A great amount of information on systemic and biologic therapies for moderate to severe psoriasis is now available. However, applying the evidence in numerous clinical scenarios has engendered debate; under these circumstances, the consensus of experts is useful.
Material and methodsA scientific committee systematically reviewed the literature relevant to 5 clinical scenarios. An online Delphi survey of dermatologists with experience treating moderate to severe psoriasis was then carried out in order to shed light on questions that remained unresolved by the available evidence.
ResultsTwenty-three dermatologists responded to the survey and consensus was reached on 37 (56%) of the 66 statements proposed. These results led to consensus on various clinical situations even though firm evidence was lacking. Thus, intermittent therapeutic regimens and strategies for reducing the intensity of treatment are considered appropriate for optimizing biologic treatment and reducing costs. The measurement of drug and antidrug antibody levels should be included routinely when following patients on biologics to treat psoriasis. Concomitant psoriatic arthritis or a history of cardiovascular conditions will influence the choice of biologic; in these situations, an agent with anti-tumor necrosis factor properties will be preferred. Tailored management is important when the patient is pregnant or intends to conceive; drug half-life and disease severity are important factors to take into consideration in these scenarios.
ConclusionsA combination of systematic review of the literature and structured discussion of expert opinion facilitates decision-making in specific clinical scenarios.
Existe gran cantidad de información sobre la terapia sistémica y biológica de la psoriasis moderada-grave. Sin embargo, pueden identificarse numerosas situaciones clínicas concretas en las que la evidencia clínica es controvertida y donde resulta útil la opinión consensuada de los expertos.
Material y métodosUn comité científico revisó, de forma sistemática, la bibliografía disponible en 5 escenarios clínicos. En aquellas cuestiones en las que la evidencia era controvertida se llevó a cabo un cuestionario on line según la metodología Delphi, realizado por dermatólogos con experiencia en el manejo de la psoriasis moderada-grave.
ResultadosEl cuestionario recogió opiniones de 23 dermatólogos y se alcanzó el consenso en 37 de las 66 aseveraciones propuestas (56%).
Los resultados permitieron consensuar propuestas en diversas situaciones clínicas, aun cuando la evidencia no fuese firme. Así, tanto el tratamiento intermitente como la desintensificación se consideraron estrategias adecuadas en la optimización de la terapia biológica y en la reducción de costes. La determinación de niveles de fármaco y de anticuerpos antifármaco debería incluirse rutinariamente en el seguimiento de los pacientes psoriásicos tratados con terapia biológica. La coexistencia de artropatía psoriásica y de antecedentes cardiovasculares condiciona la elección de la terapia biológica, prefiriéndose los fármacos anti-TNF alfa como primera elección. En pacientes embarazadas o con deseos de gestación la evaluación personalizada, la gravedad de la psoriasis y la vida media del fármaco son factores relevantes en la toma de decisiones.
ConclusionesLa combinación de una revisión sistemática de la literatura y la discusión y opinión estructurada de los expertos permite realizar propuestas para situaciones clínicas concretas.
Psoriasis is a chronic recurrent skin disease that affects approximately 2.3% of the Spanish population.1 Advances in research and pathogenesis have led to the development of a new class of drugs, referred to collectively as biologic therapy, and the advent of biologics represented a major step forward in the management of moderate to severe psoriasis. Published guidelines on the use of these drugs are based on the results of pivotal trials undertaken to provide evidence to support their approval by the regulatory agencies and their Summaries of Product Characteristics. The findings of pivotal trials provide a strong evidence base for the use of biologics to treat moderate to severe psoriasis in most of the patients who are candidates for this type of therapy.2–13 However, growing clinical experience with these drugs has revealed that the limitations of the evidence is hindering the use of biologics in a considerable number of situations that were either not covered in or specifically excluded from these trials. Although consensus statements and the results of postmarketing clinical studies partly compensate for this deficit, there are still many situations for which the evidence is scarce.
The aim of the present study was to review the evidence relating to some of these clinical situations and to complement, when the evidence was not strong, this information with the opinion of the authors structured by way of a Delphi survey, thereby creating a document that would be useful in clinical practice.
Material and MethodsCreation of the Scientific Committee and Definition of the Hypothetical Clinical ScenariosIn the first phase of the process, a 6-member scientific committee was formed. All the members were dermatologists experienced in the clinical management of moderate to severe psoriasis. Each committee member was asked to propose clinical scenarios of practical interest which, in their opinion, posed problems in the clinical management of psoriasis.
The committee members met and agreed on the 5 hypothetical clinical scenarios that would be evaluated.
Literature ReviewThe scientific committee, with the assistance of an independent external methodologist, performed a literature review and synthesized the results. They studied and assessed clinical practice guidelines and systematic reviews published between 2009 and 2013, as well as relevant clinical trials regardless of publication date. An exhaustive search of the following databases was performed: Medline, Embase, The Cochrane Library, U.S. National Guidelines Clearinghouse, Tripdatabase, and the Biblioteca de Guías de Práctica Clínica del Sistema Nacional de Salud (GuiaSalud). The search was performed in December 2013 and included only articles in Spanish or English. The level of evidence (LE) was evaluated according to the SIGN methodology.14
The other tasks of the scientific committee included a critical review of the literature, drawing up the initial Delphi questionnaire, and selecting the panel of experts who would rate the statements on the survey to investigate the issues about which, after the available evidence had been evaluated, doubts remained concerning the weight of the evidence.
The committee members drafted presentations of the most relevant findings identified by the literature review. They were also responsible for redrafting the recommendations between the first and second voting rounds of the Delphi process and for writing the final consensus document.
Meeting of the Expert Panel. Drafting and Assessment of the Survey QuestionnaireAt a meeting attended by all the participants, the members of the scientific committee presented the evidence on each of the clinical scenarios to the expert panel. The expert panel was made up of 23 dermatologists from different regions in Spain. At the meeting, each member of the scientific committee presented one of the proposed scenarios. Ample time was allowed for discussion.
Two weeks later, the members of the expert panel were invited to participate in an online survey involving 2 rounds of voting in accordance with the standard Delphi method.
The method used to reach consensus was the Delphi process modified as per RAND/UCLA recommendations.15,16 In this method a survey questionnaire containing a series of potential recommendations is drawn up and these recommendations are then evaluated and rated by an expert panel in 2 rounds of voting (Appendix B, Annex 1).
The present article presents an analysis of the evidence available on each of the clinical scenarios evaluated, the results of the Delphi process, and the discussion.
ResultsOn the first vote, consensus was reached on 32 of the 66 recommendations submitted for evaluation (positive in 26 cases and negative in 6). Five items were then reformulated by the scientific committee to eliminate ambiguities and these were resubmitted, together with the questions on which no consensus was reached in the first round, to a second vote. In total, after the 2 rounds of voting, consensus was reached on 37 of the 66 items (56%): positive in 29 cases and negative in 8 (Tables 1–4).
Results for Scenario 1: Optimization of Biologic Therapy in a Difficult Cost Environment.
| Median (IQR) | Level of Agreement | Result | |
|---|---|---|---|
| 1. Intermittent treatment is an appropriate strategy for optimizing costs in biologic therapy | 8 (7-9) | 87% | Consensus on 1st vote |
| The biologic drug with the best profile for intermittent therapy is: | |||
| 2. Etanercept | 9 (8-9) | 96% | Consensus 1st vote |
| 3. Adalimumab | 2 (1-5) | 61% | No consensus |
| 4. Ustekinumab | 2 (1-3) | 70% | Negative consensus on 2nd vote |
| 5. Infliximab | 2 (1-3) | 100% | Negative consensus on 1st vote |
| 6. De-escalation of treatment (dose reduction and/or increase in the interval between doses) is an appropriate strategy for optimizing costs in biologic therapy | 9 (9-9) | 96% | Consensus on 1st vote |
| The drug with the best profile for de-escalation (dose reduction and/or increase in the interval between doses) in biologic therapy is: | |||
| 7. Infliximab | 2 (1-3) | 78% | Negative consensus on 2nd vote |
| 8. Adalimumab | 7 (5-8) | 65% | No consensus |
| 9. Etanercept | 8 (6-9) | 70% | Consensus on 1st vote |
| 10. Ustekinumab | 7 (3-8) | 56.5% | No consensus |
| 11. The combination of a classical and a biologic drug is an appropriate strategy for optimizing costs in biologic therapy | 9 (8-9) | 91% | Consensus on 1st vote |
| The biologic drug with the best profile for use in combination with a classic systemic drug is: | |||
| 12. Adalimumab | 7 (4-7) | 56.5% | No consensus |
| 13. Infliximab | 7 (3-8) | 65% | No consensus |
| 14. Etanercept | 8 (7-9) | 78% | Consensus on 1st vote |
| 15. Ustekinumab | 5 (2-6) | 35% | No consensus |
Abbreviation: IQR indicates interquartile range.
