.69; 95% CI, 0.52-0.90; P=.0036). Likewise, a higher response rate was obtained with pembrolizumab—33.7% and 32.9%—compared with ipilimumab—11.9% (P<.001 for both comparisons).
The CheckMate 067 trial (analyzed in the next section) also supported the superiority of anti-PD1 therapy in monotherapy versus ipilimumab.26
Anti-PD1 and Anti-CTLA-4 CombinationsThe combination of ipilimumab and nivolumab was assessed in a phase 1 clinical trial in patients with metastatic melanoma. A notable decrease in tumor load was observed, usually occurring early (at 12 weeks). This decrease was profound and sustained. Although the frequency of grade 3 and 4 adverse effects was high,8 the efficacy observed was sufficiently large to justify clinical development of the combination.
A phase 2 clinical trial was designed with 142 patients with advanced melanoma who had not received any prior treatment. Patients were randomized 2:1 to receive ipilimumab 3mg/kg in combination with nivolumab 1mg/kg or placebo every 3 weeks (4 doses) followed by nivolumab 3mg/kg or placebo every 2 weeks until progression or toxicity, or ipilimumab at standard dose.27 In this study, the objective response rate in patients without the BRAF mutation was 61% (44 of 72 patients) in the combination group versus 11% (4 of 37 patients) in the group who received ipilimumab and placebo (P<.001). There was documented complete response in 16 patients (22%) in the group who received combination therapy. No complete response was observed in patients who received ipilimumab as monotherapy. The results were similar in the 33 patients with a BRAF V600 mutation. Grade 3 or 4 adverse effects were reported in 54% of the patients who received combination therapy, compared with 24% in the group who received only ipilimumab.
Finally, the phase 3 CheckMate 067 study was a double-blind study that randomized 945 patients with unresectable or metastatic stage iii melanoma (with no previous treatment) to receive nivolumab monotherapy, ipilimumab monotherapy, or a combination of the 2 drugs. The median progression-free survival was 11.5 months in the combination group (95% CI, 8.9-16.7 months) compared with 2.9 months (95% CI, 2.8-3.4 months) with ipilimumab and 6.9 months (95% CI, 4.3-9.5 months) with nivolumab. The objective response rates were 57.6% for patients treated with combination, 43.7% for nivolumab, and 19% for ipilimumab.
A subgroup analysis was undertaken and it was found that patients positive for PDL1 had a progression-free survival of 14 months in both arms that included nivolumab (both in monotherapy and for the combination) and of 3.9 months in the ipilimumab group used as a single agent. However, PDL1-negative patients appear to benefit more from the 2 drugs in combination, with a progression-free survival of 11.2 months compared with 5.3 months and 2.8 months for the nivolumab and ipilimumab arms, respectively.
Data from the CheckMate 067 trial therefore support the use of nivolumab as a first-line therapy in patients with metastatic melanoma (regardless of BRAF mutation status) and the authors concluded that combination of nivolumab and ipilimumab is a promising line of investigation for treatment of melanoma.26
Another combination of anti-CTLA-4 and anti-PD1 antibodies under study is ipilimumab at low doses (1mg/kg) in combination with pembrolizumab, with the aim of maintaining the improved efficacy while improving the toxicity profile.28 Preliminary data reported to date suggest that tolerability with this regimen is probably better than the combination of nivolumab and ipilimumab at a dose of 3mg/kg, although these data should be confirmed with longer follow-up in new studies.
