Vemurafenib is a B-raf kinase inhibitor developed to treat metastatic melanoma in patients with the BRAF V600E mutation, which is found in 40% to 60% of patients with advanced melanoma. It acts by inhibiting the BRAF/MEK/ERK mitogen-activated protein kinase (MAPK) pathway at the BRAF/MEK step. Recent studies have reported that vemurafenib is associated with a 63% reduction in risk of death.1,2 Adverse effects include joint pain, photosensitivity, alopecia, fatigue, hyperkeratosis, xerosis, nonspecific rash, keratoacanthoma, and squamous cell carcinoma.3–5 We report the case of a patient with stage IV melanoma who developed a clinically amelanotic dysplastic melanocytic nevus 2 months after starting treatment with vemurafenib.
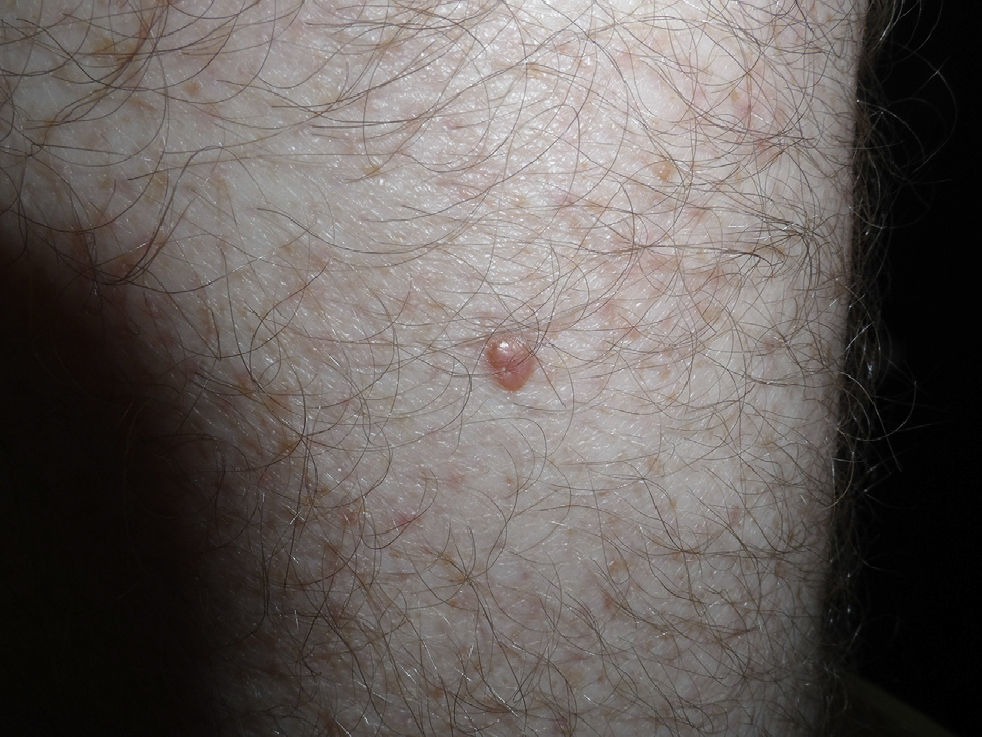
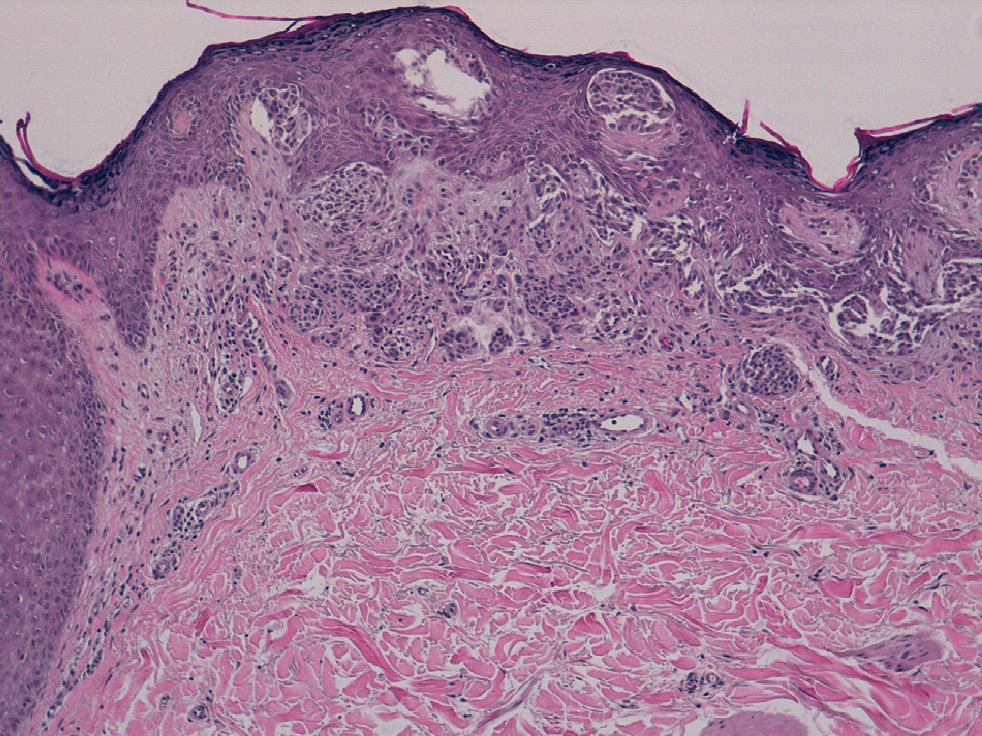
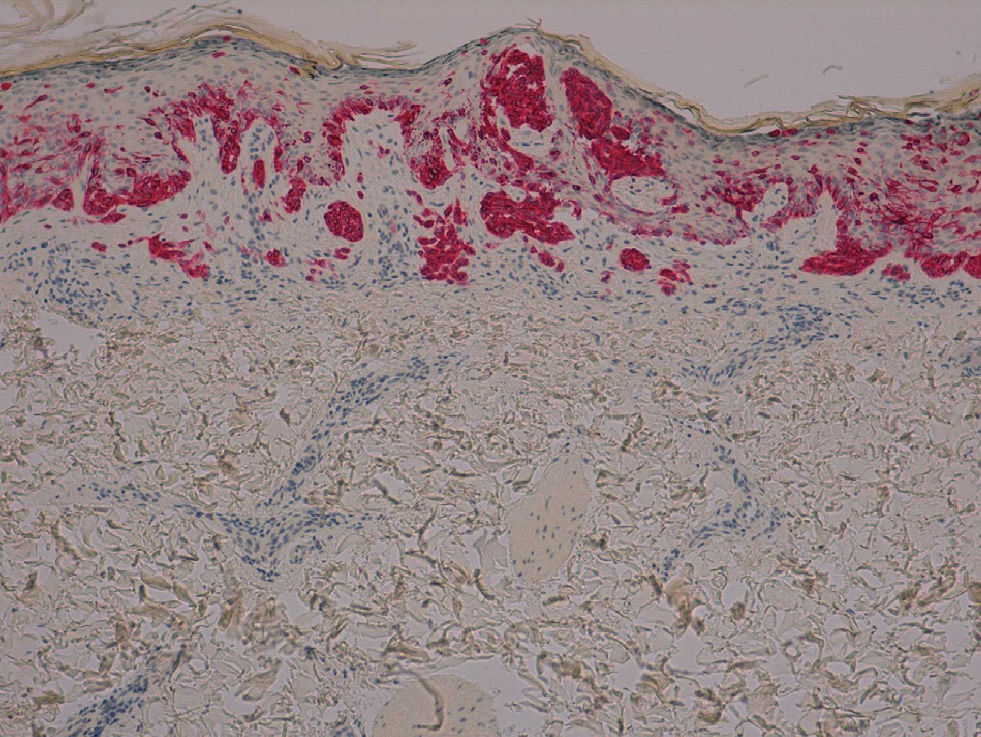
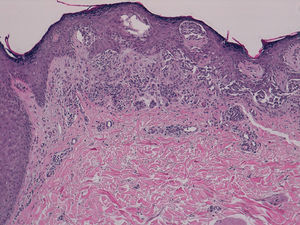
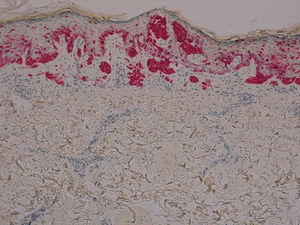
The patient was a 42-year old white man who had been diagnosed with a stage IV melanoma on the right arm 6 years earlier. Sentinel node biopsy results were positive and the patient underwent right axillary lymph node dissection followed by interferon therapy. Follow-up screening 6 years after surgery led to the detection of 2 pulmonary nodules and metastatic lesions in the C5 and T11 vertebrae, both iliac wings, and the sacrum. After confirmation of the presence of the BRAF V600E mutation, treatment was started with 960 mg of vemurafenib every 12 hours. Two months into treatment, we examined a 10 × 8-mm2 papular lesion on the left thigh that had been absent at the preceding visit, 1 month earlier, and had grown to this size gradually. The lesion was dome-shaped, skin-colored, and asymptomatic (Fig. 1). With a suspected