Cutaneous leishmaniasis is the most common form of leishmaniasis, which is endemic in Spain. The aim of this study was to evaluate the epidemiological and clinical characteristics of cutaneous leishmaniasis seen in our hospital over a period of 20 years, with a particular focus on clinical differences according to immune status and origin of infection
Materials and methodsWe performed a chart review of 67 cases of cutaneous leishmaniasis diagnosed between 1992 and 2012. Follow-up data were available for 54 patients.
ResultsFifty-four patients with cutaneous leishmaniasis were included in the study. Of these, 26 had been diagnosed between 1992 and 2002 and 28 between 2003 and 2012.
The mean age at diagnosis was 49 years, there was a predominance of male patients, and the mean time from onset of symptoms to consultation was 3 months. The most common clinical manifestations were plaques and ulcers. Most of the immunodepressed patients and patients with imported leishmaniasis had skin ulcers and/or multiple lesions. During the first decade of the study, diagnosis was based on clinical and histologic findings. These were supported by molecular techniques in the second decade. Pentavalent antimonials were the treatment of choice, producing good results and very few adverse effects
ConclusionThe number of patients with cutaneous leishmaniasis and with compromised immune status was similar in the periods 1992-2002 and 2003-2013, but more cases of imported leishmaniasis were diagnosed in the second period. Patients with ulcers and/or multiple lesions should be evaluated to rule out immunosuppression or infection by Leishmania species from other parts of the world. Both systemic and intralesional meglumine antimonate was effective and safe
La leishmaniasis es endémica en España, siendo la leishmaniasis cutánea la forma más habitual de presentación. El objetivo del estudio fue valorar las características epidemiológicas y clínicas de la leishmaniasis cutánea en las últimas 2 décadas, haciendo hincapié en las diferencias clínicas según el estado inmunitario del paciente y el origen de la infección.
Materiales y métodosSe revisaron retrospectivamente 67 historias clínicas de pacientes diagnosticados de leishmaniasis entre 1992 y 2012, de ellas 54 eran cutáneas, y con datos de seguimiento.
ResultadosSe incluyeron 54 pacientes: 26 diagnosticados entre 1992-2002 y 28 entre 2003-2012.
La edad media fue de 49 años, con un predominio en varones y un tiempo medio de evolución previo a la consulta de 3 meses. Las manifestaciones clínicas más frecuentes fueron placas y úlceras. La mayoría de pacientes inmunodeprimidos y con leishmaniasis importada presentaron lesiones ulceradas y/o múltiples. El diagnóstico se basó en los hallazgos clínico-patológicos en la primera década, asociándose el diagnóstico por métodos moleculares en la segunda. El tratamiento de elección fue los antimoniales pentavalentes, con buenos resultados y escasos efectos adversos.
ConclusiónEl número de casos y de pacientes inmunodeprimidos fueron similares en ambas décadas, diagnosticándose un mayor número de leishmaniasis importada en la segunda. En los pacientes con lesiones múltiples y/o ulceradas deberían descartarse la inmunosupresión del huésped y la infección por cepas importadas. El tratamiento con antimoniato de meglumina fue eficaz y seguro, tanto por vía sistémica como intralesional.
Leishmaniasis is a widely distributed infectious parasitic disease. It is endemic in over 70 countries, although because disease notification is mandatory in just 32 of these, its true prevalence is probably underestimated.1,2
The overall prevalence of leishmaniasis is 12 million cases worldwide, with an estimated annual incidence of 2 million cases (0.5 million cases of visceral leishmaniasis and 1.5 million cases of cutaneous leishmaniasis).3 Approximately 350 million people, many of whom are poor, are considered at risk for leishmaniasis. The countries with the greatest disease burden are Afghanistan, Brazil, Iran, Peru, Saudi Arabia, and Libya. In the Old World, prevalence is high in the Mediterranean area,4,5 and in Spain, leishmaniasis is considered to be endemic in the following autonomous communities: Andalusia, Aragon, the Balearic Islands, Cantabria, Castile and Leon, Catalonia, Valencia, Extremadura, Madrid, Murcia, Navarra, and La Rioja.3
According to the Spanish Epidemiological Surveillance Network, 1755 cases of leishmaniasis were reported in Spain between 1996 and 2011,4 corresponding to a mean annual incidence of 0.45 cases per 100 000 inhabitants. The number of cases is probably higher, as notification is mandatory only in regions in which the disease is considered endemic.
