Certain clinically and histologically recognizable skin lesions with a degree of risk of progression to squamous cell carcinoma have been traditionally grouped as precancerous skin conditions but now tend to be classified as in situ carcinomas. This consensus statement discusses various aspects of these lesions: their evaluation by means of clinical and histopathologic features, the initial evaluation of the patient, the identification of risk factors for progression, and the diagnostic and treatment strategies available today.
Bajo el término de precáncer cutáneo se han englobado tradicionalmente distintas entidades, clínica e histológicamente reconocibles, asociadas a un cierto riesgo de evolución a carcinoma escamoso cutáneo invasivo aunque en la actualidad se tiende a interpretarlas como carcinomas in situ. En este documento de consenso se abordan distintos aspectos de estas lesiones como son su evaluación a través de las características clínicas e, histopatológicas de las mismas, la evaluación inicial del paciente afecto, la identificación de los factores de riesgo para su desarrollo, los distintos métodos hoy día existentes para su estudio y diagnóstico así como las diferentes estrategias terapéuticas.
The term precancerous skin lesion has traditionally been used to refer to a set of clinically and histologically recognizable lesions with a degree of risk of progression to invasive squamous cell carcinoma of the skin. Today, these lesions are generally classified as in situ carcinomas, that is, as the intraepidermal stage of the neoplasm.1 The 2 main types of precancerous lesions are actinic keratosis (AK) and Bowen disease (BD). Much less common forms include arsenical keratoses, radiation keratoses (caused by ionizing radiation), and hydrocarbon keratosis. Other forms, which we have not discussed in this article because they affect the mucous or semi-mucous membranes rather than the skin, are leukoplakia, erythroplakia, and actinic cheilitis.
Actinic KeratosesIntroductionAKs are a type of skin lesion commonly encountered in routine clinical practice. In Spain, AK is considered to be one of the 5 most common dermatological diagnoses.2 The recent EPIQA study estimated the prevalence of AK among Spanish patients aged 45 years or older attending outpatient dermatology clinics to be 28.6%.3 Moreover, if we take into account increasingly longer life expectancy, changes in behavior relating to sun exposure that began several decades ago, and the increase in the practice of outdoor sports and recreational activities, there is no doubt that the incidence and, therefore, the prevalence of AK will continue to grow in the coming years, along with the incidence and prevalence of skin cancer. Thus, it is not surprising that nonmelanoma skin cancer, including AK, has become a public health problem and a growing financial burden for national health systems and society as a whole.4
Definition and NomenclatureAK can be regarded as a form of cutaneous squamous cell carcinoma in situ. However, some authors consider AK to be a precancerous lesion representing the first step in a continuum that starts with dysplasia of the basal keratinocytes and can progress to invasive squamous cell carcinoma (the cancerization process). In that case, AK would be the first stage in the carcinogenesis of epidermal keratinocytes caused by actinic radiation, primarily UV.5 It does not, in any case, appear to be advisable, at least when talking to patients, to use the term carcinoma in situ. The reason for this precaution is simply to avoid the unnecessary alarm and psychological and emotional impact on patients and their families that would result from the use of the term carcinoma to describe a lesion that can almost certainly be cured relatively easily and has only a very low risk of becoming an invasive tumor.
Other synonyms for AK are solar keratosis, squamous cell carcinoma in situ AK-type, keratinocytic intraepidermal neoplasia, and senile keratosis, although this last is best avoided.6
Identification of At-Risk PatientsThe chief cause of AK is exposure to nonionizing radiation, and in particular UV radiation, which directly (UV-B) or indirectly (UV-A) induces characteristic mutations in the DNA and RNA of epidermal keratinocytes as a result of photooxidative stress and the formation of cyclobutane dimers.7,8 The principle cause of these lesions is therefore chronic exposure to sunlight (more than 80% of AKs are located in chronically sun-exposed areas), and at-risk patients can be identified by exploring the factors and variables associated with chronic and intense exposure to sunlight, greater vulnerability to UV radiation, and possible defects in the ability of the patient's skin to repair damaged DNA. The following are all factors of particular interest9:
- a.
advanced age, masculine sex, outdoor occupation (farming, fishing, marine occupations, etc.), outdoor sports and recreations (tennis, golf, etc.), residence in a country with a hot climate or in a latitude close to the equator, use of artificial UV lamps. All of these factors increase the patient's long-term exposure to UV radiation.
- b.
Skin phototype. Patients with a type I or II skin phototype are more vulnerable to UV radiation.
- c.
Genetic syndromes characterized by alterations in DNA repair mechanisms, chromosomal instability, and photosensitivity (xeroderma-pigmentosum, Rothmund-Thomson syndrome, etc.).
- d.
Immunocompetence. Another at-risk group is that of immunocompromized patients, particularly solid organ transplant recipients, who are chronically immunosuppressed as a result of the therapy they receive to prevent transplant rejection.
As with any other disease, the initial assessment of a patient with AK should include a general medical history supplemented by a series of additional questions to gather information that may prove important in the design of the treatment and follow-up strategy. In the case of AK, the following additional information is of interest10:
- 1.
Prior treatments to determine the effectiveness and tolerance profile in the patient.
- 2.
Past history of nonmelanoma skin cancer.
- 3.
Exposure to sunlight at work and/or in the course of outdoor leisure activities, and UV-A sun lamp use.
- 4.
Past or current immunosuppressant therapy for any reason.
- 5.
Symptoms (itching, pain, burning, etc).
- 6.
The motive for the consultation (symptoms, concern about skin cancer, cosmetic issues).
- 7.
