The Spanish registry of Mohs micrographic surgery started collecting data in July 2013. The aim of the registry is to report on the use of this technique in Spain and the outcomes achieved. In the present article, we describe the characteristics of patients and the tumors treated.
Material and methodsThis is a prospective cohort study of patients treated with Mohs micrographic surgery. The participating centers are hospitals where at least one intervention of this type is performed each week. All patients considered for Mohs micrographic surgery in participating centers are included in the registry except those who have been declared legally incompetent.
ResultsBetween July 2013 and October 2014, data from 655 patients were included in the registry. The most common tumor involved was basal cell carcinoma, and the most common histological subtype was infiltrative basal cell carcinoma. Most of the tumors treated were located on the face or scalp, and the most common site was the nose. Almost 40% of the tumors treated were recurrent or persistent, and preoperative tumor size was similar to that reported in other European studies and in Australia. In total, 45.5% of patients had received previous surgical treatment.
ConclusionThe findings are similar to those reported in other studies, and the data collected are useful for assessing whether the results of studies carried out elsewhere are applicable in Spain.
En julio de 2013 se inició la recogida de datos del registro español de cirugía micrográfica de Mohs, que describe la aplicación y los resultados de esta técnica en España. En este artículo se describen las características del paciente y de los tumores tratados.
Material y métodosSe trata de un estudio de cohortes prospectivo en el que participan centros en los que se practica al menos una intervención semanal de cirugía micrográfica de Mohs. En cada centro se incluyen todos los pacientes que son valorados para realizar cirugía de Mohs, excepto los declarados judicialmente incapaces. En este artículo describimos las características de los pacientes y los tumores incluidos en la cohorte.
ResultadosEl número de pacientes incluidos desde julio de 2013 hasta octubre de 2014 es de 655. La mayoría de los tumores cutáneos intervenidos correspondieron a carcinoma basocelular, siendo el infiltrante el subtipo histológico más frecuente. La mayoría de las cirugías se practicaron en tumores localizados en la cara y el cuero cabelludo, siendo la localización más frecuente la nariz. Casi el 40% de los tumores operados son recurrentes o persistentes, y el tamaño tumoral prequirúrgico es similar en nuestro medio al descrito en otros estudios australianos o europeos. Hasta el 45,5% de los pacientes había recibido algún tratamiento quirúrgico previo.
ConclusiónLos datos observados son similares a los de otras series publicadas, y son relevantes para poder valorar la aplicabilidad en nuestro contexto de estudios realizados en otros medios.
Mohs micrographic surgery (MMS) is a technique for skin cancer treatment that is available at many Spanish health care facilities, both public and private. Most experiences with MMS described in the literature—including large case series with long-term follow-up data—are from the United States and Australia, although a few reports from European and Latin American hospitals have recently started to appear. In an October 2012 report, the American Academy of Dermatology attempted to unify therapeutic criteria by rating the use of MMS as “appropriate,” “uncertain,” or “inappropriate” for 270 scenarios in which this type of surgery is often considered on the basis of tumor type and patient characteristics.1 However, because there are fewer restrictions on the use of MMS in the United States than in Europe, American studies and results can have limited applicability in Spain, where the tumors treated with this technique are often larger or more complex.
A few teams of dermatologists have reported their experiences with MMS in Spain.2–4 However, because the findings of these studies correspond to individual hospitals, they may not be representative of the use of MMS at the national level.
The Spanish Registry of Mohs Surgery (REGESMOHS) was created in July 2013 and now has 15 participating hospitals. Its main objective is to describe the use of MMS in Spain and the factors that influence the outcomes achieved. In this initial report, we describe the characteristics of the patients and tumors treated with MMS in Spain.
Materials and MethodsREGESMOHS is a prospective cohort study. The 15 participating centers are public and private Spanish hospitals where MMS is performed at least once each week.
Before launching the registry, we identified the hospitals that regularly performed MMS and invited them to participate in the study. Fifteen of the 22 hospitals contacted are currently participating in REGESMOHS (Table 1) and the registry is open to the addition of new hospitals. All patients considered for MMS at the participating hospitals—except those who have been declared legally incompetent—are enrolled consecutively in the study.
