The Simplified Psoriasis Index (SPI) is a recently validated tool in Spanish that measures psoriasis severity by integrating 3 different spheres: clinical severity (SPI-s), psychosocial impact (SPI-p), and natural history (SPI-i). Our objective was to study the validity and equivalence of this new scale compared to routinely used scales such as the Psoriasis Area and Severity Index, PASI, and the Dermatology Life Quality Index (DLQI).
Materials and methodsThis was a cross-sectional and observational study that included 45 patients aged 18–74 years. Demographic data and information associated with psoriasis severity and the patients’ quality of life were collected, using PASI, DLQI, and SPI simultaneously. The correlation of reference scales (PASI and DLQI) with SPI was examined. The degree of agreement between the 2 versions of SPI completed by the physician (proSPI-s) and self-administered by the patient (saSPI-s) was also studied.
ResultsThe mean age of the study population was 51 years, with a mean psoriasis history of 14.05 years. A strong correlation was found between PASI and proSPI-s (r=0.89), as well as between DLQI and SPI-p (r=0.89), with a moderate correlation being reported between PASI and saSPI-s (r=0.52). The degree of agreement between proSPI-s and saSPI-s was moderate.
ConclusionsThese findings represent the initial results of real clinical practice using the validated Spanish version of SPI, making its use truly promising in the routine clinical practice.
El Simplified Psoriasis Index (SPI) es una herramienta recientemente validada al español, que mide la gravedad de la psoriasis integrando 3 esferas: gravedad clínica (SPI-s), impacto psicosocial (SPI-p) e historia natural (SPI-i). Nuestro objetivo fue estudiar la validez y equivalencia de esta nueva escala con las escalas que usamos de forma habitual (Psoriasis Area and Severity Index o PASI, y Dermatology Life Quality Index o DLQI).
Material y métodoEstudio observacional de corte transversal, con 45 pacientes de edades comprendidas entre los 18 y 74 años. Se recogieron sus datos demográficos y los relativos a la gravedad de la psoriasis y a la calidad de vida de los pacientes, utilizando simultáneamente estas escalas (PASI, DLQI y SPI). Se estudió la correlación de las escalas de referencia (PASI y DLQI) con el SPI. Se estudió el grado de concordancia entre las 2 versiones del SPI: versión cumplimentada por el médico (proSPI) y versión autocumplimentada por el paciente (saSPI).
ResultadosLa edad media fue de 51 años, con un tiempo de evolución media de la psoriasis de 14,05 años. Se obtuvo una buena correlación entre PASI y proSPI-s (r=0,89) y entre DLQI y SPI-p (r=0,89), y una correlación moderada entre PASI y saSPI-s (r=0,52). El grado de concordancia entre proSPI-s y saSPI-s fue moderado.
ConclusionesEstos datos suponen los primeros resultados de uso en la práctica clínica real del SPI en su versión en español validada, y hacen que el uso de esta escala sea prometedor en la práctica clínica habitual.
Psoriasis produces a wide variety of clinical signs and has a considerable impact on the patients’ quality of life, which requires tools that accurately reflect the severity of the disease and its implications.
The Psoriasis Area and Severity Index (PASI), defined by Fredriksson and Pettersson in 1978,1 has become the reference method for measuring the severity of psoriasis. It allows for standardized therapeutic decision-making in psoriasis that require systemic treatment and evaluates the response to treatment. However, this scale has never been adequately validated2 and presents limitations such as the lack of inclusion of the symptoms caused by the disease, the absence of utility in forms such as pustular psoriasis, arithmetic complexity, the need to estimate body surface area—with high intra- and inter-observer variability—lack of standardization of cutoff values for mild, moderate, and severe psoriasis, the need to resort to other scales to understand its psychosocial impact (Dermatology Life Quality Index, DLQI), and the limited importance it places on the distribution of lesions in areas with greater psychosocial or functional burden.
