The first studies on the use of imatinib in patients with metastatic melanoma were published in 2005 and 2006 and reported that the drug was not effective in this setting.1,2 However, a study published in 2006, which analyzed 102 primary melanomas, described KIT mutations or copy number increases in 39% of mucosal melanomas, 36% of acral melanomas, and 28% of melanomas arising on chronically sun-damaged skin, but in 0% of those arising on skin without chronic sun damage.3 In addition to isolated cases in which patients with metastatic melanoma who had KIT mutations achieved partial or complete regression with imatinib,4,5 results were recently published of 2 series of patients with metastatic melanoma who received imatinib after their tumors were found to contain genetic alterations (mutations or amplifications) in KIT.6,7 Carvajal and coworkers6 achieved an overall durable response rate of 16% in a group of 25 patients. In a group of 43 patients of Asian origin, Guo and coworkers7 reported a disease control rate of 53.5% (partial response in 10 patients and stable disease in 13). In a recent publication in The Lancet, Romano and colleagues8 reported that, based on their experience at Memorial Sloan-Kettering Cancer Center, mutations in exons 11 and 13 of KIT better predict response to imatinib treatment than do amplifications in this gene.8 Several studies have demonstrated that the mere immunohistochemical expression of KIT does not correlate with response to imatinib.
Given the limited experience with imatinib in the treatment of metastatic melanoma, we believe it is important to present our data concerning the frequency of KIT mutations (Table 1), and our results describing the response to imatinib of a small group of patients with confirmed mutations. We present the case of a patient treated with imatinib who achieved a temporary partial response in the form of a reduction in the number and size of visceral metastases. The key data for 2 other patients treated with imatinib are summarized in Table 2.
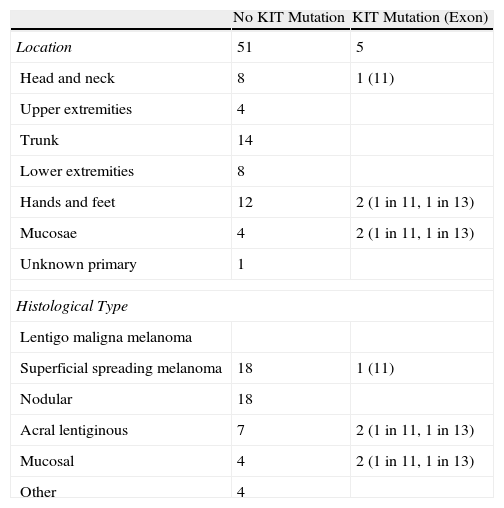
Results of the Molecular Analysis of KIT Status in 56 Patients with Melanoma by Site and Histological Type.
| No KIT Mutation | KIT Mutation (Exon) | |
| Location | 51 | 5 |
| Head and neck | 8 | 1 (11) |
| Upper extremities | 4 | |
| Trunk | 14 | |
| Lower extremities | 8 | |
| Hands and feet | 12 | 2 (1 in 11, 1 in 13) |
| Mucosae | 4 | 2 (1 in 11, 1 in 13) |
| Unknown primary | 1 | |
| Histological Type | ||
| Lentigo maligna melanoma | ||
| Superficial spreading melanoma | 18 | 1 (11) |
| Nodular | 18 | |
| Acral lentiginous | 7 | 2 (1 in 11, 1 in 13) |
| Mucosal | 4 | 2 (1 in 11, 1 in 13) |
| Other | 4 | |
DNA was extracted from the primary tumor or, when this was not possible, from any other metastatic implant, and sequencing was performed on exons 9, 11, 13 and 17.