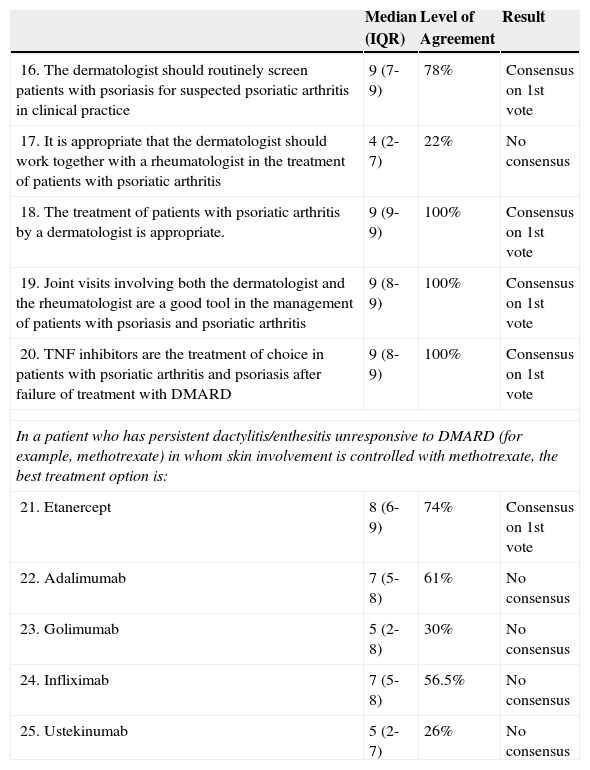
Scenario 2: Psoriatic Arthritis and Active Psoriasis After Failure of Treatment with Disease-modifying Antirheumatic Drugs (DMARD).
| Median (IQR) | Level of Agreement | Result | |
|---|---|---|---|
| 16. The dermatologist should routinely screen patients with psoriasis for suspected psoriatic arthritis in clinical practice | 9 (7-9) | 78% | Consensus on 1st vote |
| 17. It is appropriate that the dermatologist should work together with a rheumatologist in the treatment of patients with psoriatic arthritis | 4 (2-7) | 22% | No consensus |
| 18. The treatment of patients with psoriatic arthritis by a dermatologist is appropriate. | 9 (9-9) | 100% | Consensus on 1st vote |
| 19. Joint visits involving both the dermatologist and the rheumatologist are a good tool in the management of patients with psoriasis and psoriatic arthritis | 9 (8-9) | 100% | Consensus on 1st vote |
| 20. TNF inhibitors are the treatment of choice in patients with psoriatic arthritis and psoriasis after failure of treatment with DMARD | 9 (8-9) | 100% | Consensus on 1st vote |
| In a patient who has persistent dactylitis/enthesitis unresponsive to DMARD (for example, methotrexate) in whom skin involvement is controlled with methotrexate, the best treatment option is: | |||
| 21. Etanercept | 8 (6-9) | 74% | Consensus on 1st vote |
| 22. Adalimumab | 7 (5-8) | 61% | No consensus |
| 23. Golimumab | 5 (2-8) | 30% | No consensus |
| 24. Infliximab | 7 (5-8) | 56.5% | No consensus |
| 25. Ustekinumab | 5 (2-7) | 26% | No consensus |
Abbreviation: IQR indicates interquartile range.
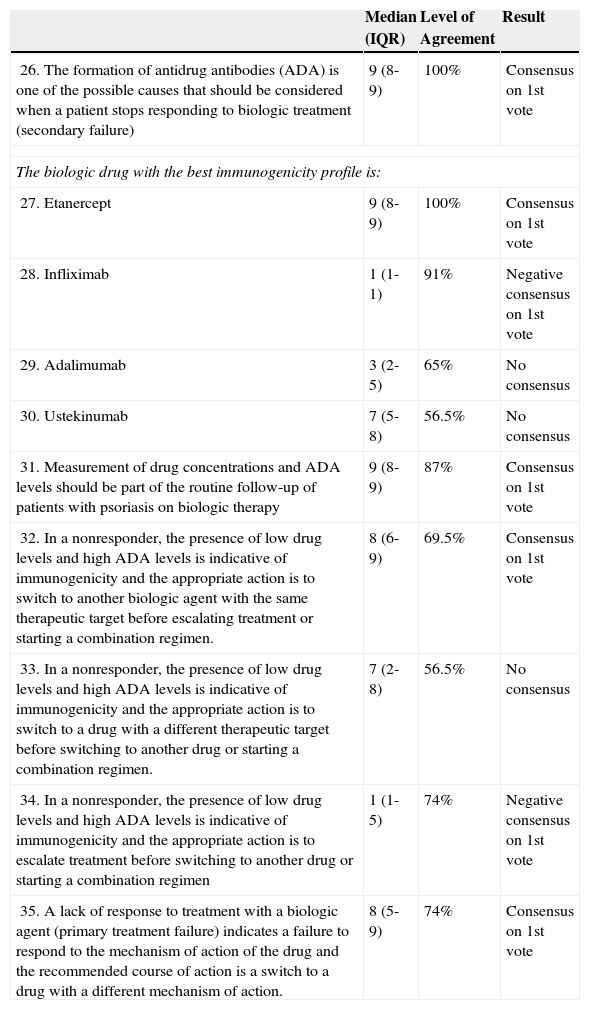
Scenario 3: Switching Biologic Agents in Psoriasis Patients Following Primary or Secondary Treatment Failure.
| Median (IQR) | Level of Agreement | Result | |
|---|---|---|---|
| 26. The formation of antidrug antibodies (ADA) is one of the possible causes that should be considered when a patient stops responding to biologic treatment (secondary failure) | 9 (8-9) | 100% | Consensus on 1st vote |
| The biologic drug with the best immunogenicity profile is: | |||
| 27. Etanercept | 9 (8-9) | 100% | Consensus on 1st vote |
| 28. Infliximab | 1 (1-1) | 91% | Negative consensus on 1st vote |
| 29. Adalimumab | 3 (2-5) | 65% | No consensus |
| 30. Ustekinumab | 7 (5-8) | 56.5% | No consensus |
| 31. Measurement of drug concentrations and ADA levels should be part of the routine follow-up of patients with psoriasis on biologic therapy | 9 (8-9) | 87% | Consensus on 1st vote |
| 32. In a nonresponder, the presence of low drug levels and high ADA levels is indicative of immunogenicity and the appropriate action is to switch to another biologic agent with the same therapeutic target before escalating treatment or starting a combination regimen. | 8 (6-9) | 69.5% | Consensus on 1st vote |
| 33. In a nonresponder, the presence of low drug levels and high ADA levels is indicative of immunogenicity and the appropriate action is to switch to a drug with a different therapeutic target before switching to another drug or starting a combination regimen. | 7 (2-8) | 56.5% | No consensus |
| 34. In a nonresponder, the presence of low drug levels and high ADA levels is indicative of immunogenicity and the appropriate action is to escalate treatment before switching to another drug or starting a combination regimen | 1 (1-5) | 74% | Negative consensus on 1st vote |
| 35. A lack of response to treatment with a biologic agent (primary treatment failure) indicates a failure to respond to the mechanism of action of the drug and the recommended course of action is a switch to a drug with a different mechanism of action. | 8 (5-9) | 74% | Consensus on 1st vote |
Abbreviation: IQR indicates interquartile range.