It is also essential to include questions in the medical history aimed at identifying any signs or symptoms indicative of progress to invasive squamous cell carcinoma (Table 1).10
Table 1.Signs Associated with Suspicion of Progression From Actinic Keratosis to Invasive Squamous Cell Carcinoma.
Inflammation or induration of the lesion Longest diameter of 1-2cm Bleeding Rapid enlargement Ulceration Lack of response to treatment (no response to treatment or rapid recurrence following an initial response)
The procedure for the physical examination of a patient with AK is as follows:
- 1.
Complete dermatological examination, paying particular attention to areas of the skin usually exposed to sunlight (face, balding scalp, ears, upper chest or neckline, and the dorsum of hands and forearms). Patients usually have multiple AKs rather than a single lesion. Patients with AKs are also more likely to have other skin tumors associated with exposure to sunlight, including basal cell carcinoma, squamous cell carcinoma, and melanoma.6
- 2.
A detailed description of the type, site, and extent of lesions is required because these factors will determine the appropriate treatment. Not all treatments are indicated in all cases or sites.
- 3.
The clinician should make a note of all signs of chronic actinic damage (telangiectasias, pigmentary changes, elastosis, and wrinkles), scars, and patches of hypopigmentation caused by previous treatments (cryotherapy, surgery, etc.), and note the visible presence, if any, of field cancerization.
AKs typically present as a macule, papule, or plaque that is rough to the touch and skin color or slightly erythematous, measuring between a few mm and 2 to 3cm. The number of AKs observed varies (single or multiple lesions) and lesions are usually located in areas of the skin characterized by signs of chronic sun damage caused by exposure to UV radiation, such as the face, the balding scalp, the upper chest, the ears, the dorsum of the hands and forearms, and the lower lip (actinic cheilitis). Since AKs generally cause no pain or discomfort or produce only mild symptoms, patients often delay consulting a dermatologist.
In addition to the classic presentation, other types of AK have been classified according to their clinical and histologic features.10
- –
Pigmented AK. Hyperkeratotic macules or flat papules that may be hyperpigmented or reticulated, but lack any erythematous component.
- –
Lichenoid AK. Clinically similar to the classic form of AK but present pronounced erythema around the base of the lesion secondary to an underlying lichenoid infiltrate.
- –
Hypertrophic or hyperkeratotic AK. These easily identifiable lesions take the form of a highly keratotic papule or plaque on an inflammatory substrate. A cutaneous horn may develop over time.
- –
