Eccrine porocarcinoma is a rare, malignant cutaneous adnexal tumor that arises from the ducts of sweat glands. Found mainly in patients of advanced age, this tumor has diverse clinical presentations. Histology confirms the diagnosis, detects features relevant to prognosis, and guides treatment. Growth is slow, but the prognosis is poor if the tumor metastasizes to lymph nodes or visceral organs. We report 7 cases of eccrine porocarcinoma, describing patient characteristics, the clinical and histopathologic features of the tumors, and treatments used. Our observations were similar to those of other published case series. Given the lack of therapeutic algorithms or protocols for this carcinoma, we propose a decision-making schema based on our review of the literature and our experience with this case series. The algorithm centers on sentinel lymph node biopsy and histologic features.
El porocarcinoma ecrino es un tumor anexial cutáneo maligno poco frecuente, que deriva de la porción ductal de las glándulas sudoríparas. Predomina en pacientes de edad avanzada, pudiendo adoptar diversas presentaciones clínicas. El estudio histológico confirma el diagnóstico y establece factores pronósticos relevantes a la hora de decidir el tratamiento. El curso evolutivo es lento, pero su pronóstico es sombrío cuando aparecen metástasis linfáticas o viscerales. Presentamos 7 pacientes diagnosticados de porocarcinoma ecrino, y describimos los aspectos epidemiológicos, clínicos, histopatológicos y los datos relacionados con el tratamiento de cada uno de ellos. Comparamos los datos obtenidos con las mayores series de casos publicadas, obteniendo similares resultados. Ante la ausencia de algoritmos terapéuticos protocolizados se propone un esquema de tratamiento basado en la literatura revisada y la experiencia personal con nuestra serie, que tiene como ejes centrales los factores pronósticos histológicos y la biopsia selectiva de ganglio centinela.
Eccrine porocarcinoma (EP) was described in 1963 by Pinkus and Mehregan,1 but it was Mishima and Morioka2 who introduced this name in 1967. EP is a rare neoplasm (0.005% to 0.01% of all malignant skin tumors).3 It arises most commonly on the lower limbs4–6 and on the head and neck7–9 of patients of advanced age,10 with no difference between the sexes.5,7 Clinical presentation is variable, and the disease can mimic many other tumors. Histology not only confirms the diagnosis but also identifies prognostic factors that will determine the therapeutic approach.4 The majority of descriptions in the literature are isolated case reports or small case series, making it difficult to draw up standardized treatment guidelines beyond surgical excision. Prognosis is very poor when metastases are present.11
Case DescriptionsWe present 7 patients diagnosed with EP, evaluating their demographic and clinical characteristics (Table 1), histopathology findings (Table 2), and data regarding treatment and clinical course (Table 3).
Most Relevant Demographic and Clinical Findings in the Series Presented.
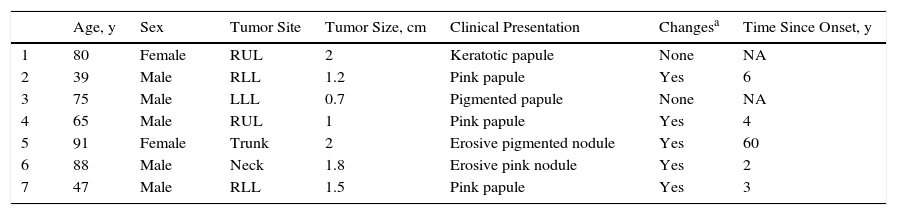
| Age, y | Sex | Tumor Site | Tumor Size, cm | Clinical Presentation | Changesa | Time Since Onset, y | |
|---|---|---|---|---|---|---|---|
| 1 | 80 | Female | RUL | 2 | Keratotic papule | None | NA |
| 2 | 39 | Male | RLL | 1.2 | Pink papule | Yes | 6 |
| 3 | 75 | Male | LLL | 0.7 | Pigmented papule | None | NA |
| 4 | 65 | Male | RUL | 1 | Pink papule | Yes | 4 |
| 5 | 91 | Female | Trunk | 2 | Erosive pigmented nodule | Yes | 60 |
| 6 | 88 | Male | Neck | 1.8 | Erosive pink nodule | Yes | 2 |
| 7 | 47 | Male | RLL | 1.5 | Pink papule | Yes | 3 |
Abbreviations: LLL, left lower limb; NA, not available; RLL, right lower limb; RUL, right upper limb.