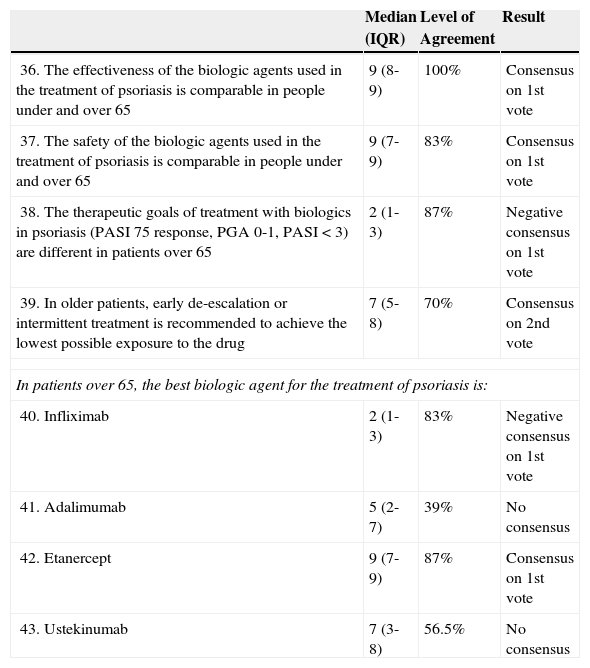
Scenario 4: Older Patients.
| Median (IQR) | Level of Agreement | Result | |
|---|---|---|---|
| 36. The effectiveness of the biologic agents used in the treatment of psoriasis is comparable in people under and over 65 | 9 (8-9) | 100% | Consensus on 1st vote |
| 37. The safety of the biologic agents used in the treatment of psoriasis is comparable in people under and over 65 | 9 (7-9) | 83% | Consensus on 1st vote |
| 38. The therapeutic goals of treatment with biologics in psoriasis (PASI 75 response, PGA 0-1, PASI < 3) are different in patients over 65 | 2 (1-3) | 87% | Negative consensus on 1st vote |
| 39. In older patients, early de-escalation or intermittent treatment is recommended to achieve the lowest possible exposure to the drug | 7 (5-8) | 70% | Consensus on 2nd vote |
| In patients over 65, the best biologic agent for the treatment of psoriasis is: | |||
| 40. Infliximab | 2 (1-3) | 83% | Negative consensus on 1st vote |
| 41. Adalimumab | 5 (2-7) | 39% | No consensus |
| 42. Etanercept | 9 (7-9) | 87% | Consensus on 1st vote |
| 43. Ustekinumab | 7 (3-8) | 56.5% | No consensus |
Abbreviation: IQR indicates interquartile range.
In a patient with longstanding psoriasis, a moderate to severe flare is controlled by biologic therapy for 1 year. Then intermittent treatment is considered to optimize costs.
The high cost of treating such a prevalent and chronic skin disease as psoriasis has become a factor that must be taken into account when making strategic decisions in the management of this condition. In patients who have a long-term optimal response to biologic therapy, some authors have suggested the possibility of treatment withdrawal, dose reduction, or a change in the dosing interval. However, there is insufficient scientific evidence to establish the ideal approach in each case.17 It is also not known whether these strategies are in fact cost-effective. However, we consider these proposals and interventions to be of clinical interest.
In the case of interrupted or intermittent treatment it is estimated that relapse occurs between 2 and 6 months following withdrawal of treatment.18 Although opinions differ on this question, as a practical guide retreatment should be considered when, after withdrawal of treatment, a patient presents a PGA > 2 and/or a PASI ≥ 5, and/or a DLQI ≥ 5, or if there is rapid disease recurrence.12
Interrupted treatment regimens are discussed in the Summary of Product Characteristics (SPC) for etanercept and there is more experience with paused treatment for this biologic agent. The use of intermittent etanercept therapy is considered safe and effective in both adults18–22 (LE 1+) and children (LE 1+).23 In the first study to examine the interruption of etanercept treatment after a satisfactory response, and subsequent retreatment, 652 patients were randomized into several groups receiving different doses.19 In 409 patients who had at least a 50% reduction in Psoriasis Area and Severity Index (PASI 50), treatment was discontinued at week 24 and resumed at the same dose once psoriasis returned. The PASI 50 response rate after 12 weeks of retreatment was between 71% and 87% depending on the dose used (LE 2+). The median time to relapse was 85 days and no serious adverse effects were observed. In another larger study, 2546 patients were randomly assigned to receive either continuous or interrupted treatment.20 All the patients received etanercept 50mg twice weekly for the first 12 weeks. The patients on continuous treatment received 50mg once weekly for a further 12 weeks, while in the intermittent group treatment was interrupted in responders (PGA ≤ 2), who were retreated with a dose of 50mg once weekly upon recurrence of disease. The proportion of responders at week 24 who achieved the primary end point (PGA ≤ 2) was greater in the continuous group than in the interrupted group (71.0% vs 59.5%; P < .001). Most patients regained the response once treatment was resumed. A limitation of this study was the short period of retreatment—between 4 and 8 weeks (LE 1+). In the open label, multicenter CRYSTEL study, 720 patients were randomized to receive continuous etanercept 25mg twice weekly or, in the intermittent treatment group, an initial dose of 50mg twice weekly for 12 weeks or until a response was obtained (PGA ≤ 2), at which time treatment was interrupted.19 In the paused group, therapy with etanercept was resumed upon relapse PGA ≥ 3) at a dose of 25mg once weekly. Both treatment regimens were effective. The mean PGA score averaged over 54 weeks was significantly lower in the continuous treatment group than in the intermittent group—1.98 vs 2.51, respectively (P < .001). The improvement in mean PASI between baseline and week 24 was also greater (68% vs 59%). A post-hoc analysis of the results for the 226 responders (PGA ≤ 2) who had paused treatment in that study showed that 83% of them recovered the response during the first cycle of retreatment (LE 2+).21 In all of these studies, the return of psoriasis was gradual following interruption of treatment; no cases involving severe adverse effects, changes in morphology of the disease, or hospitalization for exacerbations were reported.19–21 Another, more recent, analysis of the results of the CRYSTEL study reported that the disease-free interval following the second cycle of treatment was shorter.24 The median interval without treatment between cycles 1 and 2 was 11 weeks, while this interval was reduced to a median of 6 weeks between cycles 2 and 3, 3 and 4, and 4 and 5. The duration of etanercept treatment was significantly shorter in the first cycle than in the second (mean 9.8 vs 13.6 weeks, P < .001), but no significant differences in length were observed between cycles 2 and 3, or 3 and 4. The proportion of patients who said that they were very satisfied, satisfied, or somewhat satisfied decreased from 100% after the first cycle to 97% after the second, and 91% after the third.
The largest study of intermittent etanercept therapy in pediatric patients involved 211 children with moderate to severe plaque psoriasis aged between 4 and 17 years.23 In that study, 138 patients who achieved a PASI 75 response following treatment with etanercept were randomized at week 36 to either continuous treatment with etanercept or withdrawal of treatment (placebo). Of the patients who received placebo, 42% lost the response and were retreated with open-label etanercept. At the end of week 12, the PASI 75 response rate was 80% (52/65) in the group on continuous etanercept and 73.5% (50/68) in the patients whose treatment had been interrupted and resumed (LE 1+).
In the case of adalimumab, experience with intermittent therapy is limited.25–27 In the REVEAL study, patients with an acceptable response (PASI 75) at week 40 were randomized to either continuous adalimumab or a treatment-free interval of 19 weeks.25 In the interrupted therapy group, the PASI 75 response rate was 38% after 12 weeks of retreatment and 55% after 24 weeks. The response was better when treatment was resumed before the patient lost the initial PASI 50 response. In a 108-week extension period, the PASI 75 response rate was similar in both the continuous and intermittent treatment groups (75% vs 73%) (LE 1+); it should be noted that in the group on the intermittent regimen there were patients in whom psoriasis returned during the treatment-free interval and patients in whom it did not. Finally, in an open-label extension study, 347 stable responders (PGA ≤ 2) participated in an assessment of withdrawal and retreatment.26 The mean time to relapse was approximately 5 months. Of the patients who relapsed during the treatment-free interval, 69.1% (123/178) achieved a PGA ≤ 2 response after 16 weeks of retreatment. The safety profile was similar before withdrawal and during retreatment (LE 2++). In the REVEAL study it was also found that patients who lost response more slowly following withdrawal of treatment obtained a more rapid and sustained response after retreatment than those who relapsed earlier and more rapidly following interruption of treatment.27
In the case of infliximab, intermittent therapy is associated with a high rate of infusion-related reactions (LE 1+).28 This association has been demonstrated in studies such as RESTORE2,in which infusion-related reactions occurred in 15% of patients on intermittent treatment as compared to in 9% of those on continuous treatment.28 A lower proportion of infusion-related reactions has been reported in other studies, such as EXPRESS II, in which, unlike the previous study, retreatment (administered at 8-week intervals) took the form of a maintenance regimen rather than an induction dose.29
Very little has been published concerning intermittent treatment with ustekinumab. Most of the available evidence comes from the Phoenix I study in which patients with a good response were re-randomized at week 40 to either continuous ustekinumab or withdrawal followed by retreatment upon relapse.30 Of the 195 patients in the intermittent treatment group, 85.6% regained a PASI 75 response after 12 weeks of retreatment (LE 1+).