Based on the minimum data available for the period 2010-2011, leishmaniasis was the main diagnosis code recorded for 2739 hospitalized patients, 98 (3.6%) of whom had the cutaneous form of the disease.
A mean of 115 cases of leishmaniasis was reported in Spain between 2005 and 2010; this figure increased to 271 in 2011 (0.59 cases per 100000 inhabitants) due to an outbreak of cases in Madrid (2.83 cases per 100000 inhabitants).5,6
There are 2 endemic Leishmania species in the Mediterranean area: Leishmania infantum and Leishmania tropica.7L infantum is the only species in Spain. Cutaneous leishmaniasis is the most common clinical form of leishmaniasis,8,9 and it typically manifests as self-limiting papules and nodules.10
An increase in incidence of imported leishmaniasis and leishmaniasis in immunodepressed patients has been reported in recent years11 in relation to factors such as the presence of animal reservoirs, geographic mobility, and climate change, among others.2
The profile of immunodepressed patients with leishmaniasis has changed over the years, with a reduction in the number of cases involving concomitant human immunodeficiency virus (HIV) infection and an increase in the number of patients with drug-induced immunosuppression, particularly in the following settings: organ transplantation, rheumatologic diseases, cancer, and hematological disorders.11
The aim of this study was to analyze the epidemiological and clinical characteristics of patients diagnosed with cutaneous leishmaniasis in our hospital over the last 2 decades, with a particular focus on distinguishing clinical features in immunodepressed patients and patients with imported leishmaniasis.
Material and MethodsWe conducted a retrospective study in which we reviewed epidemiological, clinical, microbiologic, and histologic data for 67 patients diagnosed with cutaneous leishmaniasis at the dermatology department of a tertiary level Spanish hospital between September 1992 and December 2012. Five of the patients also had visceral leishmaniasis. Relevant data were available for 54 patients, who were all included in our analyses. We compared data for 2 consecutive decades (1992-2002 and 2003-2012) to investigate whether there had been an increase in the number of cases of leishmaniasis and whether the introduction of immunosuppressant and immunomodulatory drugs such as biologics (which emerged mainly in the second decade) might be associated with changes in clinical presentation.
ResultsFifty-four patients were included in the study. The number of cases was similar in both decades, with a slightly higher number of patients treated in the second period (28 vs 26 patients).
The median age was 43 years (range, 13-78 years), and there was a predominance of male patients (male to female ratio, 2.3:1). The mean time from onset of symptoms to the patient's visit was 3 months (range, 1 month-4 years).
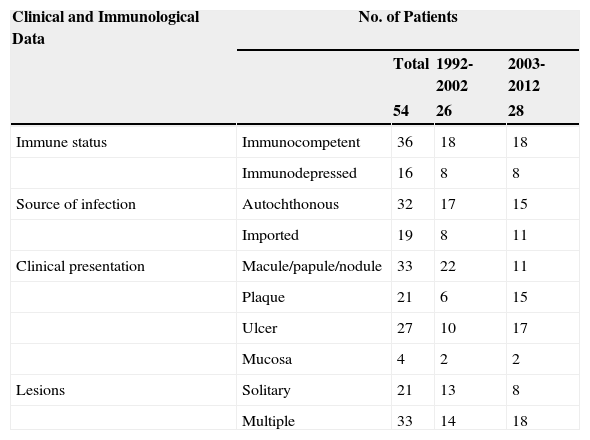
Table 1 summarizes the clinical and epidemiological characteristics of the patients analyzed.
Clinical and Epidemiological Data in 54 Patients With Cutaneous Leishmaniasis.
| Clinical and Immunological Data | No. of Patients | |||
|---|---|---|---|---|
| Total | 1992-2002 | 2003-2012 | ||
| 54 | 26 | 28 | ||
| Immune status | Immunocompetent | 36 | 18 | 18 |
| Immunodepressed | 16 | 8 | 8 | |
| Source of infection | Autochthonous | 32 | 17 | 15 |
| Imported | 19 | 8 | 11 | |
| Clinical presentation | Macule/papule/nodule | 33 | 22 | 11 |
| Plaque | 21 | 6 | 15 | |
| Ulcer | 27 | 10 | 17 | |
| Mucosa | 4 | 2 | 2 | |
| Lesions | Solitary | 21 | 13 | 8 |
| Multiple | 33 | 14 | 18 | |
Sixteen (29.62%) of the 54 patients were immunodepressed, with similar numbers seen in both decades. The causes of immunosuppression were lymphoproliferative disorders (n=3), autoimmune diseases (n=4), HIV infection (n=7), immunosuppressant therapy (n=9), and biologic therapy (n=2).