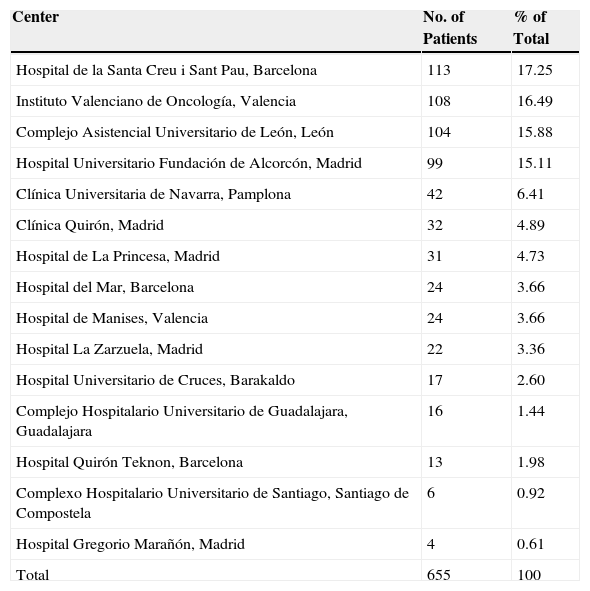
Number and Percentage of Patients From Each Participating Center.
| Center | No. of Patients | % of Total |
|---|---|---|
| Hospital de la Santa Creu i Sant Pau, Barcelona | 113 | 17.25 |
| Instituto Valenciano de Oncología, Valencia | 108 | 16.49 |
| Complejo Asistencial Universitario de León, León | 104 | 15.88 |
| Hospital Universitario Fundación de Alcorcón, Madrid | 99 | 15.11 |
| Clínica Universitaria de Navarra, Pamplona | 42 | 6.41 |
| Clínica Quirón, Madrid | 32 | 4.89 |
| Hospital de La Princesa, Madrid | 31 | 4.73 |
| Hospital del Mar, Barcelona | 24 | 3.66 |
| Hospital de Manises, Valencia | 24 | 3.66 |
| Hospital La Zarzuela, Madrid | 22 | 3.36 |
| Hospital Universitario de Cruces, Barakaldo | 17 | 2.60 |
| Complejo Hospitalario Universitario de Guadalajara, Guadalajara | 16 | 1.44 |
| Hospital Quirón Teknon, Barcelona | 13 | 1.98 |
| Complexo Hospitalario Universitario de Santiago, Santiago de Compostela | 6 | 0.92 |
| Hospital Gregorio Marañón, Madrid | 4 | 0.61 |
| Total | 655 | 100 |
For each entry added to the registry, we record patient and surgery characteristics, as well as the short-term outcome (at a postoperative appointment) and long-term outcome. Patients are followed up at least once a year so that their long-term outcomes can be described. In patients with several successive tumors, all tumors are described in the registry but follow-up data are only recorded for the first tumor.
REGESMOHS was designed so that its objectives could be achieved within 6 years if patient enrollment targets were met.
The study was approved by the research ethics committee of Navarre, the Spanish Agency of Medicines and Medical Devices (AEMPS), and all participating hospitals. It was conducted in accordance with the Declaration of Helsinki and current legislation. Written informed consent was obtained from all participating patients.
Following an established protocol, data were collected using an online data collection system (OpenClinica open source software, version 3.1) that belongs to the research unit of the Foundation of the Spanish Academy of Dermatology and Venereology. The statistical analysis was carried out using the Stata software package (version 13.1, Statacorp).
ResultsA total of 655 patients were included in REGESMOHS between July 2013, when the registry opened, and October 2014. The mean age at the time of the surgery was 68.4 (13.6) years. The 341 men who received MMS accounted for slightly more than half (52.06%) of the patients, while the 314 women accounted for 47.94%.
Patients lived in the catchment area of the hospital where the surgery was performed in 496 cases (75.73%) and lived elsewhere in 158 cases (24.12%).
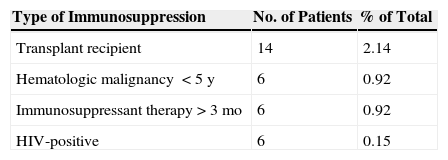
Immunosuppression was present in 27 patients (4.13%), diabetes mellitus in 80 (12.21%), and a diagnosis of some sort of multiple tumor syndrome in 28 (4.27%) (Table 2).