Consensus ResultsThe members of the expert panel considered intermittent therapy to be a valid treatment strategy for optimizing costs in biologic therapy. Among the available therapies, the panel considered etanercept to be the biologic drug with the best profile for use in intermittent therapy.
There was also consensus that neither infliximab nor ustekinumab have the best profile for use in intermittent regimens. No consensus was reached on this point in the case of adalimumab (median 2, IQR 1-5) (Table 1).
Patient with long-standing moderate to severe psoriasis and a history of inadequate response or adverse effects with phototherapy, methotrexate, and ciclosporin. Biologic therapy was started 2 years earlier and the patient has maintained a full response during this period. The strategy under consideration is de-escalation of the biologic therapy (reducing the dose or increasing the dosing interval).
Dose reduction to limit exposure to a drug may be considered when treatment is effective, although there is a theoretical risk that such a strategy could decrease the effectiveness of the therapy, and some evidence that longer intervals between doses may increase the risk of antidrug antibody formation.31 Low-dose strategies, that is, starting treatment without an induction dose and/or dose reduction, have been studied with etanercept, although the experience is not extensive (LE 1+).32–34 In the opinion of some authors, a dose reduction strategy can be considered in patients with a good response, especially when biologics are used in combination with conventional drugs; however, there may be a risk of treatment failure.31
Consensus ResultsThe panel agreed on first vote that de-escalation (reducing the dose and/or increasing the dosing interval) is a possible cost optimization strategy in biologic therapy. There was consensus that the biologic agent with the best profile for use in this strategy was etanercept. The result of voting on the question of whether ustekinumab and adalimumab have the best profile for use in this strategy was close to a positive consensus (a median of 7 in both cases with IQRs of 3-8 and 5-7, respectively). There was consensus that infliximab was not the drug with the best profile for use in de-escalation regimens (Table 1).
A patient with moderate to severe psoriasis previously treated with ciclosporin (which was associated with an increase in blood pressure) and phototherapy (which proved ineffective). Partial control of the disease has been achieved with methotrexate for 2 years. The strategy under consideration is the addition of biologic therapy to the methotrexate regimen.
Combining biologic therapy with traditional systemic drugs can improve treatment outcomes.3 In some cases, the use of such combinations can also reduce the cost of treatment by reducing the dose needed of the biologic agent, or obviating the need for a dose increase in response to a loss of efficacy; however, the evidence supporting this strategy is scant.12,35–37 The use of these combinations is not formally approved according to the SPCs of the biologic drugs.
There is evidence to support the efficacy and safety of etanercept in combination with traditional treatments, such as methotrexate (LE 1++),38–40phototherapy (LE 1+),36,41–45 acitretin (LE 1+),35,46 and ciclosporin (LE 3).47 The Spanish guidelines for the treatment of psoriasis with biologic agents consider etanercept to be particularly appropriate for use in combination regimens.2 Moreover, there is evidence that etanercept is effective even without an induction phase when it is combined with acitretin35 (LE 1+) or phototherapy (LE 2+).36
Favorable results have been reported in case series of patients with moderate psoriasis treated with adalimumab at standard doses in combination with methotrexate (LE 2–),48 phototherapy (LE 2–),49 acitretin (LE 3),50 and to a lesser extent ciclosporin (LE 3).50
Methotrexate and infliximab are frequently combined in clinical practice2; however, there is little evidence to support the effectiveness of this combination in psoriasis, and the evidence that exists is from case series (LE 2+).37,51,52 This association could be effective even when a lower-than-standard dose of infliximab is used according to the results obtained in a series of 11 patients who received doses of 3 mg/kg infliximab plus methotrexate (LE 2+).37 Ustekinumab also appears to be safe and effective at standard doses in combined therapy, although the evidence is scant (LE 3).53,54
Consensus ResultsThe panelists considered that combination therapy with traditional treatments and biologic agents was an appropriate strategy for optimizing cost in biologic therapy. There was consensus that etanercept is the biologic agent with the best profile for use in combination with a classic systemic drug. The results for adalimumab (median 7, IQR 4-7) and infliximab (median 7, IQR 3-8) were very close to consensus. No consensus was reached on the use of ustekinumab in such combinations (median 5, IQR 2-6) (Table 1).
Scenario 2. Active psoriasis and psoriatic arthritis after failure to respond to disease-modifying antirheumatic drugsPatient with plaque psoriasis and severe nail involvement. All the symptoms, except nail involvement, are well controlled by acitretin. At the most recent visit, the patient complained of generalized pain that sometimes affects the back and buttocks alternately. The patient is seen jointly by a rheumatologist and a dermatologist. Peripheral joint disease, dactylitis, and enthesitis are diagnosed. After the patient's condition fails to respond to treatment with methotrexate, biologic therapy is considered. The questions posed in this scenario were what is the most appropriate procedure for screening for psoriatic arthritis (PsA) in patients of this type, who should treat psoriatic joint disease, and what is the most appropriate therapeutic option if treatment with methotrexate fails.
PsA is a progressive joint disease that can cause permanent joint damage.4 Its prevalence increases with the duration of psoriasis.55 Up to 30% of patients with psoriasis treated in dermatology departments may have PsA (LE 2++),56 and more than a third of these may not be diagnosed (LE 2++).56 Thus early diagnosis and treatment of PsA is important.
The questionnaires used to screen psoriasis patients for PsA (Psoriatic Arthritis Screening and Evaluation [PASE], Psoriasis Epidemiology Screening Tool [PEST], and Toronto Psoriatic Arthritis Screening [ToPAS]) all have limitations owing to their poor sensitivity and specificity for patterns of arthritis other than polyarticular disease.57 Moreover, these questionnaires are rarely used in clinical practice to screen for suspected PsA (LE 4).58 The CASPAR criteria are used to classify cases of PsA once the joint disease has been diagnosed, but they are not designed to be used as a screening tool.59
Owing to the limitations of the tools available, algorithms have been developed to screen for PsA in the dermatology office on the basis of the results of a targeted medical history and physical examination (LE 4).4,60 The targeted medical history should include questions about the presence of inflammatory joint pain or current joint swelling, with particular emphasis on the knees, ankles and small joints of the hands. The patient should also be asked about the presence of inflammatory or nocturnal pain in the axial skeleton and sites of tendon attachment, especially on the heels and plantar fascia. The targeted physical examination should include inspection (to identify redness) and exploration (to test for heat, limited mobility, and pain) of the painful or swollen joints, with particular attention to the attachment sites for the Achilles tendon and plantar fascia. In addition, hands and feet should be inspected for nail changes, such as nail dystrophy, onycholysis, pitting, hyperkeratosis, and dactylitis.