diagnosis of hypertrophic actinic keratosis, complete excisional biopsy of the lesion was performed. Histologic findings included a proliferation of melanocytes in the epidermis and papillary dermis, with mild architectural disorder (Fig. 2). Melanocytes were arranged in nests in the epidermis and papillary dermis, and nuclear pleomorphism was mild. Immunohistochemical staining with Melan-A and HMB-45 showed a proliferation of melanocytes in the basal layer of the epidermis and scattered single melanocytes in the upper layers (Fig. 3). All these findings were consistent with a dysplastic nevus.
In cells with wild-type BRAF, vemurafenib increases phosphorylation of ERK (extracellular signal–regulated kinase) and thus paradoxically induces hyperactivation of the MAPK signaling pathway, promoting cell proliferation and survival. Hyperactivation is responsible for adverse effects such as keratoacanthoma, squamous cell carcinoma, and actinic keratosis. This paradoxical activation effect can also trigger progression of benign melanocytic lesions to melanoma.6 Atypical melanocytic proliferation and the development of new melanomas have been described in patients undergoing treatment with vemurafenib, and vemurafenib-induced changes in preexisting nevi have been observed by dermoscopy.7
This case in which a patient on vemurafenib developed an amelanotic dysplastic melanocytic nevus highlights the need to monitor this kind of lesion closely and perform a biopsy if there is any doubt, to ensure early detection of incipient malignancies.
Please cite this article as: Estela Cubells JR, Victoria Martínez AM, Oliver Martínez V, Alegre de Miquel V. Nevus melanocítico atípico amelanótico inducido por vemurafenib. Actas Dermosifiliogr. 2014;105:726–727.