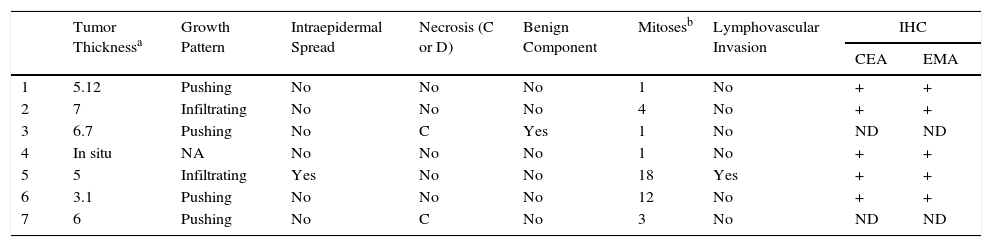
Most Relevant Histologic Findings in Our Series.
| Tumor Thicknessa | Growth Pattern | Intraepidermal Spread | Necrosis (C or D) | Benign Component | Mitosesb | Lymphovascular Invasion | IHC | ||
|---|---|---|---|---|---|---|---|---|---|
| CEA | EMA | ||||||||
| 1 | 5.12 | Pushing | No | No | No | 1 | No | + | + |
| 2 | 7 | Infiltrating | No | No | No | 4 | No | + | + |
| 3 | 6.7 | Pushing | No | C | Yes | 1 | No | ND | ND |
| 4 | In situ | NA | No | No | No | 1 | No | + | + |
| 5 | 5 | Infiltrating | Yes | No | No | 18 | Yes | + | + |
| 6 | 3.1 | Pushing | No | No | No | 12 | No | + | + |
| 7 | 6 | Pushing | No | C | No | 3 | No | ND | ND |
Abbreviations: C, comedonecrosis; D, diffuse necrosis; IHC, immunohistochemistry; NA, not applicable; ND, not determined.
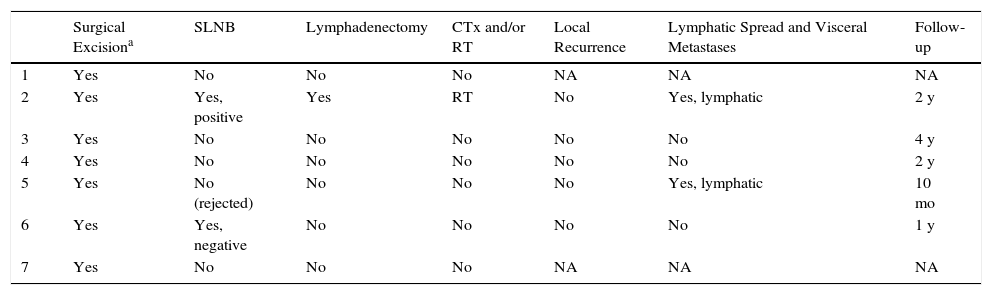
Treatment and Clinical Course of the Patients in Our Series.
| Surgical Excisiona | SLNB | Lymphadenectomy | CTx and/or RT | Local Recurrence | Lymphatic Spread and Visceral Metastases | Follow-up | |
|---|---|---|---|---|---|---|---|
| 1 | Yes | No | No | No | NA | NA | NA |
| 2 | Yes | Yes, positive | Yes | RT | No | Yes, lymphatic | 2 y |
| 3 | Yes | No | No | No | No | No | 4 y |
| 4 | Yes | No | No | No | No | No | 2 y |
| 5 | Yes | No (rejected) | No | No | No | Yes, lymphatic | 10 mo |
| 6 | Yes | Yes, negative | No | No | No | No | 1 y |
| 7 | Yes | No | No | No | NA | NA | NA |
Abbreviations: CTx, chemotherapy; Met, metastasis; NA, not available; RT, radiation therapy; SLNB, selective sentinel lymph node biopsy.