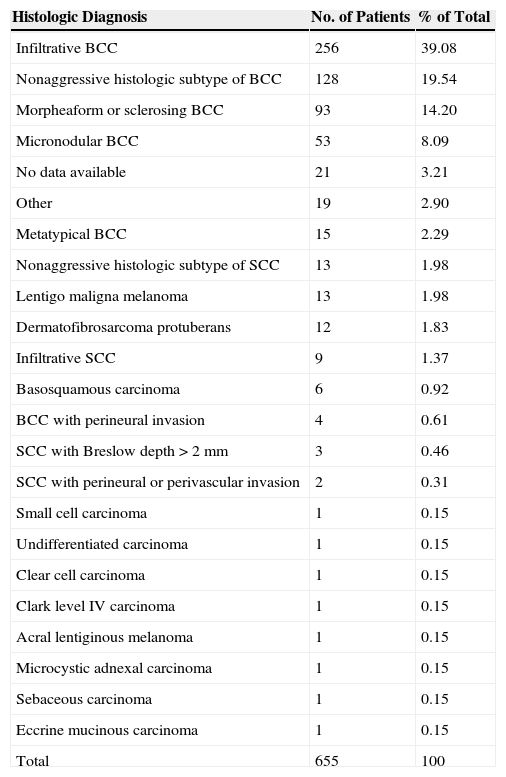
The most common type of skin tumor was basal cell carcinoma (BCC), which was present in 589 patients (89.93%), and the most common histologic subtype was infiltrative BCC, which was present in 256 patients (39.08%). The second most common tumor type was squamous cell carcinoma (SCC), which was present in 35 patients (5.64%). Table 3 shows the percentages corresponding to other histologic subtypes of BCC and SCC, as well as the other types of tumors treated.
Frequency and Percentage Distribution by Histologic Tumor Type.
| Histologic Diagnosis | No. of Patients | % of Total |
|---|---|---|
| Infiltrative BCC | 256 | 39.08 |
| Nonaggressive histologic subtype of BCC | 128 | 19.54 |
| Morpheaform or sclerosing BCC | 93 | 14.20 |
| Micronodular BCC | 53 | 8.09 |
| No data available | 21 | 3.21 |
| Other | 19 | 2.90 |
| Metatypical BCC | 15 | 2.29 |
| Nonaggressive histologic subtype of SCC | 13 | 1.98 |
| Lentigo maligna melanoma | 13 | 1.98 |
| Dermatofibrosarcoma protuberans | 12 | 1.83 |
| Infiltrative SCC | 9 | 1.37 |
| Basosquamous carcinoma | 6 | 0.92 |
| BCC with perineural invasion | 4 | 0.61 |
| SCC with Breslow depth>2mm | 3 | 0.46 |
| SCC with perineural or perivascular invasion | 2 | 0.31 |
| Small cell carcinoma | 1 | 0.15 |
| Undifferentiated carcinoma | 1 | 0.15 |
| Clear cell carcinoma | 1 | 0.15 |
| Clark level IV carcinoma | 1 | 0.15 |
| Acral lentiginous melanoma | 1 | 0.15 |
| Microcystic adnexal carcinoma | 1 | 0.15 |
| Sebaceous carcinoma | 1 | 0.15 |
| Eccrine mucinous carcinoma | 1 | 0.15 |
| Total | 655 | 100 |
Abbreviations: BCC, basal cell carcinoma; SCC, squamous cell carcinoma.
Taken together, lentigo maligna melanoma, acral lentiginous melanoma, dermatofibrosarcoma protuberans, microcystic adnexal carcinoma, sebaceous carcinoma, and eccrine mucinous carcinoma were present in 29 patients (4.41%).
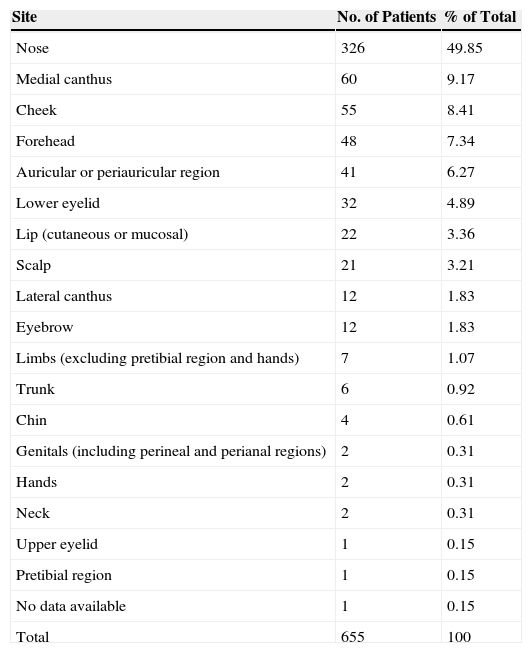
In 630 cases (96.18%), the surgery was used to treat tumors on the face or scalp, with the nose being the most frequent site (326 cases, 49.85%). Table 4 shows the frequencies and percentages of the various affected areas.