Toxicity Associated with Monoclonal AntibodiesThe adverse effects are associated with inflammatory infiltration of T lymphocytes in solid organs and increased cytokine concentrations in patient serum.29
The adverse effects observed with ipilimumab appear to be dose dependent, and may have immediate onset or appear in the long term. Onset usually occurs late, at 8 to 10 weeks after start of treatment.30 Adverse effects commonly observed with the anti-CTLA-4 antibodies ipilimumab or tremelimumab are dermatitis (pruritus, rash), enterocolitis, endocrine disorders (hypophysitis, thyroiditis), elevated liver enzymes, and uveitis.31
Adverse effects associated with the use of anti-PD1 agents are fatigue, rash, diarrhea, colitis, and endocrine disorders (hypo- or hyperthyroidism). However, these effects appear less frequently than with anti-CTLA-4 agents.32
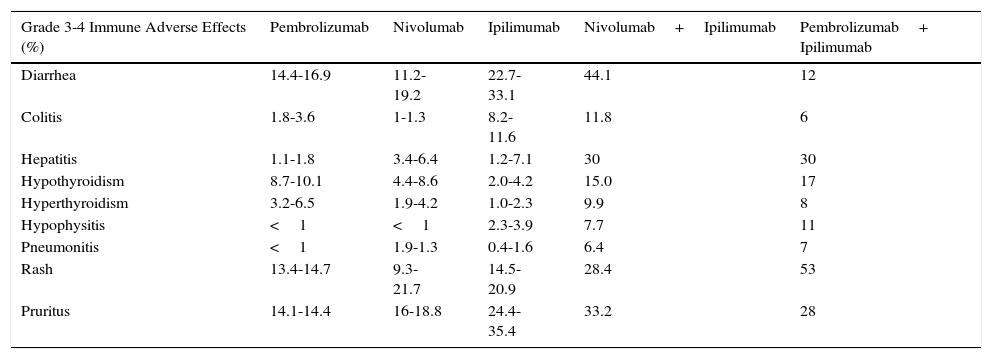
The phase 3 KEYNOTE-006 trial assessed the safety and efficacy of 2 regimens of pembrolizumab in comparison with ipilimumab.24 In general, the group that received pembrolizumab had a more favorable safety profile than ipilimumab (Table 2).
Immune-Related Toxicity with Immunomodulatory Antibodies.
| Grade 3-4 Immune Adverse Effects (%) | Pembrolizumab | Nivolumab | Ipilimumab | Nivolumab+Ipilimumab | Pembrolizumab+ Ipilimumab |
|---|---|---|---|---|---|
| Diarrhea | 14.4-16.9 | 11.2-19.2 | 22.7-33.1 | 44.1 | 12 |
| Colitis | 1.8-3.6 | 1-1.3 | 8.2-11.6 | 11.8 | 6 |
| Hepatitis | 1.1-1.8 | 3.4-6.4 | 1.2-7.1 | 30 | 30 |
| Hypothyroidism | 8.7-10.1 | 4.4-8.6 | 2.0-4.2 | 15.0 | 17 |
| Hyperthyroidism | 3.2-6.5 | 1.9-4.2 | 1.0-2.3 | 9.9 | 8 |
| Hypophysitis | <1 | <1 | 2.3-3.9 | 7.7 | 11 |
| Pneumonitis | <1 | 1.9-1.3 | 0.4-1.6 | 6.4 | 7 |
| Rash | 13.4-14.7 | 9.3-21.7 | 14.5-20.9 | 28.4 | 53 |
| Pruritus | 14.1-14.4 | 16-18.8 | 24.4-35.4 | 33.2 | 28 |
The combination of anti-CTLA-4 and anti-PD1 agents has been shown to be notably more toxic than the components of the combination separately.
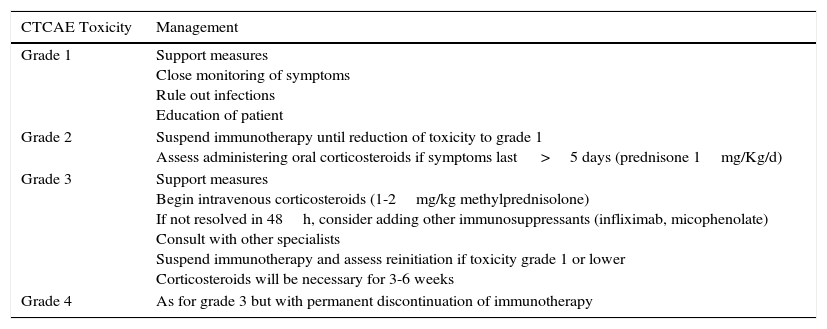
With regards the management of adverse effects, mild toxicities usually resolve spontaneously or with the use of corticosteroids. Endocrine disorders may require permanent hormone replacement therapy. In more serious situations, high-dose corticosteroids and even immunosuppressants such as anti-TNF agents may be necessary (Table 3).