In view of the difficulty of diagnosing and managing PsA, it is considered that multidisciplinary care provided by a rheumatologist and a dermatologist may facilitate diagnosis of joint disease and provide more integrated disease management in patients with psoriasis and PsA.61 Therefore, both national and international guidelines recommend that dermatologists and rheumatologists should work together closely to manage patients with severe joint and skin disease (LE 4).8,60,62–64
The Spanish guidelines on the management of biologic therapy in patients with PsA specify that, in general, biologic therapy is indicated in patients with active disease refractory to conventional treatment (nonsteroidal anti-inflammatory drugs, corticosteroid infiltrations, and disease-modifying antirheumatic drugs [DMARD]). However, they also specify that in exceptional circumstances—when the severity of the PsA (extension of skin involvement, dactylitis, enthesitis, monoarthritis, uveitis, etc.) clearly limits the patient's quality of life, leisure activities, functional and working capacity—biologic therapy may be indicated even before the possibilities of conventional treatment have been exhausted.59
Of the biologic agents currently available for PsA, the tumor necrosis factor (TNF) inhibitors have demonstrated efficacy in the 5 clinical domains of the disease: peripheral arthritis, skin and nail disease, axial involvement, dactylitis and enthesitis (LE 1+).65
The TNF inhibitors approved to treat psoriasis (adalimumab, etanercept, and infliximab) are also indicated for the treatment of active and progressive PsA in adults when the response to DMARD therapy is not adequate.4 There is evidence that these 3 biologic agents inhibit radiographic progression in patients with PsA.66–69
Ustekinumab has also been shown to be effective in the treatment of PsA (LE 1+).70 Integrated data analysis of the PSUMMIT 1 and PSUMMIT 2 trials has shown that ustekinumab inhibits radiographic progression of joint damage in PsA, although this effect was not clear from the analysis of only the data for the 312 patients in the PSUMMIT-2 trial.71 The European Medicines Agency has approved ustekinumab for the treatment of PsA, with an indication for use similar to that of the TNF inhibitors.72 In some guidelines, such as those published by the National Institute for Health and Care Excellence (NICE) in the United Kingdom, ustekinumab is only recommended for patients in whom treatment with TNF inhibitors has been unsuccessful and patients who are not suitable candidates for anti-TNF therapy.73 With respect to the recommendations in NICE guidelines, it should be remembered that, while they are based on pharmacoeconomic models supported by cost-effectiveness studies and quality-adjusted life years, they are reimbursement criteria and not clinical recommendations. According to other pharmacoeconomic models, while etanercept has been shown to be the most cost-effective biologic agent for patients with PsA and mild to moderate psoriasis, all the biologics indicated in PsA and moderate to severe psoriasis have a similar probability of being cost effective in this setting.74
No direct comparative studies have explored which is the best therapeutic option in the management of dactylitis or enthesitis. Etanercept (LE 1++),75 infliximab (LE 1+),76,77 ustekinumab (LE 1+),70,78 and golimumab (LE 1+),79) have all proven effective in the treatment of dactylitis and enthesitis. Adalimumab has proven effective in the treatment of these conditions (LE 2+)80 in many, but not all, of the studies undertaken.81
Consensus ResultsIn this scenario, no specific clinical diagnostic strategies were discussed, but the panel agreed that dermatologists in clinical practice must obtain a targeted medical history and perform a physical examination aimed at diagnosing suspected PsA. No consensus was reached on the question of whether or not it is appropriate for dermatologists to treat patients with PsA. By contrast, there was consensus on the appropriateness of dermatologists collaborating with rheumatologists in the treatment of patients with PsA. Similarly, consensus was reached on the proposal that joint dermatologist-rheumatologist visits with the patient are a good strategy for the management of psoriasis and PsA.
The panelists agreed that TNF inhibitors are the treatment of choice in patients with PsA and psoriasis when treatment with DMARD does not produce an acceptable response. In the first round of voting, the panel agreed with the statement that etanercept is the best treatment option in a patient in whom skin disease is controlled by methotrexate but who has persistent dactylitis or enthesitis unresponsive to DMARD. The score on the same statement for adalimumab and infliximab came just below the threshold of consensus; in both cases the median score was 7 and the IQR 5-8.
Scenario 3. Switching between biologic agents in patients with psoriasis following primary or secondary treatment failurePatient whose condition does not respond to treatment with biologic therapy (primary treatment failure) or in whom a good initial response is lost over time (secondary treatment failure). The question explored is how to determine the optimal treatment when a switch to another biologic drug is considered following primary or secondary treatment failure.
The PASI 75 response rate with the first biologic agent ranges from 50% to 80%.2,82 Between 75% and 85% of these patients will maintain this response in the long term.83 Success or failure of treatment is usually assessed between weeks 16 and 24, at the end of the induction phase, for all biologic agents.2
Secondary treatment failure is deemed to have occurred when the patient has an acceptable initial response to the biologic therapy, but there is a subsequent loss of response or treatment must be withdrawn due to a contraindication or because it is not tolerated.84
In the case of primary or secondary treatment failure, the alternative treatment strategies are as follows: a switch to another biologic agent, escalation of the current regimen, or using a combination regimen.2 There is no solid evidence to indicate which is the best option for starting a biologic therapy or what is the best sequence to follow when switching from one biologic to another in the case of primary or secondary treatment failure (LE 4).12,84,85
The mechanisms that result in primary or secondary failure in biologic therapy are poorly understood, but it is known that in some cases failure may be related to the development of antidrug antibodies (ADA).82,84 All biologic agents can potentially induce an unwanted immune response, whether they are human-murine chimeric monoclonal antibodies (infliximab), fusion proteins (etanercept) or fully human antibodies (adalimumab and ustekinumab).85 Since the presence of ADA may influence the levels and function of the drug in the body, this immune response can alter the efficacy of the treatment. It can also affect the drug's safety profile, mainly in the case of infliximab28 because of the possibility of infusion-related reactions (LE 1+).83,85
The recommended action when a patient on biologic therapy presents secondary treatment failure (loss of response) is to measure drug levels and determine whether ADA are present. It is also important to know that ADA can be neutralizing or non-neutralizing.5 ADA against chimeric antibodies (infliximab) and human antibodies (adalimumab, ustekinumab) are very probably, but not always, neutralizing because they interfere directly with the drug's therapeutic activity.5 The presence of neutralizing ADA does not necessarily preclude a therapeutic effect because clinical efficacy will depend on the balance between drug concentrations and ADA titers, and whether the resulting drug levels are high enough to achieve the desired clinical outcome.5
In clinical practice, the implications for safety and efficacy of the formation of ADA can vary greatly from one biologic therapy to another in patients with psoriasis. The efficacy and safety of etanercept has been shown to be independent of the presence of ADA in randomized clinical trials (LE 1+)83,86,87and in long-term extension studies (LE 2+).88 Although anti-etanercept antibodies have been detected in 18.5% of patients treated for up to 96 weeks, no link has been detected between this finding and variations in response to treatment. These findings are consistent with the apparently non-neutralizing nature of these ADA observed in laboratory studies.33
ADA are often detected in patients treated with adalimumab and in this case ADA levels correlate with drug concentrations and may influence clinical response (LE 2+).83,87,89–91 In patients on infliximab, in addition to causing a loss of clinical response, ADA levels also correlate positively with the likelihood that the patient will develop infusion reactions (LE 1+).28 The presence of neutralizing ADA has been detected in about 5% of patients treated with ustekinumab, but there is no evidence that they have any impact on the clinical response (LE 1+).83,87,92 There is evidence that concomitant methotrexate may reduce ADA formation, not only in psoriasis but also in rheumatoid arthritis, spondyloarthropathies, and inflammatory bowel disease (LE 2+).93
To facilitate clinical decisions in cases of primary or secondary treatment failure, algorithms have been developed that indicate the sequence of the actions that should be taken depending on drug levels and the presence or absence of ADA (LE 4).85 While these are not as yet definitive or standardized protocols, the available evidence, especially in the case of nonresponders, is sufficient to support recommendations.
When drug levels and ADA titers are low in nonresponders, the dose should be increased or the dosing interval shortened. When drug levels are low and ADA are medium-high, a switch to another TNF inhibitor is suggested. When drug levels are high, a switch to a drug with a different therapeutic target is recommended (LE 2++, 4).85,94
Consensus ResultsThe panelists were in agreement that ADA formation is one of the factors that should be considered when there is a loss of response to biologic therapy, and that measurement of drug levels and ADA titers should be a routine part of the follow-up procedure in patients with psoriasis treated with biologics. It was agreed, on first vote, that etanercept is the drug with the best immunogenic profile. The panel also agreed that infliximab is not the drug with the best immunogenic profile (negative consensus).83,87 No consensus was reached in the case of adalimumab (median 3, IQR 2-5). The result of the vote on whether ustekinumab is the drug with the best immunogenic profile came close to a positive consensus (median of 7, IQR 5-8).
This section also included questions that sought to validate the algorithm proposed for the management of treatment failure according to drug levels and the presence or absence of ADA.85 Consistent with the recommendation contained in the algorithm, the panelists agreed that the detection of low drug levels and high ADA titers in a non-responder is indicative of immunogenicity and that the appropriate action in such a case would be switching to another agent with the same therapeutic action rather than escalation of the regimen or the use of a combination regimen (Table 3).
Scenario 4. Older patientsA patient aged 69 years with moderate to severe psoriasis poorly controlled with DMARD. The issue under consideration is the safety and efficacy of biologic therapy in older patients.
Since older patients are usually excluded from clinical trials, specific treatment recommendations for this population are not included in clinical practice guidelines.95 Furthermore, disease management in older patients can be complicated by the presence of comorbidities, increased susceptibility to infection,95 polypharmacy (which increases the risk of drug interactions), and the particular pharmacokinetic and pharmacodynamic characteristics found in these patients.
