One of the useful measures to guide dermatologists and pharmacists is the number needed to treat (NNT), which is the number of patients who need to receive a specific therapy to achieve or prevent a clinical outcome, compared to the number needed to achieve the same outcome with an alternative treatment or placebo.1 This statistical parameter is an absolute risk measure calculated as the reciprocal of the absolute risk reduction (ARR) and was developed in the context of evidence-based medicine as a useful tool for clinical decision-making. In fact, the Consolidated Standards of Reporting Trials (CONSORT) statement recommends the use of both relative and absolute effect measures in randomized clinical trials (RCT).2 However, the latter are often underreported, while the former are widely used.
The use of NNT has been increasing in dermatology in recent years, particularly in the management of psoriasis, where very similar efficacy variables are used in various RCT. In fact, recently, the Psoriasis Working Group of the Spanish Academy of Dermatology and Venereology recommended the assessment of parameters such as NNT for efficiency-based prioritization, always using the accepted response objectives of the Psoriasis Area and Severity Index (PASI) (PASI 90, or PASI 100) as a reference.3 This is consistent with the the latest recommendations from the Permanent Pharmacy Committee of including the NNT and cost per NNT as efficiency measures in therapeutic positioning reports (TPR).4
Therefore, we present an example of how NNT facilitates the interpretation of the effectiveness of different interleukin-17 (anti-IL-17) and IL-23 inhibitors (anti-IL-23p19), and how to use the cost per NNT as an efficiency measure to categorize various treatments based on their level of efficacy.
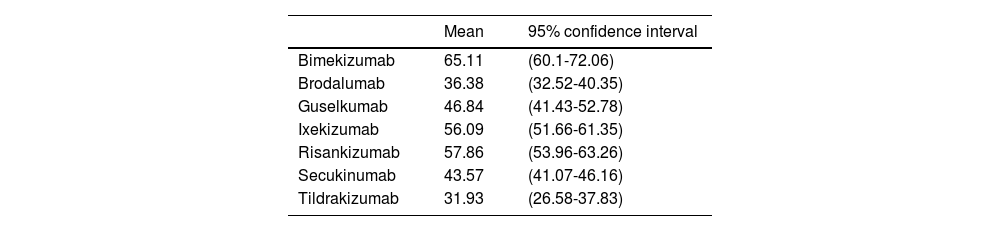
Based on the long-term efficacy results obtained by anti-IL-17 and anti-IL-23 biological therapies in their RCT, with comparable and consistent baseline patient characteristics, and using complete patient clearance (PASI 100) as a long-term efficacy measure (48 to 52 weeks), we conservatively estimated the ARR of the biological drug vs placebo assuming that the efficacy achieved by placebo on week 16 or 24 would still stands in the long run (Table 1).
Absolute risk reduction (PASI 100) of the biological drug vs placebo on weeks 48 through 52 if the efficacy achieved by placebo on weeks 16 through 24 had been kept in the long term.
| Mean | 95% confidence interval | |
|---|---|---|
| Bimekizumab | 65.11 | (60.1-72.06) |
| Brodalumab | 36.38 | (32.52-40.35) |
| Guselkumab | 46.84 | (41.43-52.78) |
| Ixekizumab | 56.09 | (51.66-61.35) |
| Risankizumab | 57.86 | (53.96-63.26) |
| Secukinumab | 43.57 | (41.07-46.16) |
| Tildrakizumab | 31.93 | (26.58-37.83) |
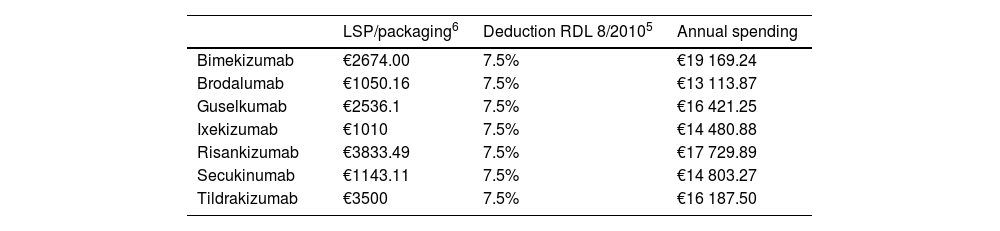
The cost per NNT was calculated by multiplying the average annual treatment spending of the 1st and following years by the NNT. Since these are hospital drugs, the costs were calculated using the notified laboratory selling price (LSP) for each pharmaceutical formulation and the deduction established in the Royal Decree-Law (RDL) 8/2010 (Table 2).5 Unit costs were obtained from the Bot Plus 2.0 database published by the General Council of Official Pharmaceutical Colleges.6 Annual spending was calculated based on these unit costs and the doses recommended in the technical data sheets of each of the anti-IL drugs under consideration.7 Since doses are higher within the 1st year of administration, the 1st and following-year averages were used.