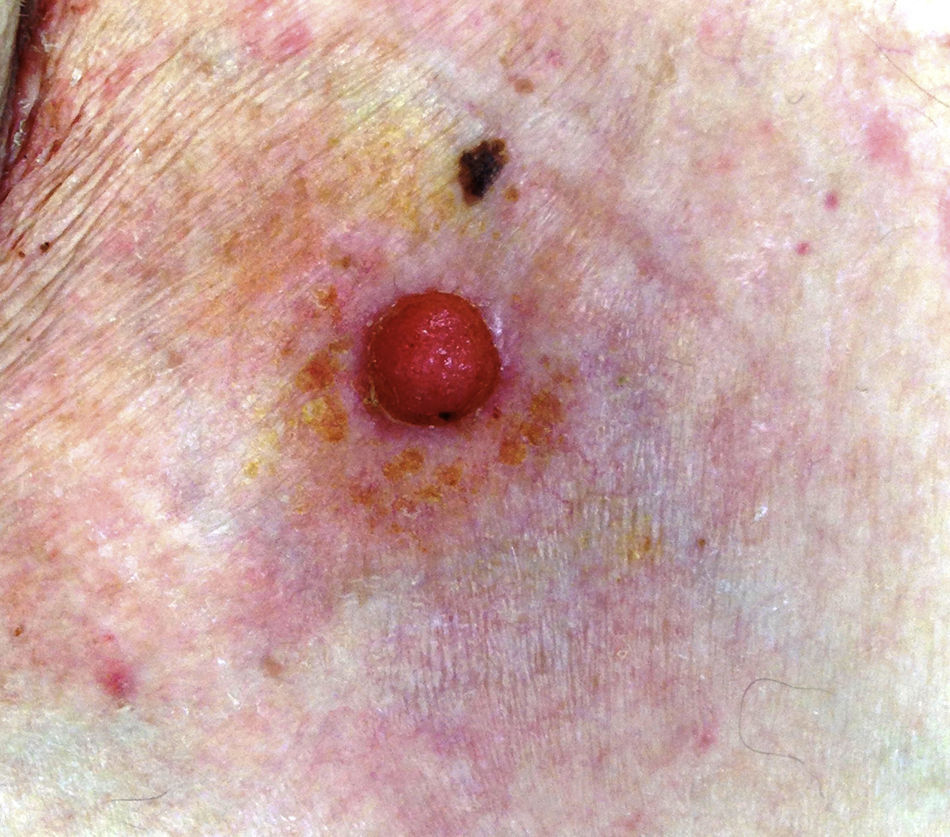
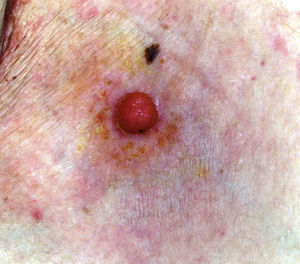
The mean age of the patients in the series was 69 years (range, 39-91 years; median, 75 years). There were 5 (71%) men and 2 (29%) women. The most common site of the tumor was on the lower limbs (3/7; 43%). Tumor diameter was equal to or less than 2cm (mean, 1.46cm; median, 1.5cm). The most frequent clinical pattern was the pink papule (3/7; 43%) (Fig. 1). Recent changes in the lesions were reported by 71% (5/7) of patients. Time since first appearance of the lesion varied between 2 and 60 years (mean, 15 years; median, 4 years). EP was only suspected in 1 case (14%).
