COVID-19 has been a challenge worldwide due to the high infectivity and pathogenicity of the virus. The use of vaccines for preventing respiratory syndromes caused by SARS-CoV-2 is promising and essential in combating the infection. Little is known about COVID-19 vaccination in patients with autoimmune skin diseases; however, cases of onset and exacerbation of cutaneous and systemic lupus erythematosus have been reported following the use of immunizers.1–9 This report describes a chronic cutaneous lupus erythematosus (CCLE) exacerbation thirty days after the second dose of ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca).
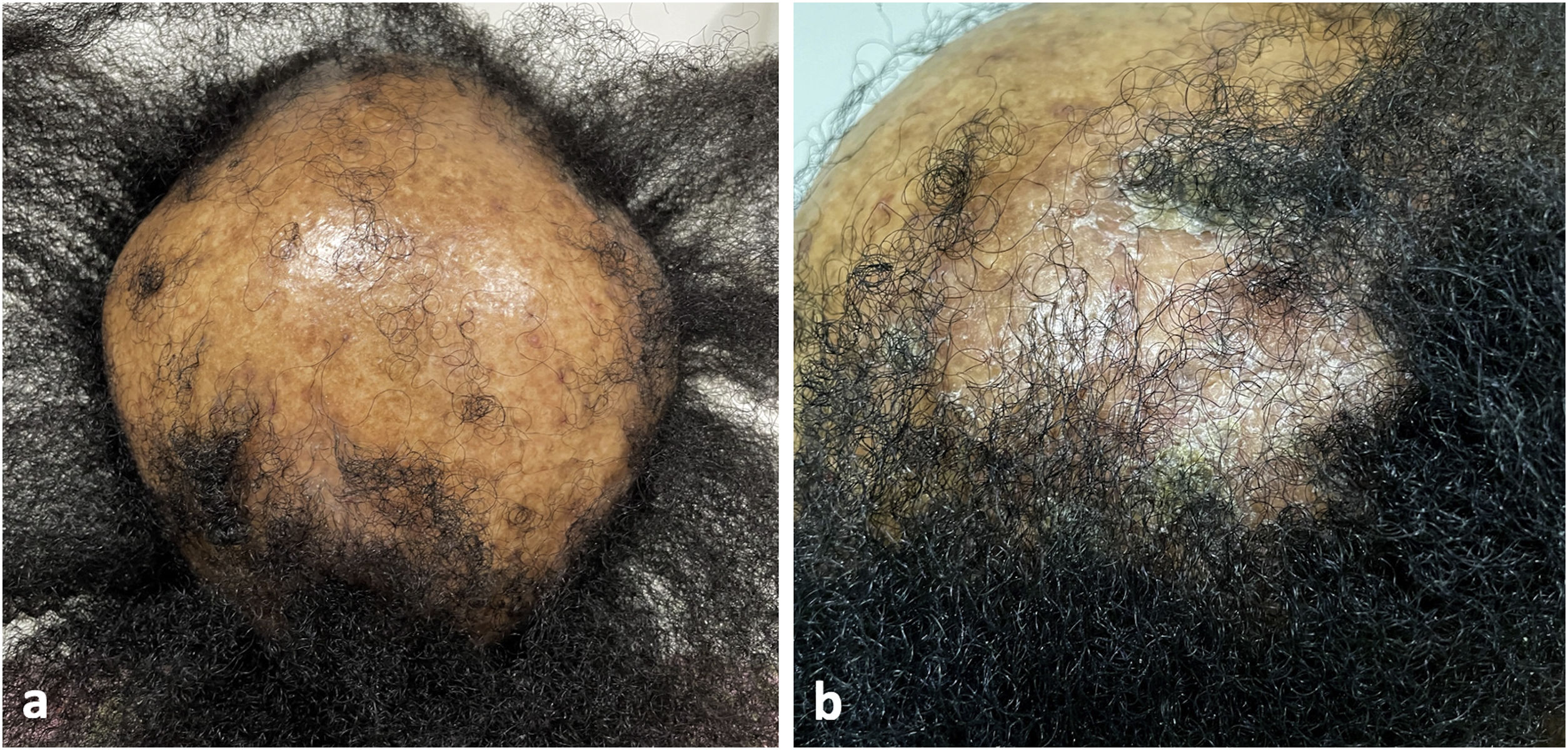
A 40-year-old female, Fitzpatrick V, had been undergoing dermatological follow-up for CCLE on the scalp, marked by an extensive frontoparietal cicatricial alopecia, stable since 2018. The patient did not exhibit other chronic medical illnesses. She presented to dermatological department in early 2022, with itching, burning and erythema on the scalp, associated with serohematic exudate. No systemic symptoms were reported. She denied possible triggering factors, such as recent infections, cigarette smoking, ultraviolet radiation exposure, and drug intake. However, the patient reported that she had received the second dose of the ChAdOx1 nCoV-19 vaccine thirty days before the onset of the symptoms.
