Actinic keratosis (AK) lesions are in situ squamous cell carcinoma. These lesions have a low risk of progressing to invasive disease but significant impact on patients’ quality of life (QoL). The aim of this study was to assess QoL and side effects in patients with AK receiving treatment with ingenol mebutate.
Material and methodsThis was a prospective, non-randomized pilot study carried out in Spain. The target population was adults with a clinical diagnosis of AK affecting any part of the body. Outcomes were assessed on the basis of a QoL questionnaire (Skindex-29), local skin response, the Treatment Satisfaction Questionnaire for Medication (TSQM 1.4), and clinical response.
ResultsA total of 19 patients were studied. Most of the participants were men (89.5%) and mean age was 76.2 years. After treatment with ingenol mebutate, significant improvement was observed in the Skindex-29 subscales relating to symptom severity (P=.041), the patients’ emotional state (P=.026), and in the overall score (P=.014). Erythema, crusting, and flaking or scaling were the local skin responses with highest median score (2.0 in all 3 cases). Imiquimod 5% and ingenol mebutate achieved higher median scores for effectiveness and global satisfaction than any other previous treatments (as measured by TSQM 1.4). In the patients’ assessment of convenience, ingenol mebutate had a higher median score than previous treatments. Over half of the patients (52.6%) had an improvement of at least 75% at month 3.
ConclusionsQoL in patients with AK improves after treatment with ingenol mebutate. The presence of side effects did not affect QoL or patient satisfaction with treatment.
Las queratosis actínicas (QA) se consideran carcinomas espinocelulares “in situ” con poca capacidad invasiva pero con un impacto significativo en la calidad de vida (CV). El objetivo fue evaluar la CV y los efectos secundarios de ingenol mebutato (IM) en pacientes con QA.
Material y métodosEstudio piloto, prospectivo, no aleatorizado, en pacientes >18 años, con diagnóstico clínico de QA de cualquier localización. Se valoraron: la CV, mediante el cuestionario Skindex- 29; la satisfacción con el tratamiento mediante el cuestionario TSQM 1.4; la respuesta clínica, y la reacción cutánea local (RCL).
ResultadosSe incluyeron 19 pacientes, el 89,5% eran hombres, con una edad media 76,2 años. Después del tratamiento con IM, se observó una mejoría significativa en las subescalas del Skindex-29 (emocional y sintomática) y en la puntuación global (p=0,026, p=0,041 y p=0,014), respectivamente). Al día 3–4, el eritema, la costra y la descamación fueron las RCLs con una mediana de puntuación más alta (2,0, en los 3 casos). La efectividad y la satisfacción global (según TSQM 1,4) presentaron puntuaciones medianas más altas con imiquimod 5% e IM comparado con los otros tratamientos previos. La valoración de la conveniencia mostró una puntuación más alta en IM comparado con los otros tratamientos previos. Más de la mitad de pacientes (52,6%) lograron una mejoría ≥75% al tercer mes.
ConclusionesLa CV en pacientes con QA mejora después del tratamiento con IM. La presencia de efectos secundarios no afecta ni a la CV ni a la satisfacción de los pacientes con el tratamiento.
A European study found the prevalence of actinic keratosis (AK) to be 15.4% in men and 5.9% in women and reported a strong association with age in both sexes, with a prevalence of 34.1% and 18.2%, respectively, in men and women aged 70 years or more.1 Approximately 10% of AKs will progress to squamous cell carcinomas, and this figure rises to 40% in immunodeficient patients.2,3
A review of published economic studies on the treatment of AK has estimated the direct cost of AK management in the US at $US1.2 billion per year, with 6% spent on topical therapy, 43% on office visits, and 51% on destructive procedures.4
Greater understanding of the pathophysiological changes leading from AK to malignancy and the increasing global prevalence of AK has led to a new focus on the importance of combining local treatments to remove individual lesions with topical or procedural field therapies that treat the entire actinically damaged field.5 According to the treatment algorithm, individually tailored treatment should be started as soon as AK is diagnosed.6 The following important considerations should be taken into account when developing a comprehensive treatment plan: treatment-related factors (efficacy, safety, tolerance, and long-term outcomes); disease- and patient-related factors (number, location, and extension of lesions, age, comorbidity, mental status, history of skin cancer, prior treatments, costs, and patient preferences); and physician-related factors (experience with the procedure, availability, and preferences).7
Several different topical treatments for AK are currently available. Outcomes in terms of clinical response and side effects vary, and most therapies require treatment over a period of weeks.8 The prolonged duration of treatment and the presence of side effects, which may include pain, discomfort, and disruption of daily activities, can affect the patient's quality of life (QoL) and reduce adherence to treatment,9–12 which is already poor in topical therapies.13 To improve adherence and, ultimately, to ensure QoL, it is important to identify options requiring a shorter treatment duration and offering more rapid resolution of local skin reactions.