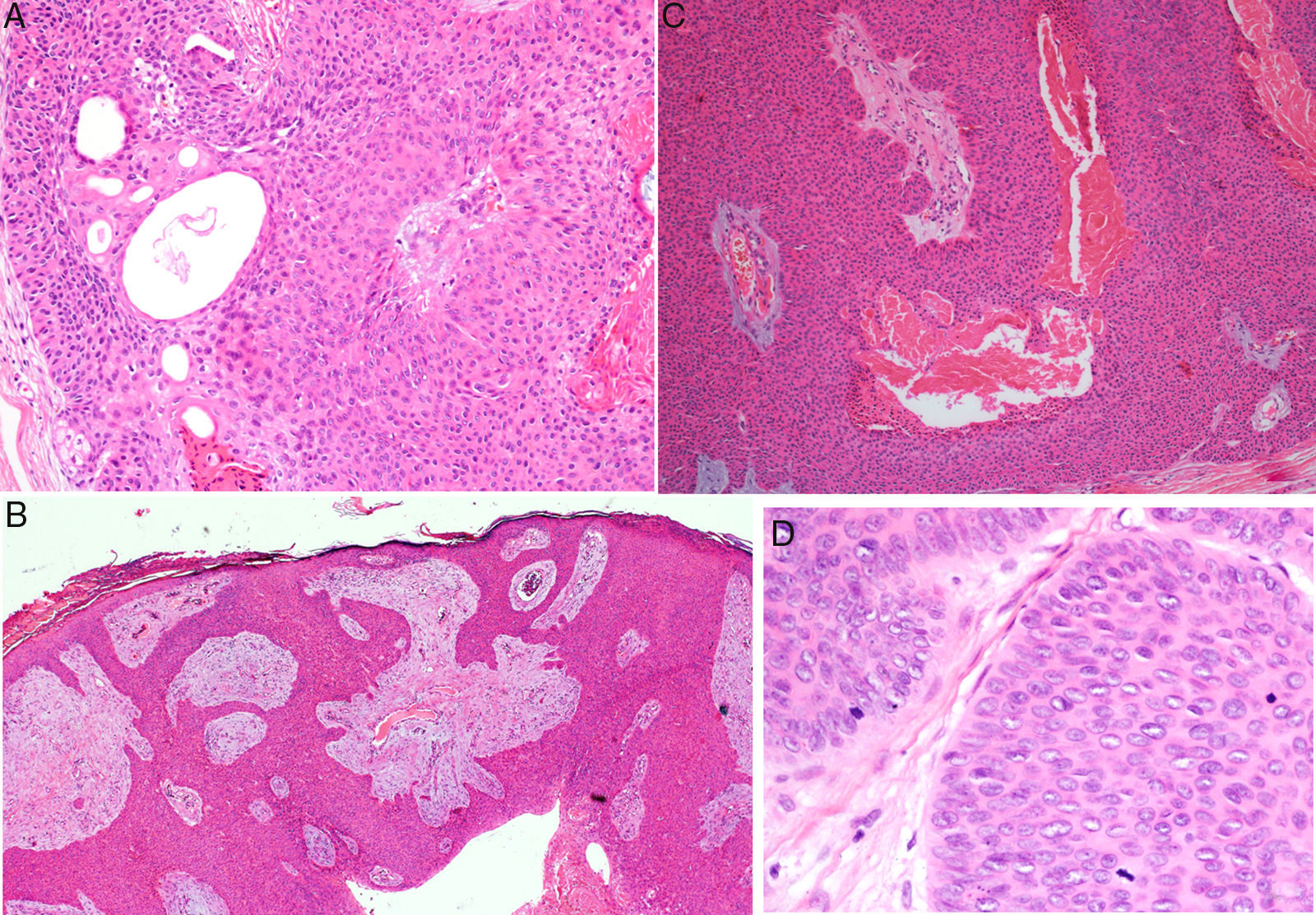
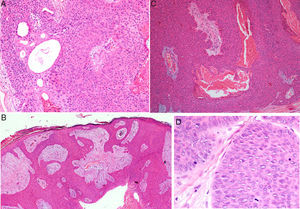
Histology confirmed the diagnosis of EP in all cases, observing broad, anastomosing cords, solid columns, and nests of polygonal basaloid cells with hyperchromatic nuclei, prominent nucleoli, and mitotic figures, with spread from the epidermis into the papillary and reticular dermis, invading the stroma asymmetrically; these changes were accompanied by dermal ductal structures (intracytoplasmic lumina or mature ducts [Fig. 2A]). In situ EP was only detected in 1 case (14%). Mean tumor thickness in the other patients was 5.49mm (range, 3.1-7mm; median, 5.56mm). The most common growth pattern was the pushing pattern (4/7; 57%; Fig. 2B), in which the cords of neoplastic cells advance by pushing the dermis, from which they are clearly separated. The infiltrative pattern was observed in 43% (3/7) of cases, with poorly defined tumor nests invading the dermis and even the hypodermis. Intraepidermal spread was observed in 1 patient (14%), the presence of necrosis in 2 (29%) (Fig. 2C), and a benign component (poroma) in 1 case (14%). The number of mitoses per square millimeter varied between 1 and 18 (mean 5.7; median, 3) (Fig. 2D). Only patient 5 presented lymphovascular invasion (1/7, 14%). Immunohistochemistry was performed in 5 patients and was positive for carcinoembryonic antigen (CEA) and epithelial membrane antigen (EMA).