Tumor Site.
| Site | No. of Patients | % of Total |
|---|---|---|
| Nose | 326 | 49.85 |
| Medial canthus | 60 | 9.17 |
| Cheek | 55 | 8.41 |
| Forehead | 48 | 7.34 |
| Auricular or periauricular region | 41 | 6.27 |
| Lower eyelid | 32 | 4.89 |
| Lip (cutaneous or mucosal) | 22 | 3.36 |
| Scalp | 21 | 3.21 |
| Lateral canthus | 12 | 1.83 |
| Eyebrow | 12 | 1.83 |
| Limbs (excluding pretibial region and hands) | 7 | 1.07 |
| Trunk | 6 | 0.92 |
| Chin | 4 | 0.61 |
| Genitals (including perineal and perianal regions) | 2 | 0.31 |
| Hands | 2 | 0.31 |
| Neck | 2 | 0.31 |
| Upper eyelid | 1 | 0.15 |
| Pretibial region | 1 | 0.15 |
| No data available | 1 | 0.15 |
| Total | 655 | 100 |
MMS was indicated for primary tumors in 410 patients (62.60%), for recurrent tumors in 164 patients (25.04%), and for persistent tumors in 81 (12.37%). The mean tumor size before surgery (among patients for whom this information was collected) was 13.9 (14.78) mm along the long axis and 9.8 (9.47) mm along the short axis.
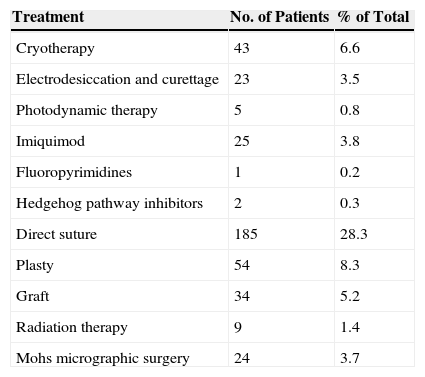
Prior to receiving MMS, 99 patients (15.2%) received medical treatment, 297 (45.5%) underwent surgery, and 9 (1.4%) received radiation therapy (Table 5). Some patients underwent more than 1 previous surgery.
Treatments Prior to Mohs Micrographic Surgery.
| Treatment | No. of Patients | % of Total |
|---|---|---|
| Cryotherapy | 43 | 6.6 |
| Electrodesiccation and curettage | 23 | 3.5 |
| Photodynamic therapy | 5 | 0.8 |
| Imiquimod | 25 | 3.8 |
| Fluoropyrimidines | 1 | 0.2 |
| Hedgehog pathway inhibitors | 2 | 0.3 |
| Direct suture | 185 | 28.3 |
| Plasty | 54 | 8.3 |
| Graft | 34 | 5.2 |
| Radiation therapy | 9 | 1.4 |
| Mohs micrographic surgery | 24 | 3.7 |
None of the patients had nodal or metastatic disease.
MMS was contraindicated in 25 patients (3.7%). The most frequent reason for contraindication, present in 7 cases (35%), was the clinical context of the patient, followed by tumor size, histologic findings, and site. Of the patients in whom MMS was contraindicated, 10 (43.48%) underwent conventional surgery, 8 (34.78%) received radiation therapy, and 1 was treated with hedgehog pathway inhibitors.
DiscussionMMS is a surgical technique designed for the specific treatment of certain types of skin cancer, mainly tumors in certain sites or with a high risk of recurrence. The technique is based on 2 main principles. Intraoperative microscopic confirmation of the surgical margins of the excised tumor specimen, properly oriented and stained, allows the surgeon to determine the precise location of the tumor foci (in cases of persistent tumors). Consequently, tumors can be completely excised in a single procedure carried out in 1 or more stages.