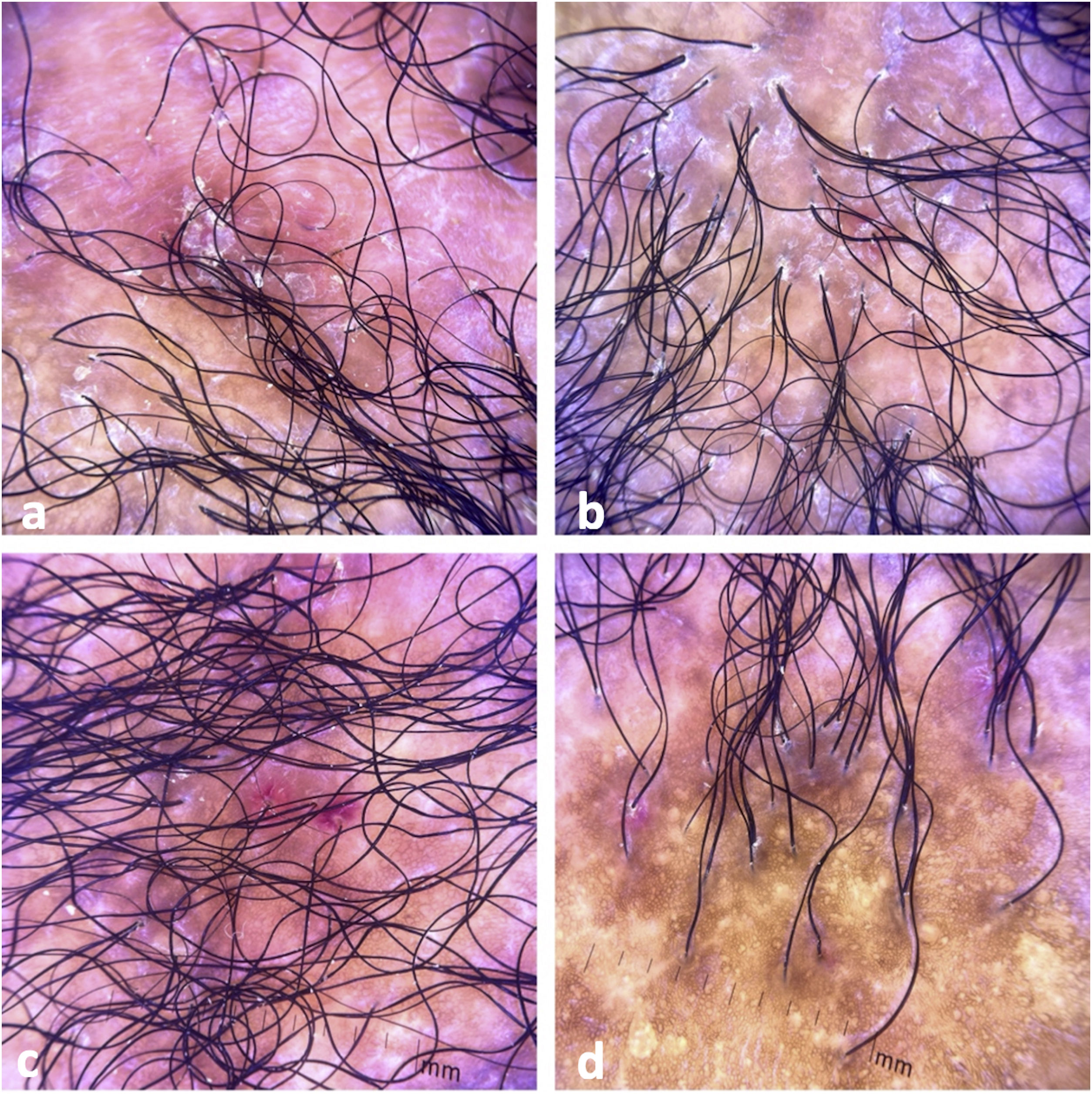
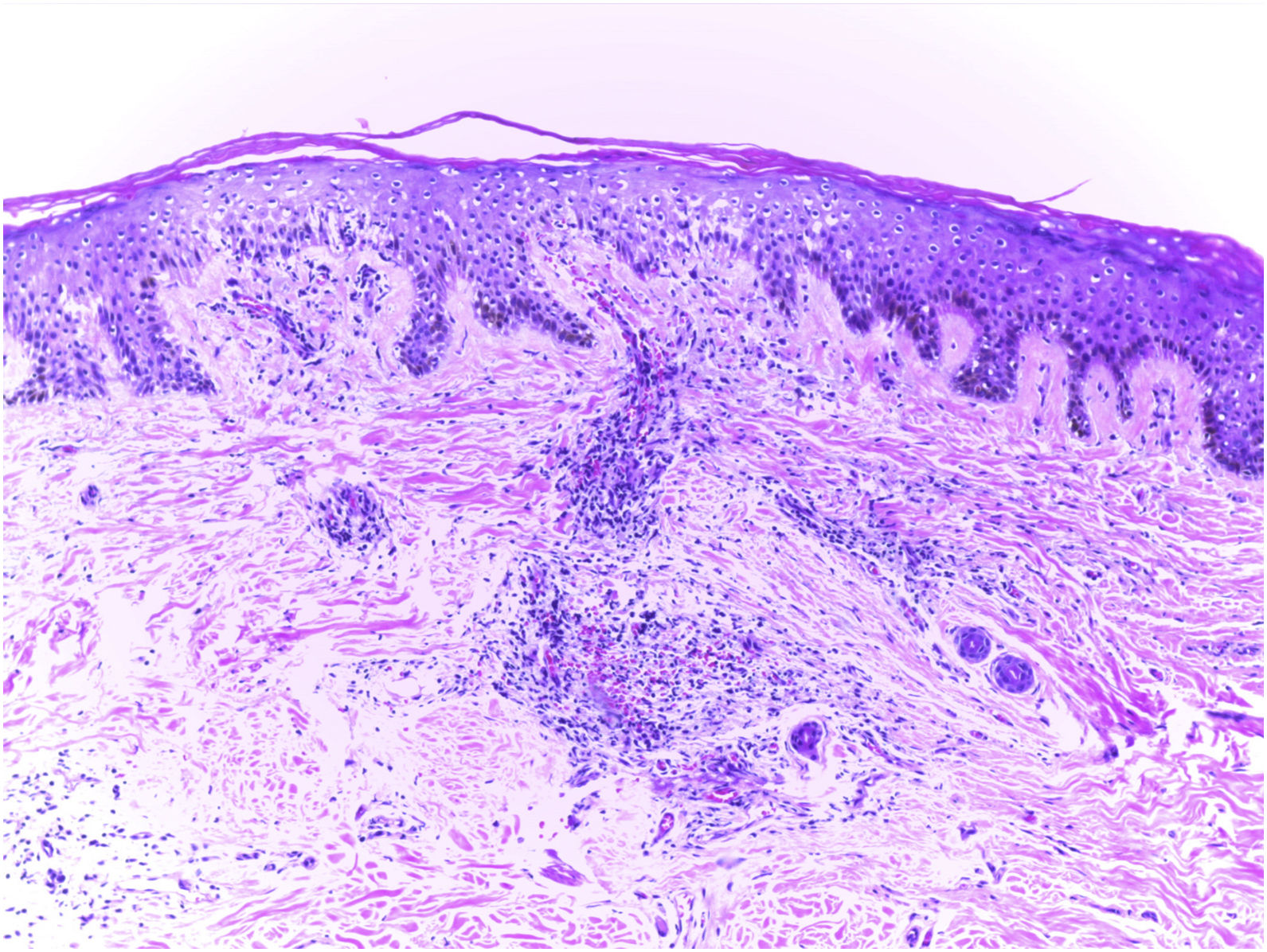
Clinical examination revealed a frontoparietal cicatricial alopecia interspersed with erythematous scaly papules and plaques, surrounded by rough and dry hair shafts of lupus hair (Fig. 1). Trichoscopic findings of disease activity included perifollicular scaling, erythema, incontinentia pigmenti signs, and pili torti, in addition to the CCLE chronic alterations, such as dystrophic hairs, milky red areas, and shiny white structures (Fig. 2). Laboratory tests showed normal blood cell count and serum chemistry. The extractable nuclear antigen panel was negative. Anatomopathological study revealed features of CCLE activity: epidermal maturation disorder with hyperkeratosis, vacuolar degeneration of the basal layer, and focal spongiosis; perivascular and periadnexal lymphohistiocytic inflammatory dermal infiltrate, with reduced follicular units, interstitial fibrosis, and extravasation of red blood cells in the dermis (Fig. 3). Alcian blue stain highlighted a deposition of dermal mucin. Clinical evaluation and complementary tests suggested the diagnosis of CCLE exacerbation triggered by COVID-19 vaccine. The lesions showed satisfactory improvement after topical corticosteroid therapy.