Histology of eccrine porocarcinoma (EP). A, Ductal structures specific to EP. Hematoxylin and eosin (H&E), original magnification×4. B, Low-power view of the tumor showing broad cords of cells that advance with a pushing pattern. H&E, original magnification×10.C, Focal necrosis or comedonecrosis. H&E, original magnification×10. D, Mitotic figures and tumor cells with signs of atypia. H&E, original magnification×20.
Initial treatment was surgical excision of the lesion, with margins of 3 to 5mm. Selective sentinel lymph node biopsy (SLNB) was considered in 3 patients (43%), based on the histologic characteristics (tumor thickness, mitoses, and vascular and lymphatic invasion). SLNB was positive in patient 2, and regional lymphadenectomy was therefore performed with adjuvant radiotherapy. Patient 5 rejected the procedure and the result was negative in patient 6. Imaging studies (ultrasound [US] and/or computed tomography [CT]) revealed lymph node metastases only in patient 5. Lymphatic spread was present in 29% (2/7) of cases at the time of diagnosis.
Of the 5 patients who attended follow-up, 1 died 10 months after diagnosis (patient 5). No local recurrence or distant metastases have been detected in the remaining patients.
DiscussionThe demographic and clinical findings in our series are similar to those reported in the most relevant publications on this disease.1–10 EP presents in patients of advanced age (71% of our patients were aged over 65 years).10 Although 71% of our patients were men, there are discrepancies regarding sex differences.5,7 The most common sites have been the lower limbs4–6 (43% in our series) and head and neck.7–9 The most common clinical presentation in our series was as a pink papule, as reported in the literature, with a diameter less than 2cm at the time of diagnosis.10,12 However, verrucous, polypoid, ulcerated, and pigmented forms exist, meaning that the differential diagnosis must include other lesions such as pyogenic granuloma, squamous cell carcinoma, and melanoma.7 Disease duration prior to diagnosis varies from months to years (mean, 15 years in our series),5 and changes such as bleeding, growth, or pruritus are common in the months prior to diagnosis (71% of our patients).