Atrophic AK. In this form of AK, the lesion is a somewhat scaly erythematous macule. Histology reveals an atrophic epidermis.
Diagnosis of AK is based primarily on the clinical findings from a skin examination. The 2 cornerstones of diagnosis are the clinical findings described above and a medical history including information on the typical risk factors.6
A three-level scale has been proposed for the classification of the severity of these lesions11:
- –
Grade 1 (mild): slight palpability (more easily felt than seen).
- –
Grade 2 (Moderate): Moderately thick and visible lesions (easily felt and seen).
- –
Grade 3 (Severe): Very thick and hyperkeratotic lesions.
The positive predictive value of a clinical diagnosis of AK ranges from 74% to 94%.12
The differential diagnosis should include invasive squamous cell carcinoma, superficial basal cell carcinoma, BD, porokeratosis, viral warts, chronic discoid cutaneous lupus erythematosus, large cell acanthoma, senile lentigo, and seborrheic keratoses. In the case of a pigmented lesion, a diagnosis of lentigo maligna or reticulated subtypes of seborrheic keratosis should be ruled out. Dermoscopy is a useful tool for this purpose, although a biopsy may sometimes be necessary.
Histopathologic diagnosisAlthough the diagnosis of AK is mainly clinical, histopathologic examination is sometimes necessary, either to rule out dermal invasion indicative of invasive squamous cell carcinoma or to exclude other possible diagnoses.
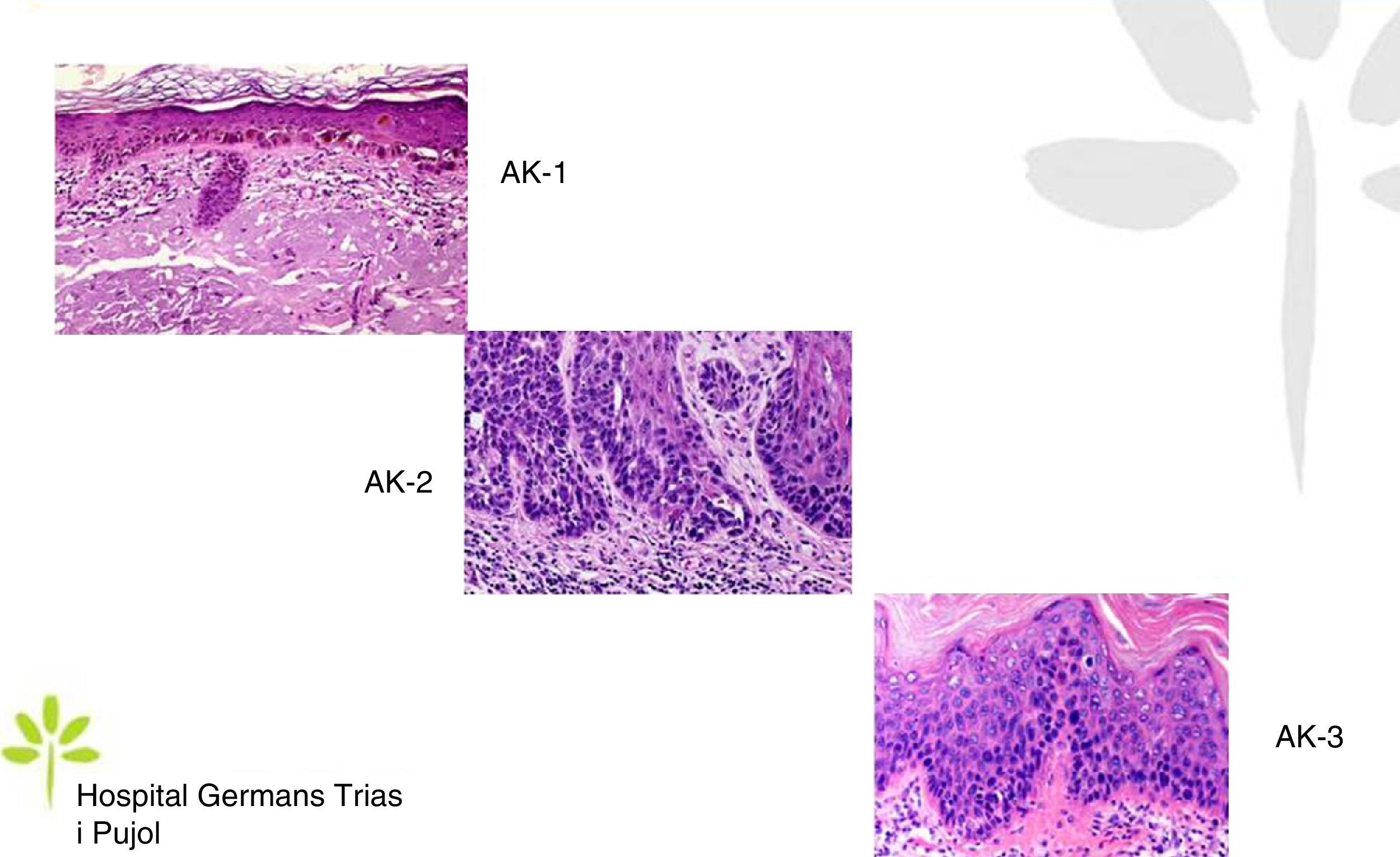
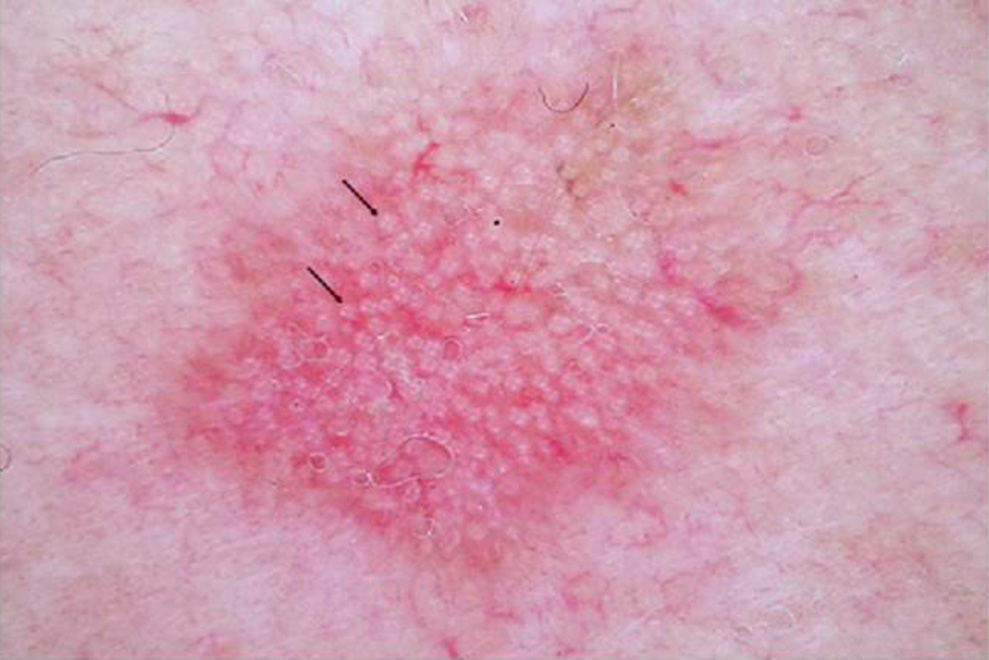
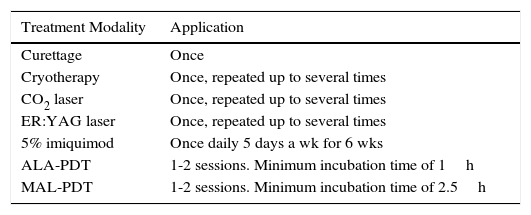
AKs are characterized microscopically by intraepidermal proliferation of atypical keratinocytes with pleomorphic and hyperchromatic nuclei characterized by a loss of polarity and increased mitoses. The atypical keratinocytes are indistinguishable from those found in invasive squamous cell carcinomas, which is why AK is considered to be an in situ squamous cell carcinoma. Depending on the degree of intraepidermal keratinocyte atypia, the lesion is rated according to the following system (Fig. 1)13,14:
- a.
Grade 1: atypia in the lower third of the epidermis
- b.
Grade 2: atypia in the lower 2-thirds of the epidermis
- c.
Grade 3: affecting the full thickness of the epidermis
In practice, however, most dermopathologists and pathologists do not include this classification in their pathology report. Although it was initially thought that this scale of intraepidermal involvement correlated with the likelihood of the lesion progressing to invasive squamous cell carcinoma, current thinking is that the risk of progression is already present in Grade 1 lesions and that progression to grades 2 or 3 is not a prerequisite for progression to invasive squamous cell carcinoma.15
Table 2 shows the histologic features16 of AK in the initial phases and Table 3 those found in fully developed lesions.