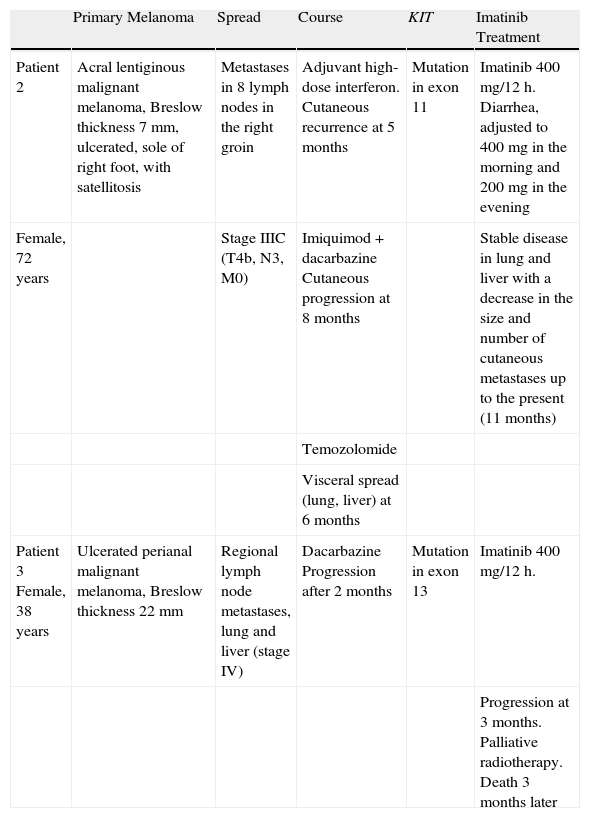
Summary of Clinical History and Response to Imatinib Treatment in Patients 2 and 3.
| Primary Melanoma | Spread | Course | KIT | Imatinib Treatment | |
| Patient 2 | Acral lentiginous malignant melanoma, Breslow thickness 7mm, ulcerated, sole of right foot, with satellitosis | Metastases in 8 lymph nodes in the right groin | Adjuvant high-dose interferon. Cutaneous recurrence at 5 months | Mutation in exon 11 | Imatinib 400 mg/12 h. Diarrhea, adjusted to 400mg in the morning and 200mg in the evening |
| Female, 72 years | Stage IIIC (T4b, N3, M0) | Imiquimod+dacarbazine Cutaneous progression at 8 months | Stable disease in lung and liver with a decrease in the size and number of cutaneous metastases up to the present (11 months) | ||
| Temozolomide | |||||
| Visceral spread (lung, liver) at 6 months | |||||
| Patient 3 Female, 38 years | Ulcerated perianal malignant melanoma, Breslow thickness 22 mm | Regional lymph node metastases, lung and liver (stage IV) | Dacarbazine Progression after 2 months | Mutation in exon 13 | Imatinib 400 mg/12 h. |
| Progression at 3 months. Palliative radiotherapy. Death 3 months later |
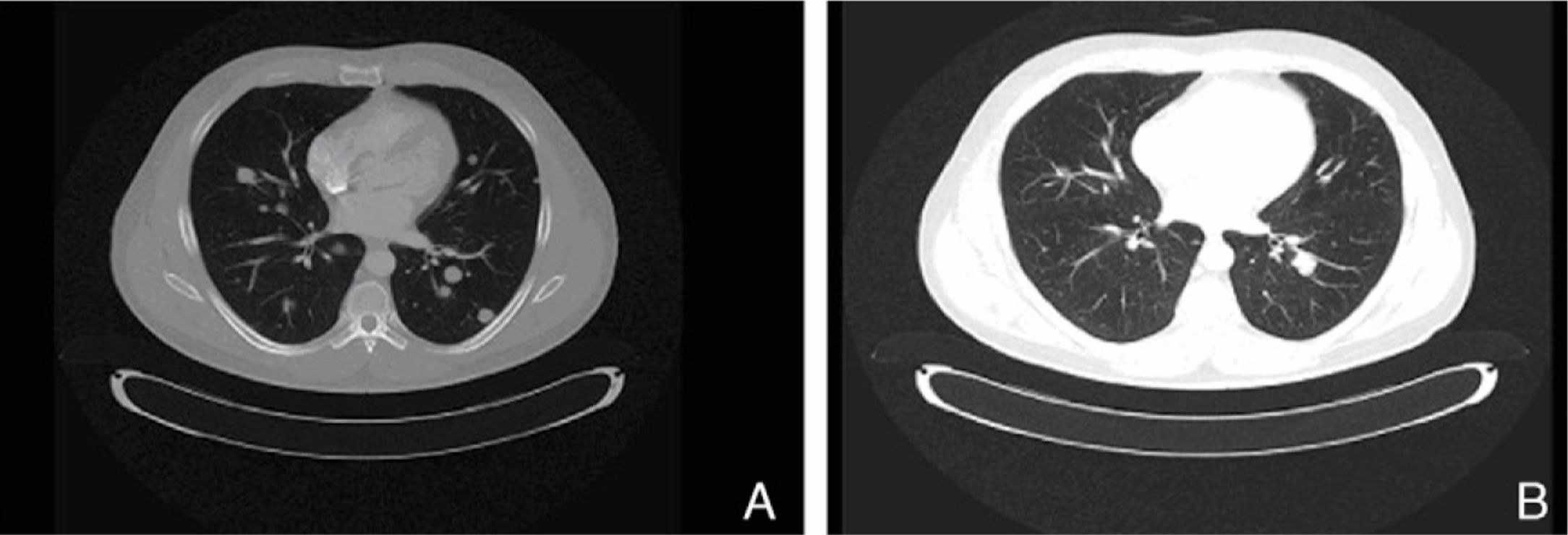
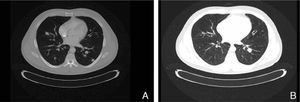
The present case report concerns patient number 1. The patient was a 49-year-old man with melanoma of the penis (9mm Breslow thickness, ulceration, and 6 mitoses/mm2) and multiple bilateral inguinal-iliac metastases (stage IIIC; pT4b, N3, M0). After surgical treatment, the patient commenced adjuvant therapy with high-dose interferon. After 7 months of interferon treatment, skin metastases were observed in the scrotum, the lung, and the lymph nodes of the left groin. The disease progressed after two lines of chemotherapy (dacarbazine followed by carboplatin). At this point, analysis of KIT in the patient's tumor using molecular biology techniques identified a mutation in exon 11, for which the patient was treated with imatinib (400mg twice a day). In the course of the disease, progressive increases were observed in the levels of S100 (up to 2.49: normal value,<0.4μg/L) and lactate dehydrogenase (LDH) (up to 434: normal value, 100-250 U/L). After 1 month of treatment with imatinib, clinical observation revealed a partial response of the skin lesions and lymph nodes, normalization of S100 levels (0.22), and a decrease in LDH levels (303). A computed tomography scan 3 months later also identified a partial response of the lung metastases (Fig. 1). However, 3 months later further increases in S100 (0.71) and LDH (378) were observed and 4 weeks later progression of lung disease and new metastases of the mediastinum were confirmed. The patient was treated as a last resort with ipilimumab. Several weeks later, multiple metastases were detected in the central nervous system and treated with palliative radiotherapy and systemic corticosteroids; the patient died 2 months later.
In view of our results (temporary partial response, a lasting stabilization of disease, and eventual progression), we believe that drugs that act by inhibiting KIT should be evaluated in patients with melanoma in whom disease has progressed despite conventional chemotherapy and in those with KIT mutations. Our results confirm the finding of other groups that this mutation occurs in acral and mucosal melanoma. Although one of the melanomas in which we detected a KIT mutation corresponded to a superficial spreading melanoma, the tumor was located on the forehead of a 55-year-old patient with moderate solar elastosis. While acral and mucous melanomas are relatively infrequent, they do not usually harbor BRAF mutations and thus are not susceptible to treatment with the new inhibitors of this tyrosine kinase, such as vemurafenib. Thus, confirmation of a KIT mutation opens the door to an additional treatment option for this group of melanomas. In the near future, these new KIT inhibitors, used both alone and in conjunction with traditional chemotherapy, will likely improve upon the results achieved to date.
Botella-Estrada R, et al. Mutaciones en KIT en una serie de melanomas y repercusiones en el tratamiento con imatinib. Actas Dermosifiliogr. 2012;103:839-41.