The SPCs of the biologic agents indicate that dose adjustments are not required in older patients and include warnings regarding the possible increase in the risk of infection in this population.72,96–98 The SPC for etanercept also indicates that Phase 3 studies with this drug in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis did not report any significant differences between patients aged under and over 65 in the frequency of adverse effects (severe or otherwise) or severe infections, or any differences in drug volume or clearance between these two populations in pharmacokinetic studies.97
The scientific evidence on the safety and effectiveness of biologic therapy in the older population is scant. With respect to etanercept, a post hoc analysis of the results of 2 randomized clinical trials found no significant differences in efficacy between older and younger patients, and this finding is supported by the evidence from small case series (LE 3).99 The changes in the patients’ quality of life, as measured by the DLQI scale, were also similar in these 2 groups. The incidence of severe adverse events was significantly higher in older patients, but these were not associated with the biologic therapy (LE 1+).100
In a study that evaluated treatment with etanercept and adalimumab in 89 patients aged over 65 years, the findings related to efficacy and safety were good, and no differences were observed between the two agents (LE 2–).101 Subgroup analysis of the results of the REVEAL study indicated that in patients with psoriasis on adalimumab efficacy was lower in those aged over 65 years, and that this difference was at the threshold of statistical significance (P = .052) (LE 1+).102
A recent meta-analysis examined the safety of biologic therapy in older patients (LE 1+).95 That study concluded that the rate of adverse effects tends to be higher in older patients than in younger patients, except with regard to injection site reactions, headache, rhinitis, allergic reactions, and upper respiratory infections, all of which are more common in younger people. While the cancer rate is higher among older patients, the number of cases is similar to what would be expected in that age group in the general population. Some studies included in that meta-analysis reported a higher rate of infections requiring hospitalization among older patients. Finally, in a study of patients with rheumatoid arthritis, the rate of adverse events was higher among older than younger patients in the groups treated with infliximab or adalimumab. By contrast, no age-related differences in the safety profile were observed in the patients treated with etanercept (LE 2).103
In a recent international consensus document, etanercept was considered to be the treatment of choice for older patients with psoriasis, largely because of its shorter half-life, which facilitates management when rapid withdrawal of treatment is necessary (owing to vaccination, surgery, etc.).104
Consensus ResultsOn first vote, there was consensus that the efficacy and safety of the biologic agents used in the treatment of psoriasis are comparable in people under and over 65, and that the objectives of treatment should be the same for both groups.
Moreover, on second vote, it was agreed that early de-escalation or intermittent treatment is advisable in older patients to minimize drug exposure. The panelists agreed that etanercept is the best biologic drug for the treatment of patients with psoriasis aged over 65 years, mainly because of its short half-life, and that infliximab was not the best option in this setting. No consensus was reached on this point for adalimumab. The score for ustekinumab as the best treatment option in this setting came close to consensus (median 7, IQR 3-8) (Table 4).
Scenario 5. Other special situationsPatient with severe psoriasis who has a history of fatty liver disease and cardiovascular disease (myocardial infarction) in addition to multiple cardiovascular risk factors (diabetes mellitus, hypertension, obesity). The topic explored is the best biologic therapy for a patient with this history.
Psoriasis is associated with increased cardiovascular risk and an increased risk of cardiovascular events (LE 1++).105 In patients with psoriasis, treatment with a TNF inhibitor could reduce the risk of acute myocardial infarction (LE 2++)106,107 and the risk of other cardiovascular events (LE 2+).108 Similarly, continuous treatment with TNF inhibitors could reduce atherosclerosis in patients with psoriatic arthritis (LE 2+).109,110 Biologic agents targeting the interleukins (IL) 12 and 23 (ustekinumab and briakinumab) have been associated with an increased risk of cardiovascular events in some studies,111 although this association was not observed by other authors (LE 1+).112,113
A relationship has also been reported between psoriasis and diseases such as nonalcoholic fatty liver disease (NAFLD),3 obesity,,114–118 diabetes mellitus,3 and hypertension,3 and also with biomarkers for cardiovascular risk.119,120
Some authors have reported an increased prevalence of NAFLD in patients with psoriasis.3 NAFLD is associated with obesity, diabetes mellitus, insulin resistance, hypertension, hyperlipidemia, and metabolic syndrome.3 In patients diagnosed with NAFLD, the greatest caution should be exercised in the treatment of psoriasis with potentially hepatotoxic drugs.3 According to their SPCs, elevated liver enzyme levels are common with infliximab (frequency ≥ 1/100 to < 1/10 patients),96 very common with adalimumab (≥ 1/10),98 and rare with etanercept (≥ 1/10,000 to < 1/1,000).97 There is no data on this effect in the SPC for ustekinumab.72
Obesity is a factor that limits response to all biologic agents. As the dosing of infliximab is weight adjusted, this agent offers the possibility of obtaining similar results in obese and nonobese patients (LE 1+).121 However, in a study of patients with moderate to severe psoriasis treated with infliximab, obesity was associated with a slower response and reduced efficacy (LE 1–).122 As the dose of etanercept is not weight adjusted, excess weight could have an effect on the therapeutic response. Patients with a body mass index (BMI) within the normal range can achieve a better response to etanercept than very obese patients (BMI > 40) (LE 1–).123 However, there are studies in which BMI was not associated with any effect on response (LE 2+).114,124 As the dosing of adalimumab is not routinely weight-adjusted, excess weight could have an effect on the therapeutic response (LE 1+).125 In subanalyses of the results of the REVEAL, BELIEVE, and CHAMPION trials, response to treatment also decreased with increasing body weight, although the reduction was often not statistically significant (LE 1+).102,126,127 A poorer response has also been observed in overweight patients receiving treatment with ustekinumab (LE 1+).128,129 The SPC for ustekinumab recommends higher doses in patients weighing more than 100kg. Moreover, treatment with adalimumab, etanercept, or infliximab can be associated with weight gain (LE 2+),114–117 but this effect has not been observed with ustekinumab (LE 2+).118
Several studies have reported an association between diabetes mellitus and psoriasis.3 In patients with rheumatoid arthritis or psoriasis, the use of an TNF inhibitor (adalimumab, etanercept, or infliximab) has been associated with a decreased risk of developing diabetes mellitus (LE 2++)130 and an improvement in insulin resistance (LE 2++).131
In obese patients with metabolic syndrome, treatment with etanercept improves fasting blood glucose compared with placebo (LE 1–).132 The SPC for etanercept contains a warning concerning the risk of hypoglycemia, indicating that in some diabetic patients a reduction in the dose of hypoglycemic agents may be required.97 Hyperglycemia is a common side effect of adalimumab according to the SPC,98 although it is rare for this effect to condition the use of this agent in clinical practice.
Although relevant studies are scarce, it is probable that TNF inhibitors have no influence on lipid metabolism (LE 2)+.131 In the SPC for adalimumab, increased lipid levels is cited as a very common adverse reaction, although no mention is made of significant differences compared to patients on placebo.98
Some studies have found a significant association between hypertension and psoriasis and report that the risk of hypertension increases with the severity of the psoriasis.3 Hypertension is a common adverse effect associated with adalimumab according to the SPC.98
Etanercept is the biologic agent for which the most evidence of a positive influence on markers of cardiovascular risk has been reported. Treatment with etanercept has been shown to reduce C-reactive protein levels and certain other biomarkers of cardiovascular risk in patients with psoriasis and psoriatic arthritis (LE 1+).119,120 However, the clinical relevance of these changes is not known.
Consensus ResultsIn the hypothetical clinical case described—a patient with severe psoriasis who has diabetes, hypertension, NAFLD, and dyslipidemia—the panelists considered that both etanercept and ustekinumab were the best options. However, in the case of a patient with severe psoriasis and a history of a major cardiovascular event (myocardial infarction or cerebrovascular accident), the consensus was that the best option was a TNF inhibitor rather than an IL-12/23 antagonist like ustekinumab (median 7, IQR 5-9).
In a patient with severe psoriasis who has a history of atherosclerosis and intermittent claudication and is unable to attend phototherapy sessions, the consensus was that the best treatment alternatives are etanercept and methotrexate. In that scenario, adalimumab and ustekinumab were close to consensus, with median scores of 7, IQR 5-8 in both cases (Table 5).