Histology is important to confirm the diagnosis of EP, and also to establish the prognostic factors that will orient management. An intraepidermal component and a dermal component are observed. The intraepidermal component is formed of nests and islands of basaloid cells that are clearly separated from adjacent keratinocytes and that extended asymmetrically into the papillary and reticular dermis, forming nests and columns of large pleomorphic cells containing variable amounts of Periodic acid–Schiff-positive glycogen and hyperchromatic nuclei with mitotic figures. Ductal structures in the form of intracytoplasmic lumina or mature ducts are visible in the dermal nests; according to some authors, these structures must be present for this diagnosis to be made.4 The pattern of tumor invasion of the dermis can be pushing (with well-defined margins), infiltrative (tumor nests that arise in the dermis and hypodermis with no clear separation from healthy tissue), or mixed.7,12 In situ forms limited to the epidermis are also seen, and other forms with intraepidermal or pagetoid spread (isolated intraepidermal tumor cells or scattered intraepidermal nests).13 Diffuse necrosis, focal necrosis (comedonecrosis),4 and areas with squamous differentiation (clear cells) have been described. Foci of eccrine poroma exist in up to 18% of cases (14% in our series)4; these areas present less cytologic atypia and fewer mitotic figures, and they show a less invasive and asymmetric growth pattern. On immunohistochemistry, the tumor is positive for pancytokeratins, and for CEA and EMA in the ductal structures.9
Robson et al.4 established the histologic characteristics with statistically significant prognostic value in their study of 69 patients; these characteristics were subsequently corroborated by other authors.6,7,13 First, the infiltrative and pagetoid growth patterns have been associated with an increased risk of local recurrence, due to the absence of a histologic tumor margin. In addition, tumor thickness equal to or greater than 7mm, an elevated number of mitoses per square millimeter (a cut-off value of 14 has been proposed), and the presence of lymphovascular invasion are associated with a higher risk of distant metastases. In contrast to the majority of publications, the pushing pattern predominated in our series (57%). A mean tumor thickness and number of mitoses below the values associated with a poor prognosis and lymphovascular invasion were observed in 14% of cases (10%-20% in other studies).5,8
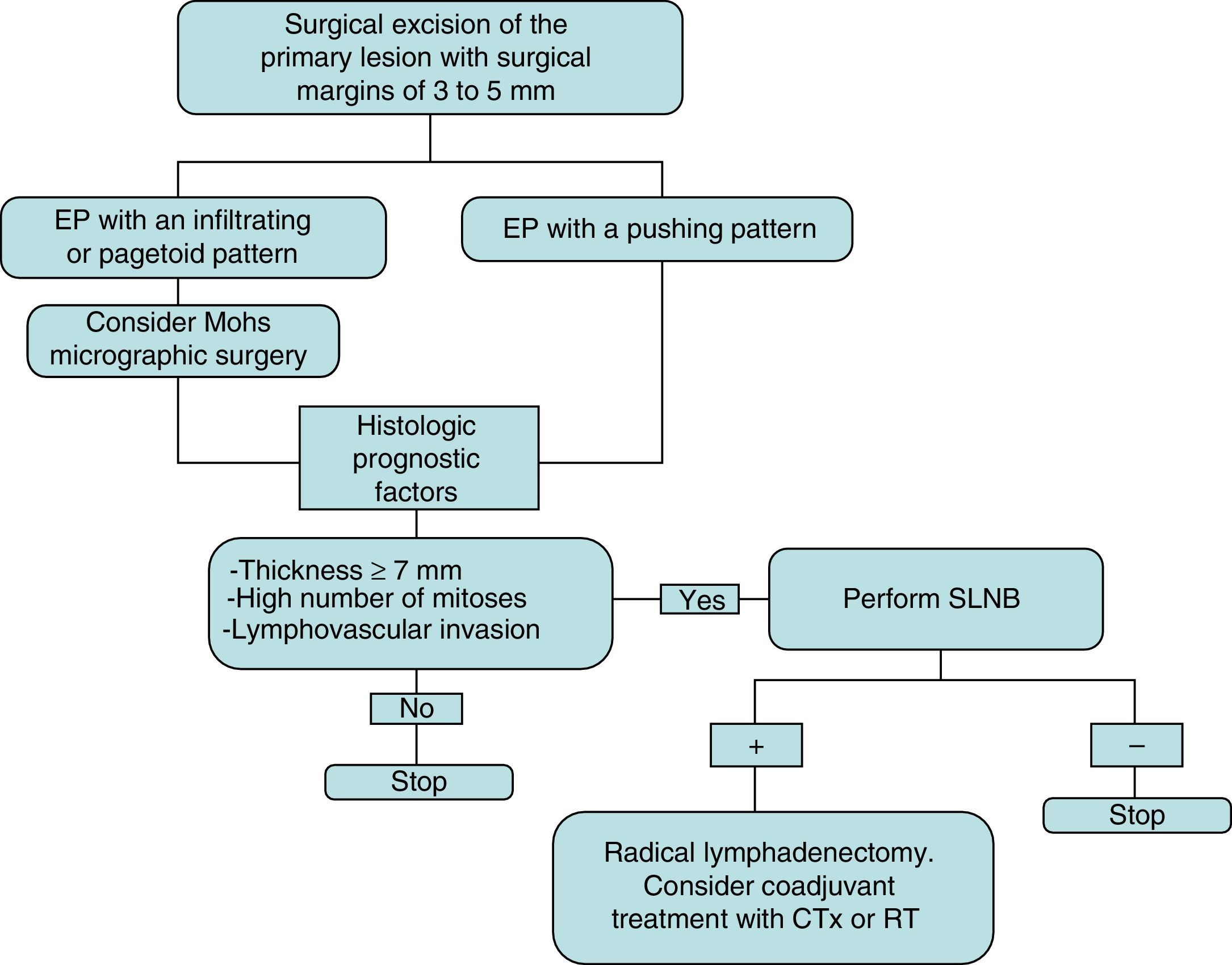
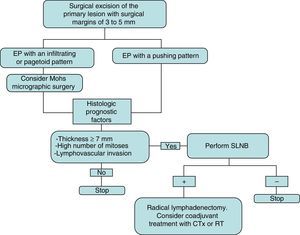