Costs associated with the assessed therapeutic alternatives.
| LSP/packaging6 | Deduction RDL 8/20105 | Annual spending | |
|---|---|---|---|
| Bimekizumab | €2674.00 | 7.5% | €19 169.24 |
| Brodalumab | €1050.16 | 7.5% | €13 113.87 |
| Guselkumab | €2536.1 | 7.5% | €16 421.25 |
| Ixekizumab | €1010 | 7.5% | €14 480.88 |
| Risankizumab | €3833.49 | 7.5% | €17 729.89 |
| Secukinumab | €1143.11 | 7.5% | €14 803.27 |
| Tildrakizumab | €3500 | 7.5% | €16 187.50 |
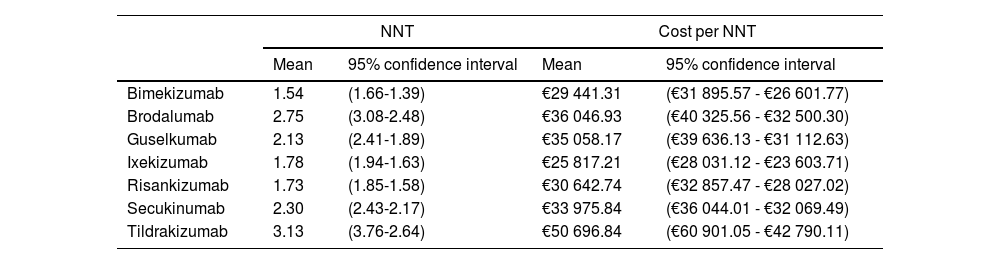
Therefore, if the optimal goal (PASI 100) is applied in the long term, bimekizumab, risankizumab, and ixekizumab achieve response rates higher than other treatments with NNTs of 1.54 (1.66; 1.39), 1.73 (1.85; 1.58), and 1.78 (1.91; 1.63), respectively (Table 3). In terms of efficiency, ixekizumab followed by bimekizumab and risankizumab are the most efficient alternatives of all. However, these results change when confidentially funded LSPs are used, being bimekizumab the most efficient treatment, followed by ixekizumab and risankizumab.
NNT and cost (Notified LSP) per NNT of the biological drug vs placebo on weeks 48 through 52 (PASI 100) if the efficacy obtained by placebo on weeks 16 through 24 had been kept in the long term.
| NNT | Cost per NNT | |||
|---|---|---|---|---|
| Mean | 95% confidence interval | Mean | 95% confidence interval | |
| Bimekizumab | 1.54 | (1.66-1.39) | €29 441.31 | (€31 895.57 - €26 601.77) |
| Brodalumab | 2.75 | (3.08-2.48) | €36 046.93 | (€40 325.56 - €32 500.30) |
| Guselkumab | 2.13 | (2.41-1.89) | €35 058.17 | (€39 636.13 - €31 112.63) |
| Ixekizumab | 1.78 | (1.94-1.63) | €25 817.21 | (€28 031.12 - €23 603.71) |
| Risankizumab | 1.73 | (1.85-1.58) | €30 642.74 | (€32 857.47 - €28 027.02) |
| Secukinumab | 2.30 | (2.43-2.17) | €33 975.84 | (€36 044.01 - €32 069.49) |
| Tildrakizumab | 3.13 | (3.76-2.64) | €50 696.84 | (€60 901.05 - €42 790.11) |
Results suggest that anti-IL-17 and anti-IL-23p19 drugs to treat adult patients with moderate-to-severe plaque psoriasis with higher efficacy (bimekizumab, risankizumab, and ixekizumab) can also have a lower NNT and higher efficiency, being bimekizumab the most efficient treatment when funded LSPs are applied. However, these results are only indicative and do not represent statistically significant differences due to the overlapping confidence intervals reported among some of the treatments.
Our study comes with some limitations too. Firstly, no long-term meta-analysis has been published to this date including all authorized anti-IL-17 and anti-IL-23p19 drugs, nor head-to-head studies among different treatments. Nevertheless, we consider our results to be robust given the homogeneity, comparability, and consistency of the patients’ baseline characteristics across the included RCTs. Secondly, we did not use hospital or health service purchase prices, as these are not public.
In conclusion, we would like to mention how these measures can help health care workers and payers alike choose the drugs that should be administered in terms of both efficacy and efficiency for the entire National Health System.
FundingThis project was funded by UCB Pharma S.A.
Conflicts of interestAlmudena González-Domínguez works at Weber, a company that has received fees for conducting this study. Nuria García-Agua has received fees for research projects from Amgen, Almirall, Archimedes Pharma, Astra-Zeneca, Boeringher Ingelheim, Bristol-Myer Squibb, Chiesi, Coloplast, Eisai Pharmaceutical, Ferrer, Genzyme, Janssen, Lundbeck, Merck Sharp Dohme, Novartis, Pfizer, Rovi, Sanofi-Aventis, Teva Pharma, UCB, and Zambon. Esteban Daudén has been engaged in the following activities: advisory board member, consultant, grant recipient, research support, clinical trial participation, and lectures fees for the following pharmaceutical companies: Abbott/Abbvie, Almirall, Amgen, Biogen, Celgene, Janssen-Cilag, Leo Pharma, Lilly, MSD, Novartis, Pfizer, UCB, Bristol-Myers, and Boehringer-Ingelheim. Pere Ventayol has received payments for attending events (courses, seminars, workshops) and acting as a speaker for Boehringuer, Gilead, Astra Zeneca, Novartis, Janssen, Clovis, Roche, Viiv, and BMS.
The authors wish to thank Carlos Dévora and Yoana Ivanova from Weber for conducting the RCT search.