Histopathologic Features of Early-Stage Actinic Keratoses.
| Atypical keratinocytes (large, pleomorphic and hyperchromatic nuclei and evidence of increased mitosis) distributed in a disorganized way in the basal layers of the epidermis. |
| Hyperkeratosis with focal parakeratosis |
| Lymphocytic infiltrate of varying density in the upper dermis |
| Actinic elastosis |
Histopathologic Features of Fully Developed Actinic Keratoses.
| Variable epidermal thickness accompanied by acanthosis or atrophy. In some cases, rete ridges are arranged in buds or columns. |
| The atypical epidermal keratinocytes may remain in the lower third of the epidermis indefinitely or may extend upwards into the superficial layers, and in some cases occupy the full thickness of the epidermis. |
| Keratinocyte atypia may extend downwards following the adnexal epithelium, both in lesions that display atypia in the basal layer and those characterized by full-thickness atypia. |
| Hyperkeratosis with frequent parakeratosis |
| Actinic elastosis in the dermis |
| Lymphocytic infiltrate of varying density in the upper dermis |
Dyskeratotic and acantholytic cells on suprabasal clefts are observed in acantholytic AK. In other cases, histology may reveal increased melanin deposition (hyperpigmentation). In the case of lichenoid AK, an intense bandlike lymphocytic infiltrate in the superficial dermis is accompanied by apoptosis of basal keratinocytes.
Other Diagnostic TechniquesAlthough the diagnosis of AK in clinical practice is primarily based on clinical findings (with histology being used primarily to resolve doubts), advances in technology have led to the use of other noninvasive and complementary diagnostic techniques. It should be noted, however, that, with the exception of dermoscopy, none of these techniques are used routinely by most dermatologists in clinical practice. It is always useful to photograph the lesions and the surrounding cancerization field to provide a baseline for subsequent assessments of response to treatment.
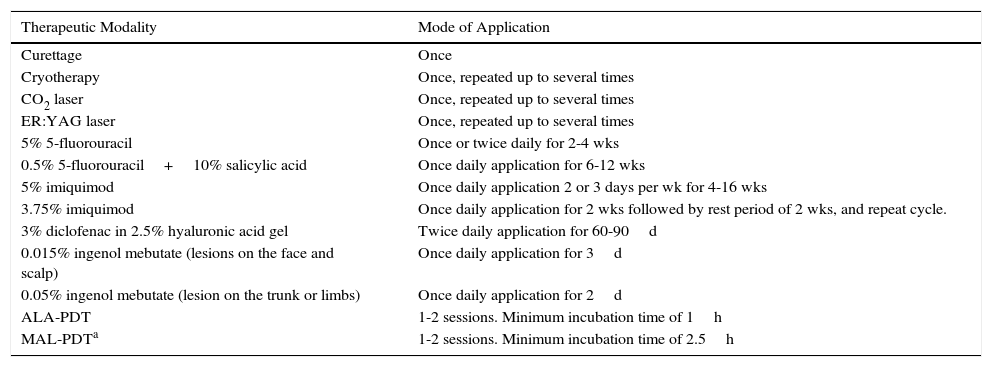
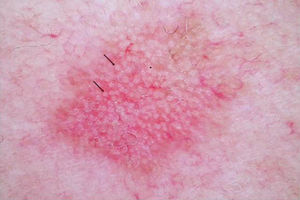
DERMOSCOPYDermoscopy is a technique used widely in dermatology to diagnose skin tumors. In the case of AK, a characteristic strawberry-like pattern has been described (Fig. 2).17 This is produced by a combination of erythema in the form of a pink-to-red pseudonetwork, associated with large wavy vessels and prominent follicular ostia surrounded by white halos. Other very characteristic features of AK are rosettes and hyperkeratosis. In the case of squamous cell carcinoma, dermoscopy allows the physician to identify structures that make a diagnostic biopsy mandatory. A dermoscopic model of progression from incipient AK to well-differentiated squamous cell carcinoma has been described and is useful in differentiating between these 2 types of lesions.18
In lesions that are difficult to diagnose on the basis of clinical findings alone, dermoscopy significantly improves diagnostic accuracy in differentiating AK from superficial basal cell carcinoma. Dermoscopy is also an essential tool in the differentiation of pigmented AKs from lentigo maligna and pigmented basal cell carcinoma, and in the decision on whether or not a biopsy is necessary.
PHOTODIAGNOSISPhotodiagnosis involves the administration of a photosensitizing agent or a precursor of a photosensitizer, which accumulates in metabolically active cells and tissues. When illuminated in the presence of oxygen with a light of the appropriate wavelength, the photosensitizer produces reactive oxygen species and free radicals, which induce photooxidation and tissue destruction. In dermatology, the photosensitizer precursors used are Δ-aminolevulinic acid (ALA) and its derivative 5-methyl aminolevulinate (MAL). In the body, these precursors are converted into protoporphyrin ix, a photosensitizer that emits a coral red fluorescence when illuminated with light of the appropriate wavelength, facilitating tumor identification. The effectiveness of photodiagnosis has not been fully confirmed, but the technique can be of use in defining the extent of the cancerization field.19 It is not available in all hospitals and consumes resources.
In the case of phototherapy, daylight is now being used rather than lamps to provide the necessary illumination in order to eliminate the need for special equipment. The area being treated has to be exposed to daylight for 2hours between 10 am and 6 pm on a dry day. In clinical trials, the outcomes obtained using daylight phototherapy have been similar to those obtained with illumination using lamps, but tolerance has been much better because daylight therapy rarely causes pain or discomfort.