Ingenol mebutate (IM), which was approved by the European Medicines Agency as a treatment for AK in November 2012, has demonstrated efficacy in clearing AK lesions on the face, scalp, trunk, and extremities.14–16
The aim of this pilot study in patients with AKs was to evaluate the following outcomes in clinical practice: effect of IM on QoL, patient satisfaction with treatment, clinical efficacy, and side effects.
Materials and methodsNineteen patients with AK were enrolled in this prospective, non-randomized pilot study. Eligible patients were adults (>18 years old) with a clinical diagnosis of AK affecting any part of the body in whom treatment with IM was not contraindicated. Patients were recruited consecutively over a 6-week period (15 November 2013 to 28 December 2013) independently of whether or not they had received previous treatment for AK. As the aim of this study was to assess the treatment in clinical practice, patients were instructed to apply the standard dose of gel over the total area affected.
The study was conducted in accordance with the Declaration of Helsinki and local laws and regulations and was approved by the Institutional Review Board of the Hospital Universitari Son Espases, Palma de Mallorca, Spain. All patients provided written informed consent.
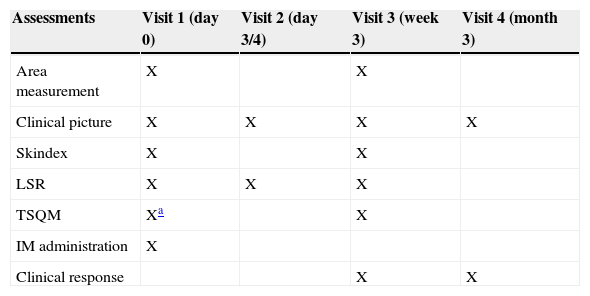
This prospective study included 4 visits: visit 1 at baseline; visit 2 on day 3 (patients with lesions on the trunk or extremities) or day 4 (patients with face and scalp lesions); visit 3 at week 3; and visit 4 after 3 months (Table 1). At visit 1 patient received IM, which they then applied for 2 consecutive days if their lesions were on the trunk or extremities or 3 consecutive days in the case of face and scalp lesions. Patients were also given fusidic acid in case inflammation occurred and were instructed in all cases to apply sun block.
Study design.
| Assessments | Visit 1 (day 0) | Visit 2 (day 3/4) | Visit 3 (week 3) | Visit 4 (month 3) |
|---|---|---|---|---|
| Area measurement | X | X | ||
| Clinical picture | X | X | X | X |
| Skindex | X | X | ||
| LSR | X | X | X | |
| TSQM | Xa | X | ||
| IM administration | X | |||
| Clinical response | X | X |
Abbreviations: IM, ingenol mebutate; LSR, local skin response; TSQM, treatment satisfaction Questionnaire for Medication.
The following data were collected at baseline: patient characteristics (sex, age and skin phototype); disease characteristics (time since initial diagnosis, prior AK treatments used in the previous 3 months; lesion size (measured using a millimeter ruler); and any clinical history of non-melanoma skin cancer (NMSC) or melanoma. The primary end points were patient QoL and treatment satisfaction. To assess QoL we used the validated Spanish version17 of Skindex-29, a questionnaire specifically designed to assess QoL in patients with dermatological conditions.18,19 Each question on the survey was rated by the patients on a 5-point scale, with response choices and corresponding scores ranging from “never” (0) to “all the time” (100); the lower the final score, the better the patient's overall QoL. The overall QoL score can be divided into 3 subscales, which correspond to the patient's emotional state, symptom severity, and functional state. Patients completed the questionnaire at baseline and at visit 3.
Patient satisfaction with treatment was assessed using the Treatment Satisfaction Questionnaire for Medication (TSQM). The TSQM 1.4 is a 14-item psychometrically robust and validated instrument comprising 4 domains20: effectiveness (questions 1–3), side effects (questions 4–8), convenience (questions 9–11), and global satisfaction (questions 12–14). TSQM 1.4 domain scores range from 0 to 100, with higher scores representing higher satisfaction in that domain. The TSQM questionnaire was completed at baseline by patients who had received any treatment in the 3 months prior to inclusion in the study and by all patients at week 3.