In our study, slightly more men (52.06%) received MMS than women (47.94%), although the difference was not statistically significant. The mean age at the time of the procedure was 68.4 (13.6) years, and immunosuppression was present in only 27 patients (4.27%), most of whom had received a solid organ transplant. Age at tumor presentation, sex distribution, and tumor type were similar to those found in case series in the United States, Australia, the United Kingdom, and the Netherlands.
In the Australian registry, which contains 11 127 patients diagnosed with BCC who underwent MMS, the procedure was slightly more common in men (52.9%) than in women (47.1%) and the mean age at the time of the surgery was 62 (14) years.5 In a series of 1263 patients diagnosed with SCC, the same authors found that the tumor was present in a higher percentage of men (74.3%) than in women (25.7%), that the differences were statistically significant (P<.0001), and that the age at presentation was similar in both sexes to that found in the previous study.6 In a British case series in which 751 patients underwent MMS for various types of skin tumors, the surgery was more common in men (53%) than in women (47%) and the mean age was 69 years (range 22-92).7
In the most recent report on the Dutch registry, MMS was more common in men than in women, and the mean age at presentation—and also at the time of surgery—was more than 65 years.8 In a Dutch series of 633 patients with 737 BCCs published in 2004, Smeets et al found that MMS was slightly more common in men (345 cases, 56%) than in women (275 cases, 44%) and that the mean age was 65 years (range 22-94).9 A recent American series, in which 12 344 patients were treated for BCCs and SCCs at Brown University in Rhode Island, was the only study to find a slightly higher mean age at the time of surgery, with women being slightly younger (70 [14] years) than men (72 [12] years).10
In the studies cited above, as in our registry, some form of immunosuppression was present in only a minority of patients, most of whom were solid organ transplant recipients.
In our series, the most common tumor type was BCC, present in 589 patients (89.93%), and the most common histologic subtype was infiltrative BCC, present in 256 (39.08%). The second most common tumor type was SCC, present in 35 patients (5.64%), followed by lentigo maligna melanoma, in 13 patients (1.98%), and dermatofibrosarcoma protuberans, in 12 patients (1.83%). Less frequent tumors—such as acral lentiginous melanoma, microcystic adnexal carcinoma, sebaceous carcinoma, and eccrine mucinous carcinoma—accounted for 4 cases (0.6%). In all 5 series cited above, BCC was the most prevalent type of skin tumor.5–10
In 630 patients (96.18%), the site of the tumor—whether primary, recurrent, or persistent—was on the face or scalp, and the most common site, in 326 patients (49.85%), was the nose. The next most frequent sites were the medial canthus, in 60 cases (9.17%), the malar region, in 55 (8.41%), the forehead, in 48 (7.34%), and the auricular and preauricular region, in 41 (6.27%). The trunk, extremities, and genitals were less frequent sites, together accounting for 20 cases (3.05%). These results are similar to those of the series mentioned above, with minor variations. In the Australian series, the most common site for BCCs and SCCs (primary and recurrent) was the nose (39.1% and 20% of cases, respectively). Among patients with BCCs, the second most common site was the periocular region for primary tumors (935 cases, 14.9%) and the malar region for recurrent tumors (1050 cases, 21.05%). The third most common site was the malar region for primary BCCs (789 cases, 12.6%) and the auricular region for recurrent BCCs (543 cases, 11.1%). Among patients with SCCs, the second most common site was the malar region for primary tumors (145 cases, 18.8%) and the auricular region for recurrent tumors (89 cases, 18.1%).5,6 In the British series, the most frequent site for both primary and recurrent tumors was the head and scalp (446 cases, 98%). The most common sites for primary tumors were the nose (39%) and the periocular region (21%), whereas the temporal region was the most common site for recurrent tumors.7 The Dutch registry had similar findings, with the most common site being the head and neck, followed by the trunk and the extremities in both sexes.8 Similarly, in the case series of Smeets et al, the most common site for BCCs was the nose (219 cases, 30%) followed by the forehead and the temporal region (167 cases, 23%).9