The initial treatment of EP is surgical, with margins of 3 to 5mm.6,13 Belin et al.13 proposed using Mohs micrographic surgery in tumors with an infiltrative or pagetoid pattern, as these tumors have poorly defined margins and a higher risk of local recurrence. EP is known to show lymphatic spread, and SLNB is therefore essential for early detection, as proposed by Bogner14 and other authors.4,6 The selection of candidates for SLNB is based on the presence of histologic prognostic factors associated with a higher risk of distant metastases.4,13 Radical lymphadenectomy of the affected territory is only performed if SLNB is positive.14 Imaging studies (US and CT) do not always identify metastases. Adjuvant chemotherapy and radiotherapy have been successful in some cases (the majority with poor prognostic factors); their administration must be individualized.15 Our management in the serie presented has been based on these guidelines (summarized as a treatment algorithm in Fig. 3). After surgical excision of the tumor, SLNB was proposed in 3 patients (43%) (elevated tumor thickness, high number of mitoses and/or lymphovascular invasion), and was finally performed in 2 of them. A positive result was obtained in 1 of these patients and regional lymphadenectomy was performed.
Treatment plan used in our series and based on a review of the literature. Initial excision is performed with margins of 3 to 5mm, and the borders must be tumor-free to continue the treatment algorithm. CTx indicates chemotherapy; EP, eccrine porocarcinoma; RT, radiotherapy; and SLNB, selective sentinel lymph node biopsy.
EP is an aggressive tumor when it presents with metastatic disease. The risk of local recurrence is of 17% to 20%,7 with lymph node metastases in 20% of cases and visceral metastases in 11%.4,11 Local recurrence has not occurred in any of our patients, but 2 (29%) presented lymph node metastases at the time of diagnosis.
The demographic, clinical, and histologic characteristics of our series were similar to those reported in larger series. In the absence of a standardized treatment algorithm, necessary for the management of a neoplasm that can give rise to lymph node and visceral metastases, we propose the treatment plan followed in our patients and based on a review of the literature.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this research.
Confidentiality of dataThe authors declare that they followed their hospital's regulations regarding the publication of patient information
Right to privacy and informed consentThe authors obtained informed consent from the patients and/or subjects referred to in this article. This document is held by the corresponding author.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gómez-Zubiaur A, Medina-Montalvo S, Vélez-Velázquez MD, Polo-Rodríguez I. Eccrine Porocarcinoma: Patient Characteristics, Clinical and Histopathologic Features, and Treatment in 7 Cases. Actas Dermosifiliogr. 2017;108:e27–e32.