Of the 7 cases of HIV and leishmaniasis coinfection, 4 were recorded between 1992 and 2002 and 3 between 2003 and 2012. In the second decade, there was a higher proportion of immunosuppressed patients receiving biologics, antitumor agents, and drugs to treat autoimmune disorders.
Nineteen patients (35%) were diagnosed with imported cutaneous leishmaniasis. They were from Jordan, Ecuador, Peru, Guatemala, Tunisia, Bolivia, Mauritania, Venezuela, Iran, Israel, India, and Morocco. The number of cases was higher in the second decade (11/19 vs 8/19) (Table 1).
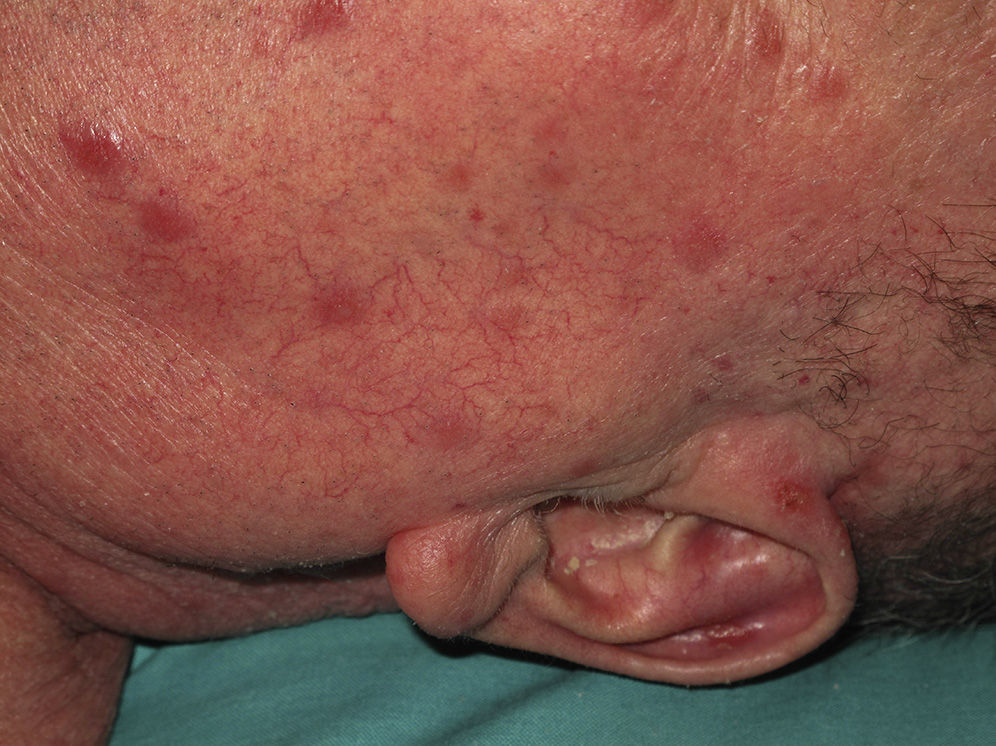
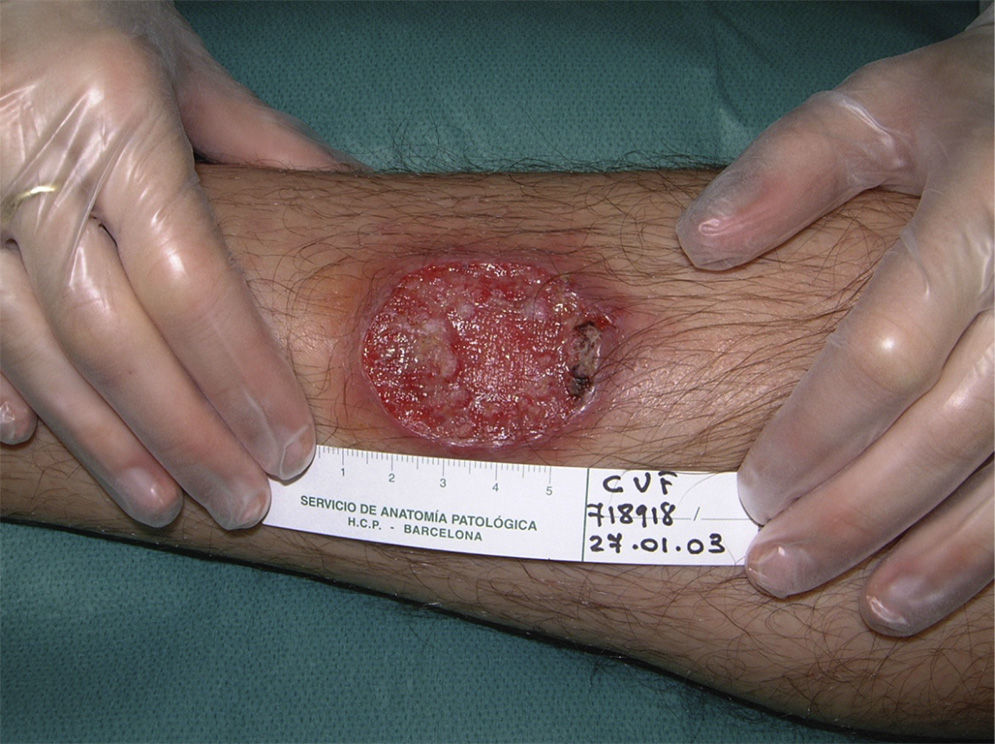
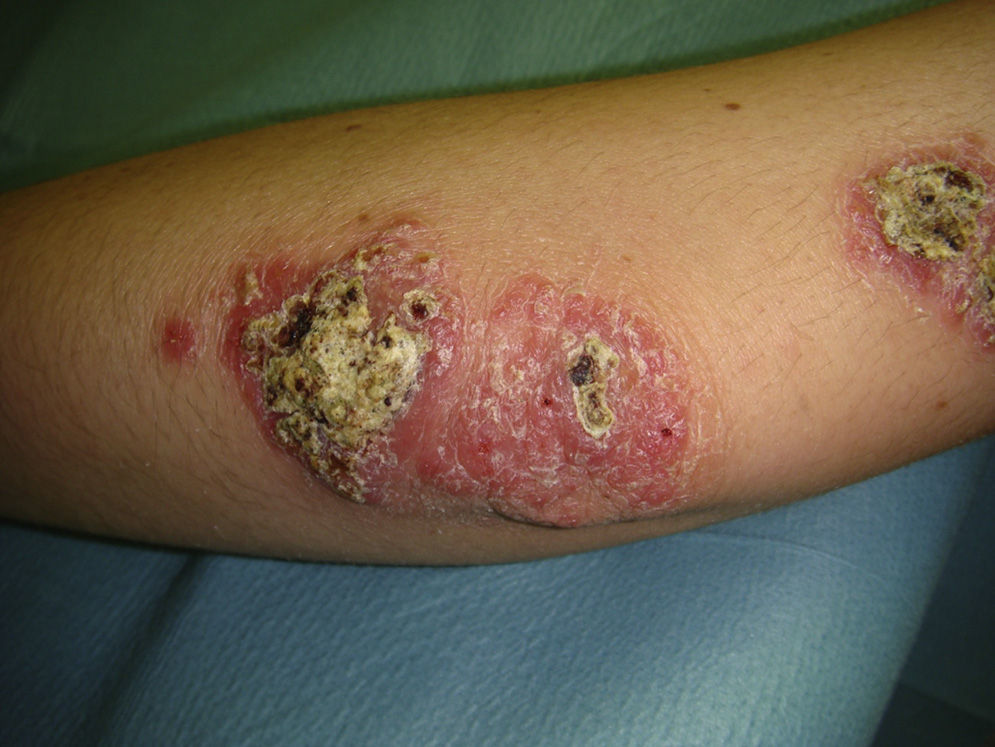
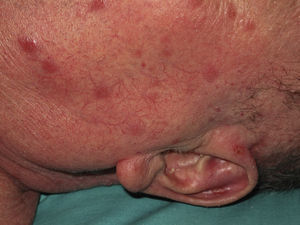
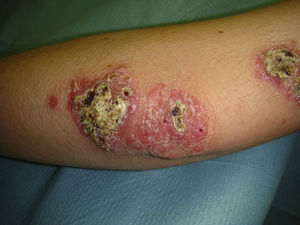
Eighty-one lesions were analyzed. Plaques and ulcers were the most common type (26% and 33%, respectively) and both were more common in the second decade. The rest of the lesions (41%) were macules, papules, or nodules. Thirty-two patients (59%) had more than 2 lesions, and were therefore considered to have the multiple form of cutaneous leishmaniasis (Fig. 1).