All this makes requires a new tool that allows for a more objective, simple, and comprehensive assessment of patients with psoriasis. The Simplified Psoriasis Index (SPI), developed in the United Kingdom,2 has been translated and validated into several languages,3–6 with promising results that encourage its use in our routine clinical practice for both children and adults.7
This scale does not require numerical calculation of the affected body surface area, includes an assessment of the patient's psychosocial sphere, and is easily reproducible. Additionally, it allows for better assessment of “special locations”; even if the patient has a small diseased surface area, these location will be categorized into the moderate or severe psoriasis group when lesions affect visible, bothersome, or hard-to-treat areas, such as face, palmoplantar area, axillary and groin folds, or genital area.
For all these reasons, this study aims to assess the clinical applicability of the SPI by studying the results obtained from using its Spanish-translated version in our real clinical practice.
Materials and methodsStudy designWe conducted a cross-sectional observational study of patients who attended the Psoriasis Unit of Hospital Universitario Virgen Macarena, Seville, Spain over a period of 2 weeks in November. After the clinical interview and physical examination of the patient, demographic variables were collected, and the PASI, DLQI, and SPI questionnaires were completed in their physician version (proSPI) and in their self-completed version by the patient (saSPI) for subsequent statistical analysis.
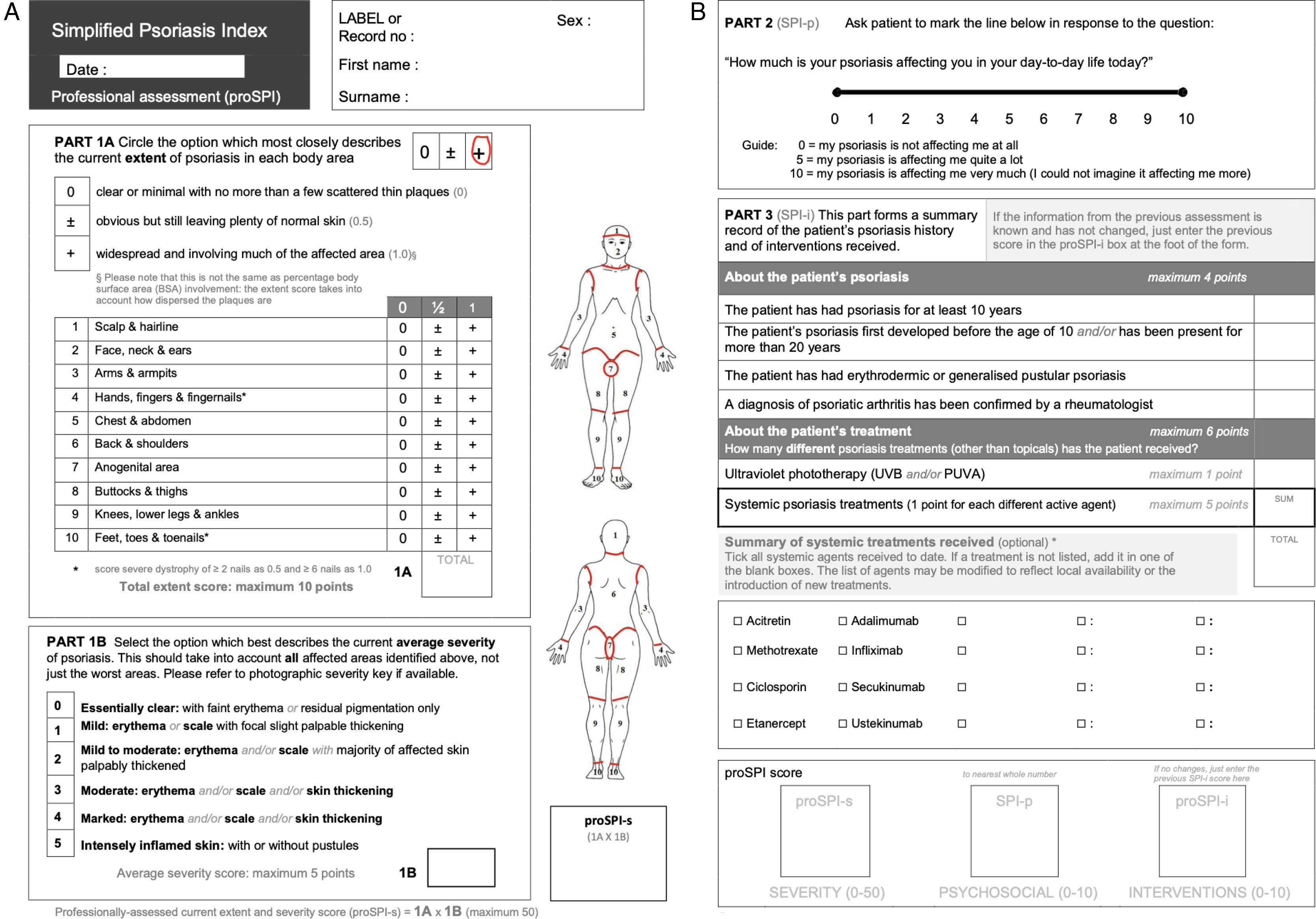
Instruments: simplified psoriasis indexThe SPI includes 3 domains:
Severity Component (SPI-severity, SPI-s): Its value range goes from 0 up to 50. It is categorized into 2 parts (1A and 1B). In the first part (1A), the extent of the lesions is assessed by assigning a score (0; 0.5 or 1) to each of the 10 locations in the questionnaire, according to the diseased surface area in each one. In the second part (1B), the mean clinical severity of the lesions is evaluated according to their characteristics.