Tricoscopic findings. (a) Perifollicular scaling, erythema, dystrophic hairs, milky red areas, and shiny white structures. (b) Perifollicular scaling, erythema, incontinentia pigmenti signs, pili torti, dystrophic hairs, and fibrotic areas. (c) Perifollicular scaling, intense erythema, and incontinentia pigmenti signs. (d) Cicatricial alopecia with incontinentia pigmenti signs, dystrophic hairs, and empty follicles.
Anatomopathological findings: epidermal maturation disorder with hyperkeratosis, vacuolar degeneration of the basal layer, and focal spongiosis; perivascular and periadnexal lymphohistiocytic inflammatory dermal infiltrate, with reduced follicular units, interstitial fibrosis, and extravasation of red blood cells in the dermis (hematoxylin–eosin, ×100).
The most common cutaneous side effects of the COVID-19 vaccines include injection site reactions (erythema, edema and pain), urticaria, and morbilliform eruptions.3 The onset or exacerbation of lupus after vaccination is rare, with few cases in the literature.1–9 The international VACOLUP study indicated that COVID-19 vaccine was well tolerated in patients with systemic lupus erythematosus. Among the 696 participants assessed, only 3% reported a medically confirmed SLE flare after the immunization.8 Considering cutaneous lupus, the authors did not find any reports about the chronic form, as in the case presented, nor about exacerbation of cicatricial alopecia in chronic cutaneous lupus erythematosus after vaccination. To date, publications with cutaneous involvement have described the subacute form, with a predominance of manifestations in the trunk and limbs.1,3,4,7
Authors suggest that the immune response generated by the immunizer results in activation of inflammatory type 1 helper T cell (Th1) and production of cytokines, with increased levels of interferon gamma (IFN-γ), interleukin-2, and tumor necrosis factor-alpha (TNF-α).3,4 This inflammatory environment contributes to the recruitment of immune cells, becoming a potential trigger for cutaneous and/or systemic lupus manifestations.
Despite the theoretical risk of lupus onset or exacerbation following immunization, the COVID-19 vaccine is recommended for patients with autoimmune diseases, regardless of the activity or severity of the underlying disorder.10 Therefore, health professionals should encourage vaccination and perform clinical surveillance of new lupus manifestations in order to provide early treatment and avoid complications.
Conflict of InterestThe authors declare that they have no conflict of interest.