General Recommendations for Management of Immune-Related Toxicities.
| CTCAE Toxicity | Management |
|---|---|
| Grade 1 | Support measures Close monitoring of symptoms Rule out infections Education of patient |
| Grade 2 | Suspend immunotherapy until reduction of toxicity to grade 1 Assess administering oral corticosteroids if symptoms last >5 days (prednisone 1mg/Kg/d) |
| Grade 3 | Support measures Begin intravenous corticosteroids (1-2mg/kg methylprednisolone) If not resolved in 48h, consider adding other immunosuppressants (infliximab, micophenolate) Consult with other specialists Suspend immunotherapy and assess reinitiation if toxicity grade 1 or lower Corticosteroids will be necessary for 3-6 weeks |
| Grade 4 | As for grade 3 but with permanent discontinuation of immunotherapy |
An extensive program to study biomarkers predictive of response is underway with the aim of selecting patients who stand to benefit from immunotherapy.
Absolute lymphocyte counts and absolute and differential eosinophil counts are potential pharmacodynamic biomarkers in patients treated with ipilimumab.33–38
In addition, in a study with patients treated with ipilimumab, exome sequencing was performed for tumor and peripheral blood samples and, after identifying somatic mutations specific to each tumor, it was possible to characterize the neoepitopes resulting from these mutations.39 With this method, the relationship between a high number of missense mutations (total mutational load) and a significant increase in clinical benefit was demonstrated. After analysis of the neoantigen characteristics, along with the histocompatibility molecules (HLA) specific to each patient, a representative pattern of tumors that respond to treatment with anti-CTLA-4 agents was established. This supports the hypothesis that some mutations represent immune determinants on which the efficacy of the treatment may depend.39
The presence of PDL1 may also play a role as a response biomarker. However, its expression is probably heterogeneous and may vary from sample to sample taken from the same tumor. Other limitations of PDL1 as a reliable biomarker include the instability of PDL1 epitopes detected by some antibodies, possible different affinities between antibodies (nonstandard techniques), and the fact that expression of PDL1 could be membrane or cytoplasmic and ubiquitous (detectable in tumor cells, natural killer T cells, T and B lymphocytes, etc).40 From the above, it follows that we are still far from considering PDL1 as a valid and reliable predictive marker.
Finally, a recent study has shown that the presence of a high density of CD8+ T cells in the invasive tumor margin, as well as colocalization of PD1 and PDL1 may predict a favorable response in patients treated with anti-PD1.41
Future Perspectives and ConclusionsSystemic therapy for advanced melanoma has been transformed in the past decade by targeted therapies and modern immunotherapy. The available data from the coBRIM, Combi-d, and Combi-v studies show that in patients with unresectable locally advanced and metastatic melanoma with the V600 BRAF mutation, the combination of a BRAF inhibitor with a MEK inhibitor is overall superior in efficacy to monotherapy with a BRAF inhibitor and should become the standard of first-line care in this subpopulation of patients, particularly in subgroups of patients with rapidly progressing disease, visceral involvement, and/or elevated lactate dehydrogenase (M1c stages) and a high tumor load. In the remaining cases, first-line treatment with immunotherapy (anti-CTLA-4 or anti-PDL) may be a reasonable alternative although the scientific evidence in this setting is weaker with immunotherapy than with targeted therapy. The introduction of immunomodulatory monoclonal antibodies in the treatment of metastatic melanoma has become a reality. These therapies have displaced the previously available standards of care. Anti-CTLA-4 and anti-PD1 therapy have notably extended overall survival with respect to standard chemotherapy, and compared with each other, anti-PD1 antibodies show a better overall benefit-risk in terms of efficacy and toxicity and so should be considered the standard of care for first-line treatment in all cases of unresectable BRAF wild-type metastatic melanoma. The role of the anti-CTLA-4 and anti-PD1 combination has yet to be determined. Although it appears more effective than anti-CTLA-4 therapy and, perhaps, perhaps not clearly demonstrated, also more effective than anti-PD1 agents in monotherapy, it is notably more toxic.
Although advances in targeted therapy and immunotherapy in melanoma have been notable in recent years, with median overall survival in excess of 24 months in recently reported studies, additional effort is still required to improve on the current results. New lines of investigation focusing on combination strategies with immunomodulatory monoclonal antibodies and targeted therapy, in association with an in depth study of predictive biomarkers and monitoring of response, would probably enable the efficacy of these new therapies in advanced melanoma to be optimized and increased.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that patient data do not appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gago MCP, Gadea OSS, de la Cruz-Merino L. Nuevos avances de tratamiento inmunobiológico en el melanoma avanzado. Actas Dermosifiliogr. 2017;108:721–728.