Psychosocial Component (SPI-p): This is assessed using a 10cm analog scale that allows the patient's response to be transformed into a score from 0 to 10.
Natural History and Interventions (SPI-i): Its value range goes from 0 to 10. Points are added based on the years of disease progression, the presence of severe clinical forms—erythroderma or pustulosis—joint symptoms, and the modality and number of treatments used.
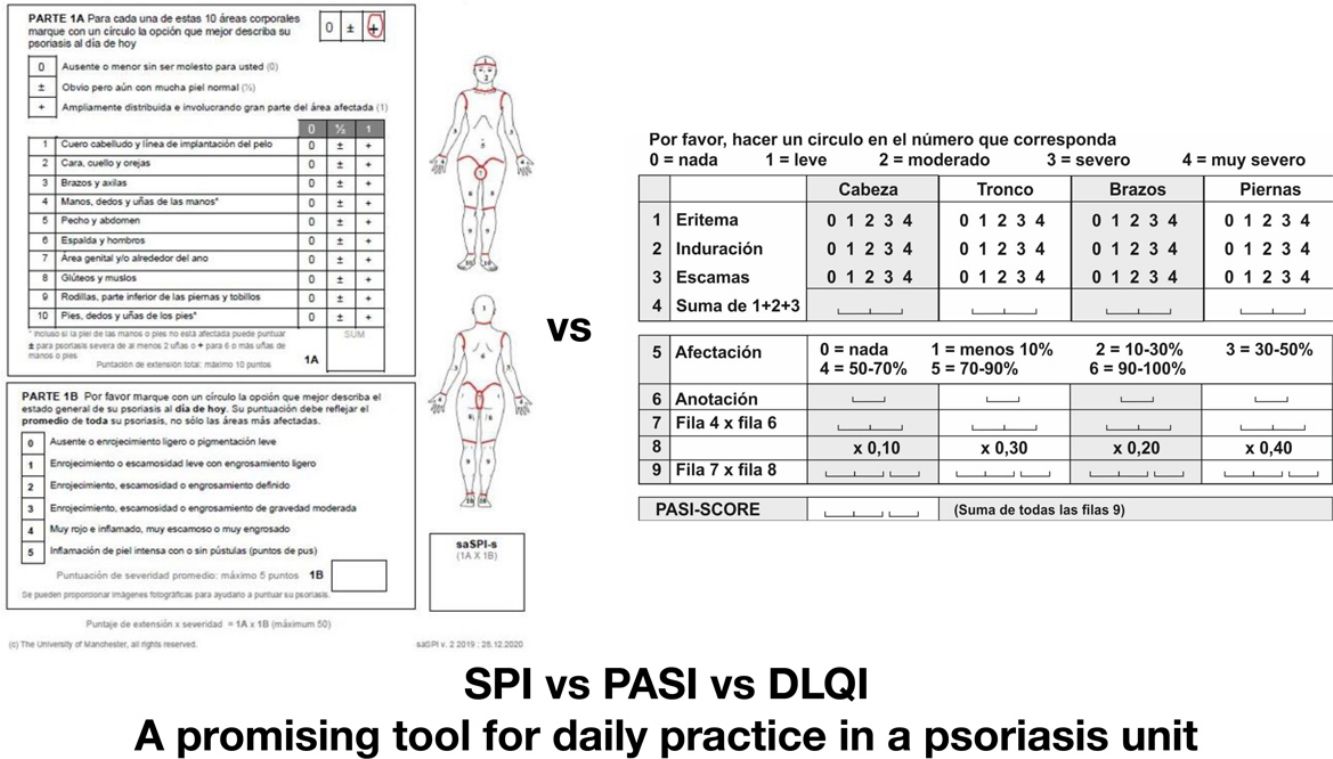
Additionally, there are 2 versions available—saSPI and proSP—that differ in the absence of technical language in the patient version (Figs. 1 and 2).