IN VIVO REFLECTANCE CONFOCAL MICROSCOPYIn vivo reflectance confocal microscopy is a noninvasive skin examination technique that uses an 830nm laser microscope The technology allows real-time skin imaging with cellular resolution and a penetration depth of 200 to 300μm. Handheld microscopes offer the possibility of rapid examination of multiple lesions in a few minutes.
Reflectance confocal microscopy has demonstrated high diagnostic sensitivity and specificity, particularly in pigmented lesions and when dermoscopic findings do not conclusively rule out basal cell or squamous cell carcinoma. In Spain, this technique is more often used in research than in routine clinical practice because the microscopes are unfortunately only available in very few hospitals. Because it can identify pleomorphism and architectural disruption in the stratum spinosum, reflectance confocal microscopy is particularly useful for detecting subclinical AK in the cancerization field or in areas of severe actinic damage.20
OPTICAL COHERENCE TOMOGRAPHYOptical coherence tomography is a noninvasive technique that uses infrared light waves to create skin images using the principle of interference (interferometry). It has lower resolution than reflectance confocal microscopy but greater tissue penetration, from 500μm to several mm depending on the device used and the tissue under examination. This technique has been used in research settings to study AKs and skin cancer and to monitor the response to treatment of these lesions.21
Field cancerizationSince the presence or absence of a cancerization field is an important consideration in the design of a treatment strategy, this factor must always be evaluated in patients with AK.
The cancerization field is the area of actinically damaged skin that may surround each AK and display similar genetic alterations. The field may contain clinically visible AKs, subclinical AKs (only visible under a microscope), groups of keratinocytes with genetic mutations only detectable using molecular biology methods, and normal skin.22,23
The problem associated with the evaluation of field cancerization is that it is impossible to clearly delimit the area clinically, making the delimitation of the size of the field somewhat arbitrary. Reflectance confocal microscopy could be very useful in delimiting the cancerization field, but the equipment required is not usually available in clinical practice, at least in Spain.
The presence of field cancerization has important implications for treatment. If we only treat clinically visible lesions, the targeted lesions will be eliminated but the field will continue to pose a problem, giving rise to new AKs over time. However, by treating the cancerization field, we can eliminate not only the visible AKs but also the subclinical lesions, and possibly the clones of cells that would eventually become AKs.
In any case, the treatment of field cancerization is still a more theoretical than practical concept given the impossibility of reliably establishing the clinical boundaries of the field and the lack of any evidence that treating field cancerization decreases the risk of invasive squamous cell carcinoma.
Natural History and Need for TreatmentAK is currently regarded as a chronic disease with the potential to progress to invasive squamous cell carcinoma, to persist unchanged, or to spontaneously regress to normal.24
However, the available data on rates of progression and regression are limited, unreliable, and affected by important methodological limitations. Furthermore, no prognostic factors have been identified that could be used to predict the likely outcome of a particular AK.
Thus, since there is no way to predict progression and given the chronic nature and inherent potential for progression to invasive squamous carcinoma of the lesions, treatment is recommended.24
Cosmetic considerations are another reason for treating AKs and occasionally the need to alleviate symptoms, although these are rare.
Treatment StrategiesTherapeutic modalitiesThe therapeutic armamentarium is extensive and includes ablative, non-ablative, and mixed modalities.
Destructive methods are generally used to treat solitary lesions or small numbers of lesions, but not to treat field cancerization.10 They include curettage with or without electrocoagulation, cryotherapy, CO2 laser, Erbium-doped yttrium aluminum garnet (Er:YAG) laser, and surgery. Most of the nondestructive and mixed methods are indicated for the treatment of lesions and cancerization fields.6,10 Commercial preparations available in Spain include 0.5% 5-fluorouracil+10% salicylic acid; 3.75% and 5% imiquimod; 3% diclofenac in 2.5% hyaluronic acid gel; 0.05% and 0.015% ingenol mebutate; 5% 5-fluorouracil can be obtained as a customized compound.10 Mixed modality approaches include photodynamic therapy, which often requires pretreatment curettage of the AK to facilitate penetration of the photosensitizer (ALA or MAL).
Details of the application of the different therapeutic modalities are shown in Table 4.
Therapeutic Modalities for Actinic Keratosis.
| Therapeutic Modality | Mode of Application |
|---|---|
| Curettage | Once |
| Cryotherapy | Once, repeated up to several times |
| CO2 laser | Once, repeated up to several times |
| ER:YAG laser | Once, repeated up to several times |
| 5% 5-fluorouracil | Once or twice daily for 2-4 wks |
| 0.5% 5-fluorouracil+10% salicylic acid | Once daily application for 6-12 wks |
| 5% imiquimod | Once daily application 2 or 3 days per wk for 4-16 wks |
| 3.75% imiquimod | Once daily application for 2 wks followed by rest period of 2 wks, and repeat cycle. |
| 3% diclofenac in 2.5% hyaluronic acid gel | Twice daily application for 60-90d |
| 0.015% ingenol mebutate (lesions on the face and scalp) | Once daily application for 3d |
| 0.05% ingenol mebutate (lesion on the trunk or limbs) | Once daily application for 2d |
| ALA-PDT | 1-2 sessions. Minimum incubation time of 1h |
| MAL-PDTa | 1-2 sessions. Minimum incubation time of 2.5h |
Abbreviations: ALA, 5-aminolevulinic acid; CO2, carbon dioxide; ER:YAG, erbium-doped yttrium aluminium garnet; MAL, methyl aminolevulinate; PDT, photodynamic therapy
It is important to note that destructive and nondestructive therapies are not incompatible. In fact, the combination of a lesion-directed therapy and a field-directed therapy is common, often well tolerated, and achieves greater efficacy in terms of total clearance and prevention of recurrence than the use of either method alone.25–27
Treating field cancerization as well as clinically visible lesions is to a certain extent a preventive strategy because it stops subclinical lesions from progressing to clinically visible lesions which could eventually become squamous cell carcinoma.28 Moreover, by preventing new lesions or delaying their onset, field-directed therapy can serve to increase the interval between follow-up visits.