Secondary end points were the results of the assessment of local skin responses (LSRs) and clinical response. To ensure uniform reporting, LSRs were assessed quantitatively using a grading scale and photographic guide images, a method similar to that used in published studies with IM.14,15,21 The following responses were assessed on a scale that ranged from 0 to 4 (with higher numbers indicating more severe reactions): swelling, vesiculation or pustulation, erosion or ulceration, flaking or scaling, crusting, and erythema. The composite LSR score was defined as the sum of the 6 individual scores (maximum composite score, 24).21 LSR were assessed at visits 2 and 3.
Clinical response was assessed at visit 3 and at the end of the study (visit 4). Response was assessed by the investigator using a 6-point scale, as follows: worsening or no change compared to baseline (0); improvement of less than 25% (1); improvement of 25–49% (2); improvement of 50–75% (3); improvement greater than 75% (4), and complete response, that is, complete clinical disappearance of the lesion (5). The degree of infiltration, erythema, and flake injury was also considered.
The data were analyzed using SPSS version 21.0 (IBM Corporation). The qualitative variables were described using absolute frequencies and percentages. Standard descriptive statistics, such as median, interquartile range (Q1–Q3), minimum, and maximum, were calculated. Non-parametric statistics for paired data were used for comparisons. Missing data were not imputed.
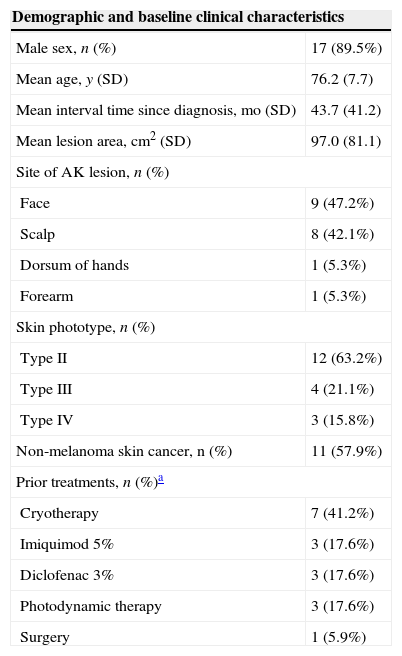
ResultsThe study enrolled 19 subjects (17 men and 2 women). The mean age was 76 years and the mean interval since diagnosis was 44 months. Almost half of the participants had facial lesions and 58% had a clinical history of NMSC. The 13 patients who had AK lesions which had proven refractory to earlier treatments completed the TSQM questionnaire at baseline and visit 3; the other 6 patients were treatment-naive and completed the TSQM questionnaire only at visit 3 (Table 1). The demographic details and baseline clinical characteristics of the patients are shown in Table 2.
Demographic and clinical characteristics of the study population.
| Demographic and baseline clinical characteristics | |
|---|---|
| Male sex, n (%) | 17 (89.5%) |
| Mean age, y (SD) | 76.2 (7.7) |
| Mean interval time since diagnosis, mo (SD) | 43.7 (41.2) |
| Mean lesion area, cm2 (SD) | 97.0 (81.1) |
| Site of AK lesion, n (%) | |
| Face | 9 (47.2%) |
| Scalp | 8 (42.1%) |
| Dorsum of hands | 1 (5.3%) |
| Forearm | 1 (5.3%) |
| Skin phototype, n (%) | |
| Type II | 12 (63.2%) |
| Type III | 4 (21.1%) |
| Type IV | 3 (15.8%) |
| Non-melanoma skin cancer, n (%) | 11 (57.9%) |
| Prior treatments, n (%)a | |
| Cryotherapy | 7 (41.2%) |
| Imiquimod 5% | 3 (17.6%) |
| Diclofenac 3% | 3 (17.6%) |
| Photodynamic therapy | 3 (17.6%) |
| Surgery | 1 (5.9%) |
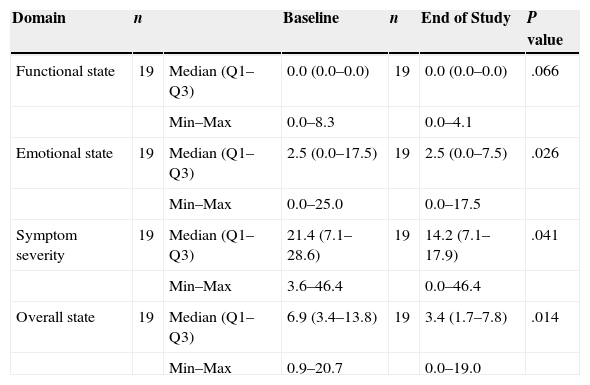
The change in QoL was measured by comparing the results of the Skindex-29 questionnaire obtained at baseline and at the end of the study (Table 3). After IM treatment, significant improvement was observed in the subscales relating to the patients’ emotional state (P=.026) and to symptom severity (P=.041), as well as in the overall score (P=.014). A trend toward improvement in functional state was also observed (P=.066).