With this instrument, criterion validity and concordance are evaluated. Criterion validity is the degree to which a specific instrument adequately reflects the reference scale. To achieve this, we compared the degree of correlation of the SPI domain that assesses clinical severity (proSPI-s and saSPI-s) with the PASI, and similarly, the degree of correlation of the SPI ppsychosocial domain (SPI-p) with the DLQI. On the other hand, concordance quantifies the degree of reliability among multiple evaluators. To do this, the correlation between the professional's assessment and the patient's self-assessment was measured: proSPI-s and saSPI-s, in a generalized manner and broken down by body areas.
Statistical analysisThe collected data were analyzed using the IBM® SPSS® version 21 software. Spearman rank correlation coefficient was calculated across various measurement tools for the physical and psychosocial severity of psoriasis—proSPI, saSPI, PASI, SPI-p, DLQI—while the intraclass correlation coefficient was calculated for each of the body areas presented in the SPI-s, using the values obtained from the proSPI-s and saSPI-s scales.
ResultsThe study included a total of 45 patients, with a mean age of 51 years (95%CI, 46.11–55.90) and a mean duration of psoriasis of 14.05 years (95%CI, 9.07–19.03). Additionally, 26.6% exhibited associated psoriatic arthritis. Regarding clinical severity, they had mean PASI values of 4.51 (95%CI, 2.72–6.30) and mean proSPI-s and saSPI-s scores of 3.78 (95%CI, 2.36–5.21) and 5.12 (95%CI, 3.11–7.13), respectively. The mean DLQI value was 6.62 (95%CI, 4.21–9.03) and the SPI-p was 2.95 (95%CI, 2.23–3.68).
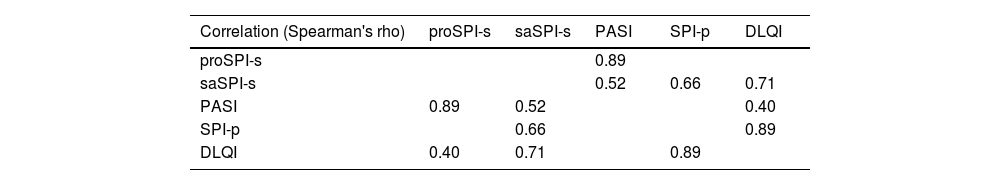
To evaluate criterion validity, the Spearman rank correlation coefficient was calculated between the gold standard and the SPI different modalities: a good correlation was observed between the PASI and proSPI-s (r=0.89) and between the DLQI and SPI-p (r=0.89). However, the correlation level between PASI and saSPI-s (r=0.52) was only moderate in the obtained sample (Table 1).
Correlations.
| Correlation (Spearman's rho) | proSPI-s | saSPI-s | PASI | SPI-p | DLQI |
|---|---|---|---|---|---|
| proSPI-s | 0.89 | ||||
| saSPI-s | 0.52 | 0.66 | 0.71 | ||
| PASI | 0.89 | 0.52 | 0.40 | ||
| SPI-p | 0.66 | 0.89 | |||
| DLQI | 0.40 | 0.71 | 0.89 |
The highlighted values correspond to the most important values discussed in the text.
DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index; proSPI-s: severity component of the Simplified Psoriasis Index completed by the professional; saSPI-s: severity component of the Simplified Psoriasis Index self-completed by the patient; SPI-p: psychosocial impact of the Simplified Psoriasis Index.