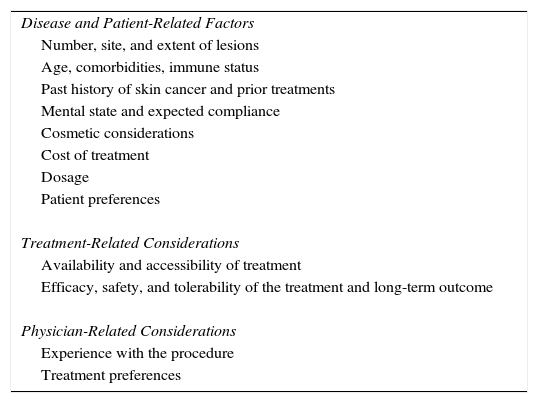
Considerations affecting the choice of treatmentThe decision on whether to use a lesion-directed or field-directed therapy and which modality to use will depend on many factors that must be evaluated on a case-by-case basis. The factors that affect the choice of treatment are shown in Table 5.
Considerations When Choosing A Therapy for Actinic Keratoses.
| Disease and Patient-Related Factors |
| Number, site, and extent of lesions |
| Age, comorbidities, immune status |
| Past history of skin cancer and prior treatments |
| Mental state and expected compliance |
| Cosmetic considerations |
| Cost of treatment |
| Dosage |
| Patient preferences |
| Treatment-Related Considerations |
| Availability and accessibility of treatment |
| Efficacy, safety, and tolerability of the treatment and long-term outcome |
| Physician-Related Considerations |
| Experience with the procedure |
| Treatment preferences |
Given the large number of factors, it would be very difficult to design a treatment algorithm covering all clinical situations. In fact, the different strategies that have been proposed in the clinical practice guidelines of different countries vary somewhat.6,10,24,29,30 However, a useful starting point is to establish a strategy based on the number of lesions to be treated,6 their distribution (whether they are isolated or grouped), and the presence or absence of field cancerization. The different options can then be modeled on the basis of other factors.
In patients with between 1 and 5 ungrouped lesions located in different anatomical areas, therapies that target individual lesions or topical pharmacological treatments can be used (Table 4). In Spain, and many other countries, cryotherapy is undoubtedly the most widely used treatment in this setting. However, this does not mean that it has been shown to be the most effective or the most efficient option, and the recurrence rate after cryotherapy is high.10,31 It is interesting to note that cryotherapy was strongly recommended in recent European evidence-based guidelines despite the paucity of evidence and scant number of comparative clinical trials.6 In patients with a few isolated lesions, the use of other lesion-directed, ablative treatments (photodynamic therapy and CO2 or Er:YAG laser therapy) is limited in Spain due to the high cost and lack of equipment. Finally, the risk of scarring limits the use of curettage, electrocoagulation therapy, and surgery. These destructive modalities are, nonetheless, necessary in some cases, for example in highly hyperkeratotic lesions or when histopathologic study is required to resolve diagnostic doubt.10
In other situations, topical therapies can be used. If the lesion is moderately or very hyperkeratotic, 0.5% 5-fluorouracil in 10% salicylic acid should be used, as indicated in the Summary of Product Characteristics and because it has been shown to obtain good results, as assessed by both patient and clinician.32 Other topical treatments can be used to treat lesions without hyperkeratosis.10 When there is little or no hyperkeratosis, 3% diclofenac in 2.5% hyaluronic acid gel is less effective than other topical treatments33 but is the best tolerated therapy34,35 The other topical drug therapies can provoke intense local inflammatory reactions. These local reactions include erythema, scaling, scabbing, edema, vesicles, pustules, erosions, ulceration, and scarring with hyperpigmentation or hypopigmentation of greater or lesser severity. In general, adverse reactions appear a few days after start of treatment and persist throughout treatment and for a few days or weeks after completion.33 They often reduce adherence to treatment. In the case of ingenol mebutate, the duration of treatment is shorter and it is the only therapy in which the local reaction develops after the patient has completed the treatment regimen; as a result, adverse reactions rarely influence adherence.36 Moreover, some of these therapies, including sodium diclofenac and photodynamic therapy, can cause photosensitivity, and others, such as imiquimod, can produce systemic symptoms, including general malaise and flu-like syndrome associated with the local skin reaction.6 In light of the immune-mediated mechanism of action of several of these drug therapies, the application of topical corticosteroids is not recommended for the treatment of these localized skin reactions.
If the lesions are numerous (6 or more) and affect different anatomical areas, the principles are the same as those detailed above except that in this case topical drug therapies are the recommended option because of the pain associated with destructive modalities and the risk of superinfection, pigmentation disorders, and scar formation.6
Another clinical situation is that of a patient with fewer than 6 AKs, which are grouped in a single anatomical area or are associated with hyperkeratosis and marked changes due to chronic actinic damage in the surrounding area (field cancerization). In this situation, destructive approaches are not appropriate, and the choice of photodynamic therapy or topical drug therapy will depend on what is agreed between the physician and the patient after analysis of the advantages and disadvantages of each modality, seeking the best balance between efficacy and tolerance and the best cost-benefit profile.37 In these situations, ablative treatments should only be used to treat an isolated lesion that does not respond to topical treatment or photodynamic therapy or is highly hyperkeratotic; they should never used to treat field cancerization.