Four patients (2 in each decade) had oral and/or nasal mucosal involvement due to the contiguity of the facial lesions.
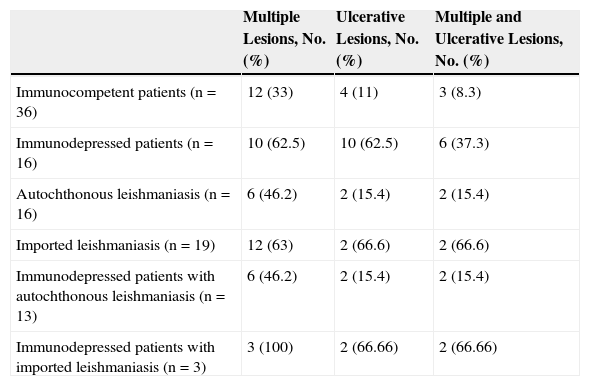
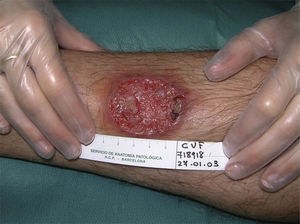
All these cases occurred in patients who did not mention having traveled abroad. L infantum was identified by polymerase chain reaction (PCR) in 1 case. The patients were therefore diagnosed with mucocutaneous leishmaniasis due to L infantum. Two of the patients were immunocompetent and 2 were immunodepressed. Table 2 summarizes the characteristics of the patients by immune status. Of the 16 immunodepressed patients, 10 (62.5%) had multiple lesions and the same number had ulcerative lesions. In the group of 19 patients with imported leishmaniasis, 12 (63%) had multiple lesions and 15 (78%) had ulcers (Figs. 2 and 3).
Classification of 54 Cases of Cutaneous Leishmaniasis According to Source of Infection and Immune Status.
| Multiple Lesions, No. (%) | Ulcerative Lesions, No. (%) | Multiple and Ulcerative Lesions, No. (%) | |
|---|---|---|---|
| Immunocompetent patients (n=36) | 12 (33) | 4 (11) | 3 (8.3) |
| Immunodepressed patients (n=16) | 10 (62.5) | 10 (62.5) | 6 (37.3) |
| Autochthonous leishmaniasis (n=16) | 6 (46.2) | 2 (15.4) | 2 (15.4) |
| Imported leishmaniasis (n=19) | 12 (63) | 2 (66.6) | 2 (66.6) |
| Immunodepressed patients with autochthonous leishmaniasis (n=13) | 6 (46.2) | 2 (15.4) | 2 (15.4) |
| Immunodepressed patients with imported leishmaniasis (n=3) | 3 (100) | 2 (66.66) | 2 (66.66) |
Twelve (33%) of the 36 immunocompetent patients had multiple lesions and 4 (11%) had ulcerative lesions. The corresponding numbers in the group of 32 patients with autochthonous leishmaniasis were 12 patients with multiple lesions (31%) and 2 with ulcerative lesions (6.25%).
Diagnosis in the first decade was based on clinical and pathologic findings in all cases. Hematoxylin-eosin staining was performed in 41 cases and Giemsa staining in just 3.
In the second decade, diagnosis was confirmed by molecular techniques in 11 patients. PCR analysis identified the species in 6 patients, 5 of whom were infected with L infantum. The sixth patient, from Jordan, was infected with L tropica.
The patients were treated with pentavalent antimonials, namely, intralesional meglumine antimonate in 32 cases and intramuscular meglumine antimonate (20mg/kg/d for 21 days) in 16 cases.
Intralesional meglumine antimonate was used to treat patients with single lesions measuring less than 3cm and patients with 5 or fewer lesions. Systemic treatment was used in all other cases.
Just 1 patient, who was treated with intramuscular meglumine antimonate, developed serious adverse effects, consisting of supraventricular arrhythmia, elevated liver and pancreatic enzyme levels, and acute kidney failure. Treatment was therefore discontinued at 2 weeks, but despite this, the lesions resolved. We do not know if the infection was cured, as the patient died due to worsening of his underlying condition. Treatment with meglumine antimonate was completed without complications in the remaining patients.