Skindex-29 quality-of-life questionnaire.
| Domain | n | Baseline | n | End of Study | P value | |
|---|---|---|---|---|---|---|
| Functional state | 19 | Median (Q1–Q3) | 0.0 (0.0–0.0) | 19 | 0.0 (0.0–0.0) | .066 |
| Min–Max | 0.0–8.3 | 0.0–4.1 | ||||
| Emotional state | 19 | Median (Q1–Q3) | 2.5 (0.0–17.5) | 19 | 2.5 (0.0–7.5) | .026 |
| Min–Max | 0.0–25.0 | 0.0–17.5 | ||||
| Symptom severity | 19 | Median (Q1–Q3) | 21.4 (7.1–28.6) | 19 | 14.2 (7.1–17.9) | .041 |
| Min–Max | 3.6–46.4 | 0.0–46.4 | ||||
| Overall state | 19 | Median (Q1–Q3) | 6.9 (3.4–13.8) | 19 | 3.4 (1.7–7.8) | .014 |
| Min–Max | 0.9–20.7 | 0.0–19.0 |
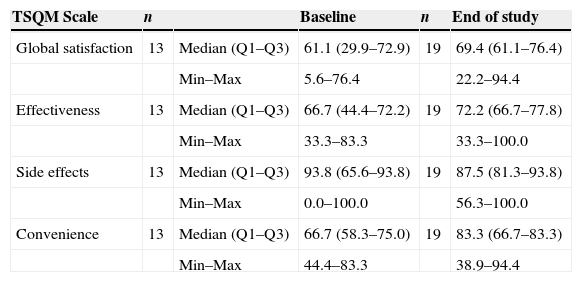
The TSQM 1.4 results from visit 3 were compared with those obtained at the baseline visit in patients who had received prior AK treatments (Table 4). In this group, median scores for effectiveness, convenience, and global satisfaction were higher at week 3—after IM treatment—than at baseline (assessment of prior treatment). The greatest differences between the assessment of prior treatment and IM were observed in convenience (median scores of 66.7 vs. 83.3, respectively) and global satisfaction (median scores of 61.1 vs. 69.4, respectively). The differences observed were not statistically significant in any of the 4 domains, but a trend toward a better global satisfaction score with IM as compared to previous treatments was observed (P=.059).
TSQM 1.4 scores by domain at baseline and the end of the study.a
| TSQM Scale | n | Baseline | n | End of study | |
|---|---|---|---|---|---|
| Global satisfaction | 13 | Median (Q1–Q3) | 61.1 (29.9–72.9) | 19 | 69.4 (61.1–76.4) |
| Min–Max | 5.6–76.4 | 22.2–94.4 | |||
| Effectiveness | 13 | Median (Q1–Q3) | 66.7 (44.4–72.2) | 19 | 72.2 (66.7–77.8) |
| Min–Max | 33.3–83.3 | 33.3–100.0 | |||
| Side effects | 13 | Median (Q1–Q3) | 93.8 (65.6–93.8) | 19 | 87.5 (81.3–93.8) |
| Min–Max | 0.0–100.0 | 56.3–100.0 | |||
| Convenience | 13 | Median (Q1–Q3) | 66.7 (58.3–75.0) | 19 | 83.3 (66.7–83.3) |
| Min–Max | 44.4–83.3 | 38.9–94.4 |
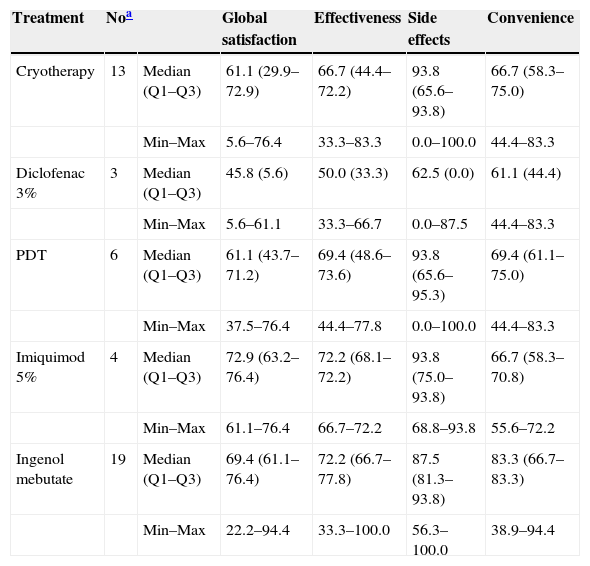
The TSQM 1.4 results for IM were also compared to those corresponding to each previous treatment (Table 5). Imiquimod 5% and IM had the highest median scores for effectiveness, 72.2 in both cases. Imiquimod 5%, cryotherapy, and photodynamic therapy had the highest scores for satisfaction with respect to side effects (a median of 93.8, in all 3 cases). IM had the highest score related to satisfaction with the convenience of treatment (median of 83.3). Finally, the highest score for global satisfaction was observed in patients treated with imiquimod 5% and IM, with median scores of 72.9 and 69.4, respectively (Table 5).