Another anomalous clinical situation is that of immunocompromized patients with AK, who often present multiple lesions. In this group of patients—given the possibility of more rapid progression to invasive squamous cell carcinoma and, in some cases, the difficulty of diagnosis—the most usual approach is to use a combination of lesion-directed and field-directed therapies. The most common approach in such cases is a destructive lesion-directed therapy in combination with topical drug treatment or photodynamic therapy.32 The appropriateness of using immune boosting drugs on large areas in iatrogenically immunosuppressed patients should be considered with particular attention because of the potentially negative impact of such treatment on the patient's underlying condition. Nevertheless, 5% imiquimod, 5% 5-fluorouracil, 3% sodium diclofenac, and photodynamic therapy have all been shown to be safe in this group of patients.32
Preventive and/or Adjuvant TherapyPhotoprotectionThe treatment of AKs should always be accompanied by sun protection measures, irrespective of the treatment and the clinical situation.10
Since UV radiation is the main cause of the condition, protecting the skin from exposure to the sun forms an integral part of the overall treatment of AKs. The following sun protection measures should be considered: protection from solar radiation by seeking shade during peak hours of UV radiation (10 am to 3 pm); wearing a wide brimmed hat, sunglasses, and photoprotective clothing; applying sunscreen with a sun protection factor of 30 or higher on a regular basis. Several studies have demonstrated the usefulness of daily application of sunscreen not only in preventing the development of new AKs but also in the remission of existing lesions.38,39
ChemoprophylaxisIn at-risk patients with multiple lesions who develop multiple invasive squamous cell carcinomas (a common clinical situation in solid organ transplant recipients), chemoprophylaxis with oral retinoids can be considered. This type of treatment has been shown to effectively reduce the number of new lesions (both squamous cell carcinoma and AK).40 Nevertheless, it is rarely used and remains controversial because of the poor tolerance associated with really effective doses, the scant number of studies on the topic, and the inconsistent results obtained by existing studies.10,38,41,42
Follow-upAK should be regarded as a chronic disease and patients should be followed up and monitored regularly. The number and frequency of check-ups will depend on the patient's age at onset, risk factors, and history of nonmelanoma skin cancer.6 During follow-up visits, the clinician should stress the need for self-examination, encouraging the patient to ensure early detection of suspicious lesions. Follow-up can be done by a primary care physician, who should refer the patient to a specialist if any diagnostic doubt arises or help is needed to identify the most appropriate therapeutic strategy.10
Bowen DiseaseIntroductionBowen disease (BD), traditionally called keratinizing squamous cell carcinoma in situ of the skin, is much less common than AK, although no data on prevalence is available. However, the incidence of BD, like that of AK, will increase owing to increases in life expectancy and the growing popularity of outdoor pursuits.
Definition and NomenclatureIn BD, the term squamous cell carcinoma in situ refers to an intraepithelial lesion characterized by clonal proliferation of atypical keratinocytes occupying the full thickness of the epidermis. Like AKs, these lesions can, if left untreated, progress to invasive squamous cell carcinoma.43
Identification of At-Risk PatientsUnlike AK, BD does not only develop on sun-exposed areas of the skin because its pathogenesis includes other types of radiation (ionizing) in addition to UV radiation, certain toxic substances (arsenic), and even infection with oncogenic strains of the human papillomavirus.44 Thus, the risk factors for developing BD are the same as those listed for AK but also at risk are individuals who, because of their sexual behavior, could have acquired a high-risk oncogenic serotype of the human papillomavirus.45
Initial Assessment of Patients with BDMedical HistoryAs in any other disease, the initial assessment of a patient with BD should include a general medical history. This should include a series of additional questions to obtain information that could be important in the design of a treatment and follow-up strategy. The following additional information is of interest:
- 1.
Details of prior treatments to assess their efficacy and the patient's tolerance in each case.
- 2.
Prior history of nonmelanoma skin cancer.
- 3.
Sun exposure in the course of work or outdoor leisure activities and the use of UV-A lamps, etc.
- 4.
Current immunosuppressant therapy or history of such therapy for any reason.
- 5.
Associated symptoms (itching, pain, burning sensation, etc.).
- 6.
The reason for the consultation (symptoms, anxiety about skin cancer, cosmetic concerns).
- 7.
Questions designed to identify the signs of suspected progression of a BD to invasive squamous cell carcinoma, basically to ascertain whether the base of the lesion is infiltrated, whether there is a tumoral lesion on the surface, or whether the lesion is ulcerated.
- 8.
Finally, the clinician should ask about the patient's sexual habits and the diagnosis in a partner of human papilloma virus infection or intraepithelial cervical neoplasia.46
The following steps should be taken in any patients suspected of having BD:
- 1.
A complete dermatologic examination because the lesions can appear in any part of the integumentary system, in both exposed and covered areas. An exhaustive examination of the skin is also necessary because of the high incidence of prior or concomitant nonmelanoma skin cancers, primarily basal cell carcinoma, in patients with BD.47
- 2.
Also essential is a detailed description of the BD, usually a solitary lesion, including color, grade of hyperkeratosis, presence of ulceration, spontaneous bleeding, infiltration of the base, etc.
- 3.
An association between BD and internal malignancy was reported for many years, and patients with BD have been advised to undergo assessment to rule out this possibility (directed medical history and further investigation). More recent studies have not, however, confirmed this association.48
BD almost always presents as a slow-growing solitary lesion that takes the form of a well-defined scaly or crusted annular plaque with irregular borders measuring 1 to 2cm in diameter. The lesion is erythematous and not infiltrated. In some cases, it is characterized by marked hyperkeratosis and it may be pigmented. Lesions can sometimes grow to several centimeters in diameter and some patients present several lesions.