In the second decade, 7 patients were treated on days 1 to 5, 14, and 21 with intravenous liposomal amphotericin B at a dose of 3 to 4mg/kg/d, depending on the patient's immune status.12 The lesions healed in 3 cases. The 4 nonresponders were subsequently treated with intramuscular meglumine antimonate at the standard dose. Complete response was observed in 3 of these patients. The fourth patient, who had severe HIV-associated immunosuppression, showed improvement, but died from reasons unrelated to leishmaniasis or leishmaniasis treatment.
Thirty-nine patients (74%) were followed clinically for at least 14 months. Cure was confirmed in 35 of these (65%), all of whom were asymptomatic at the 1-year follow-up visit. All of the patients who were not seen in follow-up had a single cutaneous lesion.
DiscussionLeishmaniasis is a parasitic infection that is endemic in the Mediterranean area. The number of cases is expected to rise in coming years due to an increase in animal reservoirs (infected domestic dogs),3 immunodepressed hosts, migratory movements, and global warming.11
It has been estimated that approximately 75% of new cases of leishmaniasis are cutaneous.13
In our study, which compared data for 1992-2002 and 2003-2012, we observed a slightly higher incidence of cutaneous leishmaniasis cases in the second decade (28 vs 26 cases)
There was a predominance of male patients in our series, supporting previous reports.13 Although leishmaniasis is considered a predominantly infantile disease, all the patients in our series were adults, as we do not treat children at our hospital.
The incidence of leishmaniasis in immunodepressed patients was similar in both decades; this observation contrasts with previous reports that predicting an increase in cases in this setting.11 Antiretroviral drugs were introduced into Spanish hospitals—ours included—at the end of 1996, with full implementation in 1997. It is therefore possible that the number of patients with severely compromised immune systems due to HIV infection leveled off in the second decade, explaining the reduction in cases of cutaneous leishmaniasis observed in this group of patients. Our small sample size could also explain why we did not observe significant differences. The prevalence of leishmaniasis imported from the Old World in our series (60%) also contrasts with reports by many authors describing more cases of leishmaniasis imported from the New World.14
Typical skin manifestations due to L infantum infection in immunocompetent patients are self-limiting solitary or multiple papules, plaques or nodules, which may develop ulcers.10 We observed similar skin manifestations in our series, but it is worth noting that the majority of immunodepressed patients and patients with imported leishmaniasis had multiple and/or ulcerative lesions. We therefore recommend ruling out immunodepression and infection by imported strains in patients with multiple and/or ulcerative skin lesions.
Delayed diagnoses are common in leishmaniasis.6 In our series, the mean time from the onset of symptoms to diagnosis was 3 months. The differential diagnosis is broad and includes skin conditions such as insect bites, bacterial and mycobacterial infections, and even granulomatous disorders. Leishmaniasis is diagnosed by histology, culture, and molecular analysis. Histologic features include Leishmania amastigotes in macrophages in the upper dermis and a lymphoplasmacytic inflammatory infiltrate. A diagnosis can be reached with hematoxylin-eosin staining, but Giemsa staining is also useful. Novy-MacNeal-Nicolle medium is the standard culture medium used to diagnose leishmaniasis. This medium, however, is not widely used at our hospital due to its low yield and the slow growth of Leishmania species. Molecular techniques, and PCR in particular, are among the most sensitive techniques for the diagnosis of cutaneous leishmaniasis.15
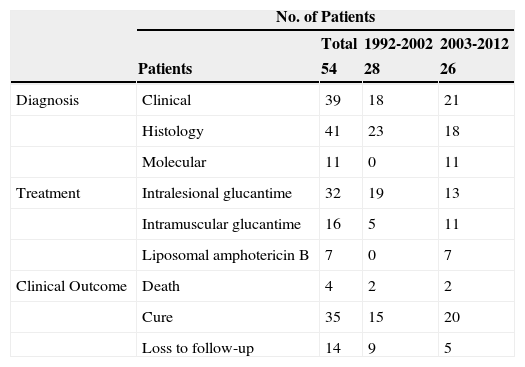
Diagnosis was confirmed by histology in the first decade of our study. In the second decade, 20% of cases were confirmed by molecular analysis, which additionally identified the species in 54% of these. Species identification is particularly important in the case of imported leishmaniasis, as it helps to guide treatment and predict clinical course. Leishmaniasis originating in Central America requires systemic treatment to prevent mucosal involvement and lymphatic spread (Table 3). When molecular techniques are not available, however, a thorough history and histologic examination are essential diagnostic tools.16
Diagnosis, Treatment, and Clinical Outcome in 54 Patients With Cutaneous Leishmaniasis.