In the American series, SCCs were more common in men (24%) than in women (16%) (P<.01), a finding consistent with data in the literature. The most common site for primary BCCs and SCCs was the nose (27% in women, 19% in men), followed by the auricular region in men (15%) and the malar region in women (14%) and, in third place, the malar region in men (14%) and the forehead in women (12%). In general, the American study found that men were more likely to present nonmelanoma skin cancer on the scalp and auricular region, whereas women were more likely to have tumors in the central facial area and lower limbs. The same study found that superficial BCCs were slightly more common in women (P<.001), whereas the British and Dutch series found that this tumor type was more common in men. The American authors suggested that more frequent clinical check-ups might explain the higher frequency of superficial BCCs in women, as these check-ups would allow the early detection of smaller and less invasive tumors. They also suggested that intense intermittent exposure to UV radiation—closely related to the development of superficial BCCs—may be more prevalent in women.10
In our series, MMS was used to treat primary tumors in 410 cases (62.60%), recurrent tumors in 164 cases (25.04%), and persistent tumors in 81 cases (12.37%). These data are comparable to those of the Australian series, in which primary tumors were the most common indication for MMS in BCCs (56.2% of cases) and in SCCs (61.1% of cases); in cases in which MMS was used to treat recurrent tumors, the most common previous treatment was simple surgery.5,6 In the Dutch series of Smeets et al, very similar percentages of patients received MMS for primary BCCs (365 cases, 50.8%) and for recurrent or persistent BCCs (355 cases, 49.2%), and the most frequent prior treatment in recurrent tumors was simple surgical excision, used in 115 cases (45.3%).9
The most common prior treatment in the REGESMOHS registry was cryotherapy, used in 43 patients (6.6%), followed by topical imiquimod, used in 25 patients (3.8%). Two patients (0.3%) were treated with hedgehog pathway inhibitors. Among REGESMOHS patients who had previously received surgical treatment, the most common technique, used in 185 cases (28.3%), was simple surgical excision, whereas only 24 patients (3.7%) had previously undergone MMS.
As mentioned above, the mean tumor size before surgery—taking into account all tumor types—was 13.9 (14.78) mm along the long axis and 9.8 (9.47) mm along the short axis. These data are also comparable to those of other series.
MMS was contraindicated in only 25 cases (3.7%), mainly because of the clinical context of the patient (multiple conditions, multiple-drug regimens, difficulty of movement, or high degree of dependence for basic activities of daily living).
Advantages of the study: Compared with previous studies, ours is much more representative of the current use of MMS in Spain because we collected data in a prospective, standardized manner from a majority of the Spanish hospitals that perform the procedure.
Limitations of the study: Some hospitals are missing from the registry and the current sample size does not allow the most common variables to be quantified with great precision.
ConclusionOur data show that BCCs are the tumors most frequently treated with MMS, that nearly 40% of treated tumors are recurrent or persistent, and that tumor size before surgery in Spain is similar to that reported elsewhere in Europe and in Australia. These data will be useful in assessing whether studies conducted in other countries are applicable to the Spanish context.
Ethical DisclosuresProtection of persons and animalsThe authors declare that no experiments were performed on humans or animals for the purpose of this study.
Data confidentialityThe authors declare that they followed their hospitals’ regulations regarding the publication of patient information.
Right to privacy and informed consentThe authors obtained informed consent from the patients and/or subjects referred to in this article. The signed forms are in the possession of the corresponding author.
FundingREGESMOHS is funded by the Foundation of the Spanish Academy of Dermatology and Venereology, with the collaboration of Roche Farma. The funding laboratory did not participate in the preparation of the article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The following REGESMOHS members participated in data collection: Lucía Ascanio Armada and Dolores Caro Gutierrez (Hospital Universitario Fundación Alcorcón); Carlos Serra Guillén, Eduardo Nagore Enguidanos, Beatriz Llombart Cussac, and Celia Requena Caballero (Instituto Valenciano de Oncología); Victoriano Morales Gordillo (Hospital Quirón, Pozuelo, Madrid); Luis Hueso Gabriel and Antonio Martorell (Hospital de Manises); M. José Seoane Pose (Complexo Hospitalario Universitario de Santiago); Ricardo Suárez and Natividad Cano (Hospital Gregorio Marañón, Madrid); and Beatriz Pérez Zafrilla (registry monitor). We also thank the staff of all the departments participating in the registry.
Please cite this article as: Ruiz-Salas V, Garcés JR, Miñano Medrano R, Alonso-Alonso T, Rodríguez-Prieto MÁ, López-Estebaranz JL, et al. Descripción de los pacientes intervenidos mediante cirugía de Mohs en España. Datos basales del registro español de cirugía de Mohs (REGESMOHS). Actas Dermosifiliogr. 2015;10:562–568.