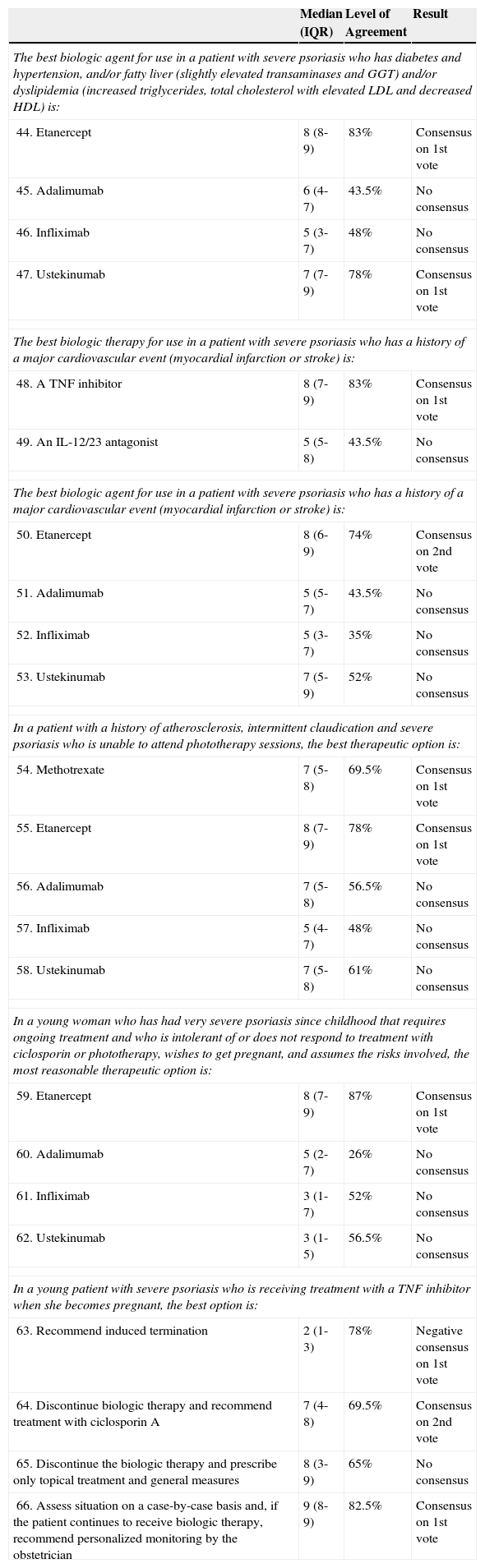
Scenario 5:Other special situations.
| Median (IQR) | Level of Agreement | Result | |
|---|---|---|---|
| The best biologic agent for use in a patient with severe psoriasis who has diabetes and hypertension, and/or fatty liver (slightly elevated transaminases and GGT) and/or dyslipidemia (increased triglycerides, total cholesterol with elevated LDL and decreased HDL) is: | |||
| 44. Etanercept | 8 (8-9) | 83% | Consensus on 1st vote |
| 45. Adalimumab | 6 (4-7) | 43.5% | No consensus |
| 46. Infliximab | 5 (3-7) | 48% | No consensus |
| 47. Ustekinumab | 7 (7-9) | 78% | Consensus on 1st vote |
| The best biologic therapy for use in a patient with severe psoriasis who has a history of a major cardiovascular event (myocardial infarction or stroke) is: | |||
| 48. A TNF inhibitor | 8 (7-9) | 83% | Consensus on 1st vote |
| 49. An IL-12/23 antagonist | 5 (5-8) | 43.5% | No consensus |
| The best biologic agent for use in a patient with severe psoriasis who has a history of a major cardiovascular event (myocardial infarction or stroke) is: | |||
| 50. Etanercept | 8 (6-9) | 74% | Consensus on 2nd vote |
| 51. Adalimumab | 5 (5-7) | 43.5% | No consensus |
| 52. Infliximab | 5 (3-7) | 35% | No consensus |
| 53. Ustekinumab | 7 (5-9) | 52% | No consensus |
| In a patient with a history of atherosclerosis, intermittent claudication and severe psoriasis who is unable to attend phototherapy sessions, the best therapeutic option is: | |||
| 54. Methotrexate | 7 (5-8) | 69.5% | Consensus on 1st vote |
| 55. Etanercept | 8 (7-9) | 78% | Consensus on 1st vote |
| 56. Adalimumab | 7 (5-8) | 56.5% | No consensus |
| 57. Infliximab | 5 (4-7) | 48% | No consensus |
| 58. Ustekinumab | 7 (5-8) | 61% | No consensus |
| In a young woman who has had very severe psoriasis since childhood that requires ongoing treatment and who is intolerant of or does not respond to treatment with ciclosporin or phototherapy, wishes to get pregnant, and assumes the risks involved, the most reasonable therapeutic option is: | |||
| 59. Etanercept | 8 (7-9) | 87% | Consensus on 1st vote |
| 60. Adalimumab | 5 (2-7) | 26% | No consensus |
| 61. Infliximab | 3 (1-7) | 52% | No consensus |
| 62. Ustekinumab | 3 (1-5) | 56.5% | No consensus |
| In a young patient with severe psoriasis who is receiving treatment with a TNF inhibitor when she becomes pregnant, the best option is: | |||
| 63. Recommend induced termination | 2 (1-3) | 78% | Negative consensus on 1st vote |
| 64. Discontinue biologic therapy and recommend treatment with ciclosporin A | 7 (4-8) | 69.5% | Consensus on 2nd vote |
| 65. Discontinue the biologic therapy and prescribe only topical treatment and general measures | 8 (3-9) | 65% | No consensus |
| 66. Assess situation on a case-by-case basis and, if the patient continues to receive biologic therapy, recommend personalized monitoring by the obstetrician | 9 (8-9) | 82.5% | Consensus on 1st vote |
Abbreviation: IQR indicates interquartile range.
A woman of childbearing age with severe psoriasis who requires treatment with a biologic agent and wishes to get pregnant or a woman on TNF inhibitors who becomes pregnant. The question posed is what is the most appropriate therapeutic approach for severe psoriasis in women of childbearing age and pregnant women.
The management of psoriasis in pregnant women and in women of childbearing age who wish to become pregnant presents a challenge because of the need for reliable contraception with some of these therapies, the possible direct relationship between psoriasis and low birth weight babies and premature births,133 and because complications may arise during pregnancy due to the association between psoriasis and various comorbidities, including obesity, hypertension, and depression as well as alcohol and tobacco addiction.134
The course of psoriasis during pregnancy is highly variable135: the condition improves in 50% of patients, remains unchanged in 25%, and gets worse in the remaining 25%.
Among the topical treatments, corticosteroids and vitamin D derivatives (calcipotriol) may be used and tacrolimus could also be considered, but tazarotene is contraindicated.136 Phototherapy is another options that can be considered (UV-B or narrowband UV-B). Among the traditional systemic treatments, methotrexate and acitretin are contraindicated. Ciclosporin falls into FDA category C (animal reproduction studies have shown an adverse effect on the fetus and there are no adequate studies in humans), although many reports suggest that it is relatively safe during pregnancy.137
Biologic treatments used in psoriasis fall into FDA category B (animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate studies in pregnant women). It has been suggested that it is unlikely that these drugs will cross the placenta until the end of the second trimester of pregnancy and it is, therefore, considered that they do not pose any risk to the embryo or fetus during the first 2 trimesters.138
The half-life of the drug may be one of the determining factors in the choice of biologic therapy in a woman of childbearing age. The biologic with the fastest elimination is etanercept with a half-life of 3 days97 as compared to 10 days for infliximab,96 15 days for adalimumab,98 and 3 weeks for ustekinumab.72 Contraception is recommended during treatment and for a further 3 weeks following withdrawal of treatment with etanercept,97 a further 15 weeks with ustekinumab,72 5 months with adalimumab,98 and 6 months following discontinuation of infliximab.96
Consensus ResultsThe panel considered that in the case of a young woman of childbearing potential with very severe psoriasis since childhood who requires continuous treatment, is intolerant or does not respond to treatment with ciclosporin or phototherapy, wishes to become pregnant, and assumes the risk this entails with respect to the course of her disease, the most reasonable choice is etanercept because its short half life will facilitate withdrawal.
In the case of a young woman with severe psoriasis on anti-TNF therapy who becomes pregnant during treatment, the panel considered, on first vote, that the best option would be to assess each case individually and, if biologic treatment were continued, to recommend specific monitoring by the obstetrician. On second vote, consensus was also reached on another option: withdrawal of biologic therapy and recommendation of treatment with ciclosporin A. The panelists also agreed that induced termination should not be recommended in such cases.