| No. of Patients | ||||
|---|---|---|---|---|
| Total | 1992-2002 | 2003-2012 | ||
| Patients | 54 | 28 | 26 | |
| Diagnosis | Clinical | 39 | 18 | 21 |
| Histology | 41 | 23 | 18 | |
| Molecular | 11 | 0 | 11 | |
| Treatment | Intralesional glucantime | 32 | 19 | 13 |
| Intramuscular glucantime | 16 | 5 | 11 | |
| Liposomal amphotericin B | 7 | 0 | 7 | |
| Clinical Outcome | Death | 4 | 2 | 2 |
| Cure | 35 | 15 | 20 | |
| Loss to follow-up | 14 | 9 | 5 | |
Treatment with meglumine antimonate was effective in most cases. Multiple adverse effects have been described in patients treated with systemic meglumine antimonate17 and include myalgia, joint pain, increased pancreatic enzyme levels, exanthema, nausea, abdominal pain, fatigue, headache, increased liver transaminases, leukopenia, and nonspecific ST segment elevation on electrocardiography.18 Meglumine antimonate proved safe and effective in our series of patients and only needed to be discontinued in 1 patient who developed serious adverse effects due to systemic therapy.
Pentavalent antimonials remain the treatment of choice for both localized and generalized cutaneous leishmaniasis, regardless of the patient's immune status.10,19,20
Liposomal amphotericin B is particularly effective in visceral leishmaniasis, and in our series it was partly effective in cutaneous leishmaniasis.
Four of the 7 patients treated with liposomal amphotericin B showed no improvement, but they did show partial response to subsequent treatment with intramuscular meglumine antimonate.
It has been known since the 1990s that a high percentage of cases of cutaneous leishmaniasis from the Old Word heal spontaneously. Furthermore, good results have been reported in studies of local treatments such as cryotherapy, paromomycin ointment, and intralesional antimonials. Intralesional meglumine antimonial administered at a dose of 0.5 to 3mL three times a week over 4 to 5 weeks is currently considered the first-line treatment for most cases of leishmaniasis and has proven effective in L infantum infections in the Mediterranean area (recommendation and evidence rating BII).21 Adverse effects reported for intralesional meglumine antimonate include local allergic reactions, pain, swelling, pruritus, and transient erythema. Intralesional therapy is not recommended for certain locations such as the fingers, ears, lips, and nose.22
Combined local and systemic treatment has proven superior to either of these treatments administered alone. Oral treatments, namely azoles and miltefosine, reduce costs associated with hospitalization. Response to these drugs, however, is limited, and current knowledge is confined to a series of patients in whom other treatments had previously failed.23
Systemic treatment is the treatment of choice for New World cutaneous leishmaniasis, although local treatment could be considered in specific situations. The effectiveness of each drug depends on the species of Leishmania and the geographic region. Pentavalent antimonials are the most widely used drugs, but they have many adverse effects and require long treatment periods. The recommended treatment for mucocutaneous leishmaniasis from the New World is meglumine antimonate in association with pentoxifylline for a period of 30 days.15,21,23
In our series, 25% of patients were lost to follow-up. Most of these had a single skin lesion and had been treated with intralesional meglumine antimonate and/or cryotherapy. We therefore assume that cure would have been achieved in most of these cases. Four patients died during the study period, but none of the deaths were related to leishmaniasis or leishmaniasis treatment.
In conclusion, we did not detect an increase in the number of cases of cutaneous leishmaniasis or an increase in the number of immunodepressed patients with leishmaniasis over the last 2 decades. We recommend assessing immune status and ruling out infection by imported Leishmania species in patient with multiple and/or ulcerative lesions.
Ethical DisclosuresProtection of humans and animalsThe authors declare that no tests were carried out in humans or animals for the purpose of this study.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors declare that no private patient data appear in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Giavedoni P, Iranzo P, Fuertes I, Estrach T, Alsina Gibert M. Leishmaniasis cutánea. Experiencia de 20 años en un hospital español de tercer nivel. Actas Dermosifiliogr. 2015;106:310–316.