Genital BD is associated with human papillomavirus virus infection and develops on the pubis, the penile shaft, or in the perianal area. In such cases, it usually presents as one or more well-defined, hyperkeratotic plaques. BD affecting the genital mucosa is called eythroplasia of Queyrat, and the lesion presents in the form of an erythematous macule-plaque, which is difficult to delimit and may have small superficial erosions. Another variant associated with human papillomavirus infection is BD affecting the periungual area, which presents as a scaly erythematous plaque around the cuticle of the nail and may be accompanied by onycholysis or discoloration of the nail.49
Diagnosis of BDClinical diagnosisA definitive diagnosis of BD usually requires a skin biopsy. The clinical presentation, which is described above, is very indicative. Dermoscopy can aid diagnosis by revealing glomeruloid vessels and scales,50 but a biopsy is generally required to exclude other possible diagnoses. The main differential diagnoses are infiltrating squamous cell carcinoma, superficial basal cell carcinoma, nummular eczema, condylomata acuminata, porokeratosis, chronic discoid cutaneous lupus erythematosus, and seborrheic keratosis.
Histopathologic diagnosisBD is characterized microscopically by full-thickness intraepidermal proliferation of atypical keratinocytes that may include the intraepidermal portion of skin appendages. Typically, the atypical keratinocytes are pleomorphic and hyperchromatic. They can also be vacuolated and have a clear and prominent cytoplasm reminiscent of Paget cells. Other common findings are cellular dyspolarity, loss of maturation, and the presence of numerous mitotic figures. Other typical features include hyperkeratosis, to a greater or lesser degree, and parakeratosis, as well as acanthosis and complete architectural disorder. A chronic inflammatory infiltrate composed of lymphocytes, plasma cells, and histiocytes is usually found in the superficial dermis. Several histologic variants have been described: psoriasiform, atrophic, and acantholytic BD.
Natural History and Need for TreatmentUnlike AK, BD usually present as solitary and persistent lesions. If left untreated, the lesion will continue to grow superficially and may eventually invade the deeper dermis and become invasive squamous cell carcinoma. The estimated risk of malignant progression is 3% to 5%. In the case of eythroplasia of Queyrat, the risk is slightly higher, at around 10%.44
Treatment StrategiesTherapeutic ModalitiesThe therapeutic armamentarium for BD, like that of AK, includes ablative, non-ablative, and mixed modalities. The choice of treatment will depend on size and site of the lesion and the preferences of both physician and patient. However, mixed modality approaches using a combination of destructive and nondestructive methods are not often required in the case of Bowen disease.
Destructive treatments include surgery, curettage (shave) with electrocoagulation, cryotherapy, and CO2 or Er:YAG laser therapy. The nondestructive treatment options and mixed modalities currently approved in Spain for the treatment of BD are 5% imiquimod and photodynamic therapy with MAL or ALA. However, in clinical practice, physicians tend to use the same therapies for BD that they use for AK.
A recent study published by the Cochrane group identified only 9 randomized trials on BD.51 The authors concluded that photodynamic therapy with MAL was an effective treatment, with the evidence showing it to be more effective than cryotherapy and not significantly different from 5-fluorouracil. Efficacy improved, however, when ALA was used instead of MAL.51
The modes of application for the different therapeutic modalities are shown in Table 6.
Therapeutic Modalities for the Treatment of Bowen Disease.
| Treatment Modality | Application |
|---|---|
| Curettage | Once |
| Cryotherapy | Once, repeated up to several times |
| CO2 laser | Once, repeated up to several times |
| ER:YAG laser | Once, repeated up to several times |
| 5% imiquimod | Once daily 5 days a wk for 6 wks |
| ALA-PDT | 1-2 sessions. Minimum incubation time of 1h |
| MAL-PDT | 1-2 sessions. Minimum incubation time of 2.5h |
Abbreviations: ALA, 5-aminolevulinic acid; CO2, carbon dioxide; ER:YAG, erbium-doped yttrium aluminium garnet; MAL, methyl aminolevulinate; PDT, photodynamic therapy
The decision on whether to use surgery, another ablative therapy or topical treatment will depend on several factors, as is the case with AK. These factors must be considered on a case-by-case basis for each patient.44
Surgical excision with pathological examination of the sample is the only procedure that can guarantee lesion-free margins and confirm that the lesion was localized to the epithelium. When other treatment options are used, we do not have 100% certainty that the lesion was solely intraepithelial. For this reason, before other options are chosen, a biopsy should be performed to confirm the diagnosis and to obtain samples of all of the areas that could provide evidence that the lesion has invaded the dermis.
Conflicts of InterestCarlos Ferrándiz has received sponsorship for conference attendance and/or consultancy fees from Almirall, Leo Pharma and Spherium Biomed. Maite Fernández-Figueras has received honoraria as an invited speaker and/or research funding from Almirall, Galderma, Leo Pharma, Novartis, and Roche. Carla Ferrándiz-Pulido has received speakers fees and/or consultancy fees and/or sponsorship to attend conferences from Almirall, Leo Pharma, ISDIN, Galderma, and IFC. Carlos Guillén has received consulting fees and/or speaker's fees from Galderma, Almirall, Meda, Leo Pharma, Bio Frontera, ISDIN. J. Malvehy has received consultancy fees and/or funding for research from Almirall, Leo Pharma, Meda, Amgen, ISDIN, Novartis, Roche, Scibase, GSK, Bristol Meyers-Squibb, Cantabria, and MAVIG.
Please cite this article as: Ferrándiz C, Malvehy J, Guillén C, Ferrándiz-Pulido C, Fernández-Figueras MT. Precáncer cutáneo. Actas Dermosifiliogr. 2017;108:31–41.