DiscussionThe evaluation of expert opinion on the scenarios under consideration (cost optimization, psoriatic arthritis, primary and secondary treatment failure, the treatment of older patients, comorbidities, and pregnancy) shows that clinical experience can make a valuable contribution to the decisions made in daily clinical practice.
Intermittent therapy could be a strategy used to optimize resources in high-cost therapies such as biologic agents.12 Etanercept was the drug considered to be the ideal choice for intermittent regimens on the basis of the available clinical evidence, perhaps on account of the greater experience with this drug in the treatment of adults18–22 and children.23Adalimumab26,27 and ustekinumab30 could also be valid options, even in the absence of a larger body of scientific evidence. The higher incidence of adverse reactions associated with intermittent treatment with infliximab28 may have influenced the consensus that this drug is not suitable for intermittent treatment.
Although very little evidence of reduced dose regimens is cited in the SPCs of the biologics and the safety and efficacy of such regimens are not well-established, these non-standard options are recognized as valid by most of the experts consulted.31 Although some differences emerged in the rating of these regimens, etanercept, adalimumab and ustekinumab were all considered good choices. Infliximab was not considered an appropriate choice in this scenario, perhaps because of its immunogenicity, which could be exacerbated by sub-therapeutic doses.31 Combination therapies were also considered a valid alternative for cost optimization, in line with certain published guidelines.2 The 3 anti-TNF agents were all rated by the expert panel as appropriate options in this setting, with variations in ratings that were probably due to the available evidence and the personal experience of the experts. It is also likely that the scant evidence available in the case of ustekinumab was a factor in the experts’ decision to rate this option as less desirable.53,54 It should be noted that although the Delphi discussants considered that intermittent treatment and reduced-dose regimens could be appropriate strategies for reducing costs in a tough economic climate, there is no firm evidence that this is so and these opinions must be weighed with due caution. The panel did not take into account the cost or possible side effects of combination therapy. In any case, it would be worthwhile investigating these approaches in a prospective study.
In light of the responses received, dermatologists expert in psoriasis recognize the importance of psoriatic arthritis and its role as a key factor in the decisions made in routine clinical practice. This was confirmed by the consensus among the panelists that screening for joint disease should be included in the routine questions asked when updating the medical history of these patients and that collaboration with a rheumatologist is appropriate. Despite the recent approval of ustekinumab as a treatment for psoriatic joint disease,67 the discussants currently prefer anti-TNF therapy as the first-line option for these patients.
Despite the current lack of a standardized protocol, the experts considered the measurement of drug concentrations and ADA titers in the routine management of biologic therapy to be appropriate, and agreed that treatment decisions should be informed by these findings, particularly in the case of secondary treatment failure. It is, therefore, likely that this approach will become routine whenever the necessary technical resources are available. The opinion was that etanercept is the least immunogenic biologic83,86,87 and infliximab is the biologic therapy most likely to trigger the formation of ADA.83,87
While the panel agreed that the treatment goals and prospects for biologic therapy should not be any different in patients aged under or over 65 years, there was also a consensus that safety should be prioritized in older patients and that intermittent treatment and the use of doses lower than the standard regimen specified in the SPC were appropriate strategies in this population. Both ustekinumab and etanercept—the latter presumably due to its short half-life and good safety profile—were considered the best options in this scenario.
In general, the introduction of biologic agents represents an advance over conventional treatments in terms their influence on the comorbidities that together make up metabolic syndrome, a condition often associated with psoriasis.131 In the case of cardiovascular morbidity, the potential anti-inflammatory benefits for atherosclerosis and intermittent claudication119,120 are reflected in the choice of biologic therapy (etanercept, followed by ustekinumab and adalimumab) in combination with methotrexate in this scenario. However, the results of the meta-analyses published on this topic105,111 probably influenced the experts’ preference for TNF inhibitors rather than ustekinumab in patients with a history of myocardial infarction.
As a growing number of patients are receiving treatment with biologics, the possibility that pregnancies will occur in patients on biologic therapy should be seen as increasingly likely. The explicit contraindication in the SPC to the use of biologics during pregnancy72,96–98 and the lack of evidence in the literature could explain the panel's responses concerning this scenario and the recommendations on the use of phototherapy or ciclosporin. When the use of a biologic agent is deemed absolutely necessary in a woman of childbearing potential, the use of a drug with a short half-life, such as etanercept,97 could offer an advantage if pregnancy should occur during treatment.
The limitations of this study are those inherent in the Delphi process, including the difficulty of clarifying and refining the individual opinions of panel members. The possible influence on the voting of the members of the scientific committee, who reviewed the literature, was limited since they did not vote in the Delphi process. Although it was exceptional for its size, expertise, and the representativeness of its members, the opinions developed and expressed by the expert panel do not necessarily reflect the majority opinion of dermatologists in Spain.
This Delphi consensus was undertaken to improve knowledge about the use of biologic therapy in the different scenarios considered, which were chosen because of their clinical interest and the lack of firm evidence in the literature on these topics. The structured opinion of the expert panel can be seen as an additional element in the effort to develop a standardized approach and achieve excellence in the management of psoriatic disease.
FundingThis Delphi consensus was sponsored by Pfizer Spain, which financed the online survey and both face-to-face meetings and teleconferences. No Pfizer employees participated in any of the expert panel discussions or in the drafting of the text.
Conflicts of InterestThe following authors declare that they have received support and funding for research, consulting and speaking fees, and honoraria for participation in clinical trials from the following companies: Abbvie (José Manuel Carrascosa Carrillo, Isabel Belinchón, Pablo de la Cueva Dobao, Rosa Izu Belloso, Jesús Luelmo Aguilar, and Ricardo Ruiz-Villaverde), Almirall (Isabel Belinchón and Pablo de la Cueva Dobao), Celgene (José Manuel Carrascosa Carrillo), Janssen-Cilag (José Manuel Carrascosa Carrillo, Isabel Belinchón, Pablo de la Cueva Dobao, Rosa Izu Belloso, Jesús Luelmo Aguilar, and Ricardo Ruiz-Villaverde), Leo Pharma (Isabel Belinchón and Pablo de la Cueva Dobao), Lilly (José Manuel Carrascosa Carrillo and Pablo de la Cueva Dobao), MEDA (Pablo de la Cueva Dobao), MSD (José Manuel Carrascosa Carrillo, Isabel Belinchón, Pablo de la Cueva Dobao, Jesús Luelmo Aguilar, and Ricardo Ruiz-Villaverde), Novartis (José Manuel Carrascosa Carrillo, Isabel Belinchón, Pablo de la Cueva Dobao, Rosa Izu Belloso, and Jesús Luelmo Aguilar), and Pfizer (José Manuel Carrascosa Carrillo, Isabel Belinchón, Pablo de la Cueva Dobao, Rosa Izu Belloso, Jesús Luelmo Aguilar, and Ricardo Ruiz-Villaverde).
All of the authors consider that they have acted with total independence with respect to the drafting of this article.
Ethical DisclosuresProtection of persons and animals.The authors declare that no experiments were performed on humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
The authors would like to extend special thanks to all the dermatologists who participated in the Delphi consensus process and express their appreciation of the work done. The panelists were as follows: Mariano Ara Martín, Susana Armesto Alonso, Xavier Bordas Orpinell, Gregorio Carretero Hernández, Carlos de la Torre Fraga, Emilia Fernández López, Marta Ferrán Farres, Manuel Galán Gutierrez, Carmen García Donoso, Francisco Guimera Martín-Neda, Enrique Jiménez Carpio, Rafael Jiménez Puya, Enrique Jorquera Barquero, Leandro Martínez Pilar, Jaime Notario Rosa, Raquel Rivera Díaz, Cristina Rubio Flores, Jose Carlos Ruiz Carrascosa, Diana Patricia Ruiz Genao, Jose Luis Sanchez Carazo, M Caridad Soria Martínez, David Vidal Sarro, and J Ignacio Yanguas Bayona. The authors would also like to thank the editorial support in the drafting of this article received from Nature Publishing Group Iberoamérica, Dr. Marta Díaz and Dr. Pablo Rivas.
Please cite this article as: Carrascosa JM, Belinchón I, de-la-Cueva P, Izu R, Luelmo J, Ruiz-Villaverde R. Recomendaciones de expertos para el tratamiento de la psoriasis en situaciones especiales. Actas Dermosifiliogr. 2015;106:292–309.