To evaluate the concordance of the 2 modalities of the SPI scale, the intraclass correlation coefficient (ICC) was calculated between the proSPI-s and saSPI-s values of each of the studied locations, yielding values<0.75 (moderate reliability) in the face, neck, and ears (0.72); arms and armpits (0.63); chest and abdomen (0.74); anogenital area (0.68); buttocks and thighs (0.72); knees, lower legs, and ankles (0.74).
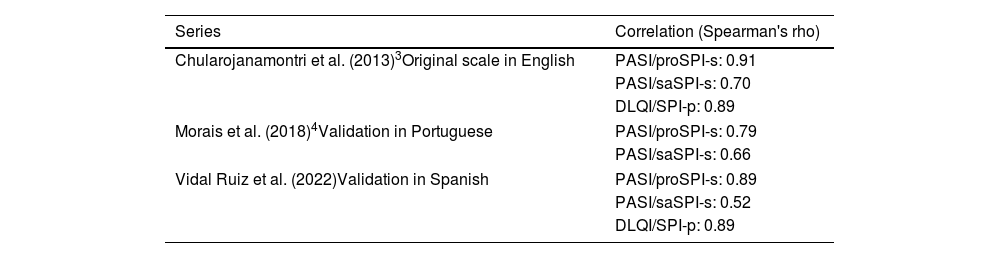
DiscussionThe use of the validated Spanish version of the SPI scale in then routine clinical practice allows for a comprehensive assessment of psoriasis by simplifying the calculation of the diseased surface area in each location by giving equal weight to each. Additionally, it includes an assessment of the psychosocial impact in the form of a visual analog scale, which is simple and quick. In the initial development by Chularojanamontri et al., a good correlation between the proSPI—criterion validity—and the reference criterion—PASI—was observed, as well as a good correlation between SPI-p and the DLQI, which is consistent with the results seen in this study. However, the correlation observed between saSPI-s and PASI is not as intense, not even compared to the validation study of the scale in Portuguese (Table 2).
Comparison of correlation values between PASI/proSPI-s and saSPI-s, and DLQI/SPI-p across different published series.
| Series | Correlation (Spearman's rho) |
|---|---|
| Chularojanamontri et al. (2013)3Original scale in English | PASI/proSPI-s: 0.91 |
| PASI/saSPI-s: 0.70 | |
| DLQI/SPI-p: 0.89 | |
| Morais et al. (2018)4Validation in Portuguese | PASI/proSPI-s: 0.79 |
| PASI/saSPI-s: 0.66 | |
| Vidal Ruiz et al. (2022)Validation in Spanish | PASI/proSPI-s: 0.89 |
| PASI/saSPI-s: 0.52 | |
| DLQI/SPI-p: 0.89 | |
To understand these results, we considered the possibility that some locations were being systematically undervalued by the professional. The SPI scale breaks down into 10 body areas, allowing special locations to have a greater weight than in PASI. When calculating the ICC between proSPI-s and saSPI-s for each of the studied locations, we obtained moderate reliability values in certain locations. Notably, the ICC was lower, on one hand, in the anogenital area (0.68), a region that is often not thoroughly explored in the routine clinical practice despite the impact it has on disease severity; and on the other hand, although less expected due to being a much more accessible location for examination, in arms and armpits (0.63). This disparity could partly justify the lower correlation reported between saSPI-s and PASI, although the published evidence to date does not include this information, so further studies with a larger number of patients and a breakdown by locations would be necessary to corroborate this hypothesis.
Limitations include the small number of patients included in the study sample and the lack of prospective follow-up, which prevents us from studying the scale across the patient's clinical progression. Additionally, the good disease control that the participants included in the study generally present implies that they have a relatively low PASI. This circumstance may have partly conditioned the results obtained.
ConclusionsThis study describes the first results of the use of the validated Spanish version of the SPI in real clinical practice, showing a good correlation between the SPI-s performed by the professional and the PASI scale, and between the psychosocial assessment and the DLQI. The simplicity of its calculation and its integrative nature, along with the evaluation of the disease natural history make the use of this scale promising for our clinical practice. The use of the saSPI scale could be a useful tool for remote monitoring of patients with psoriasis, although its poorer correlation with the PASI poses a limitation.
Conflicts of interestNone declared.