TSQM 1.4 results for the study treatment and each previous AK treatment.
| Treatment | Noa | Global satisfaction | Effectiveness | Side effects | Convenience | |
|---|---|---|---|---|---|---|
| Cryotherapy | 13 | Median (Q1–Q3) | 61.1 (29.9–72.9) | 66.7 (44.4–72.2) | 93.8 (65.6–93.8) | 66.7 (58.3–75.0) |
| Min–Max | 5.6–76.4 | 33.3–83.3 | 0.0–100.0 | 44.4–83.3 | ||
| Diclofenac 3% | 3 | Median (Q1–Q3) | 45.8 (5.6) | 50.0 (33.3) | 62.5 (0.0) | 61.1 (44.4) |
| Min–Max | 5.6–61.1 | 33.3–66.7 | 0.0–87.5 | 44.4–83.3 | ||
| PDT | 6 | Median (Q1–Q3) | 61.1 (43.7–71.2) | 69.4 (48.6–73.6) | 93.8 (65.6–95.3) | 69.4 (61.1–75.0) |
| Min–Max | 37.5–76.4 | 44.4–77.8 | 0.0–100.0 | 44.4–83.3 | ||
| Imiquimod 5% | 4 | Median (Q1–Q3) | 72.9 (63.2–76.4) | 72.2 (68.1–72.2) | 93.8 (75.0–93.8) | 66.7 (58.3–70.8) |
| Min–Max | 61.1–76.4 | 66.7–72.2 | 68.8–93.8 | 55.6–72.2 | ||
| Ingenol mebutate | 19 | Median (Q1–Q3) | 69.4 (61.1–76.4) | 72.2 (66.7–77.8) | 87.5 (81.3–93.8) | 83.3 (66.7–83.3) |
| Min–Max | 22.2–94.4 | 33.3–100.0 | 56.3–100.0 | 38.9–94.4 |
Abbreviation: PDT; photodynamic therapy.
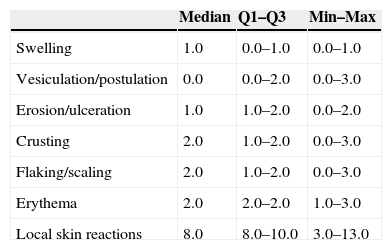
IM was applied for 2 consecutive days on lesions on the trunk or extremities and for 3 consecutive days on facial and scalp lesions. Side effects were assessed on day 3 or 4, that is, the day after the treatment was completed (Table 6). The LSRs with the highest median score were erythema, crusting, and flaking or scaling (2.0, in all 3 cases). The median composite LSR score was 8.0. All LSRs had resolved by week 3.
Side effects of treatment at visit 2 (Day 3 or 4).a
| Median | Q1–Q3 | Min–Max | |
|---|---|---|---|
| Swelling | 1.0 | 0.0–1.0 | 0.0–1.0 |
| Vesiculation/postulation | 0.0 | 0.0–2.0 | 0.0–3.0 |
| Erosion/ulceration | 1.0 | 1.0–2.0 | 0.0–2.0 |
| Crusting | 2.0 | 1.0–2.0 | 0.0–3.0 |
| Flaking/scaling | 2.0 | 1.0–2.0 | 0.0–3.0 |
| Erythema | 2.0 | 2.0–2.0 | 1.0–3.0 |
| Local skin reactions | 8.0 | 8.0–10.0 | 3.0–13.0 |
Average lesion size was 97.0cm2 before treatment and 42.2cm2 after treatment, an average reduction of 54.8cm2 (95% CI, 25.1–84.4; P=.001).
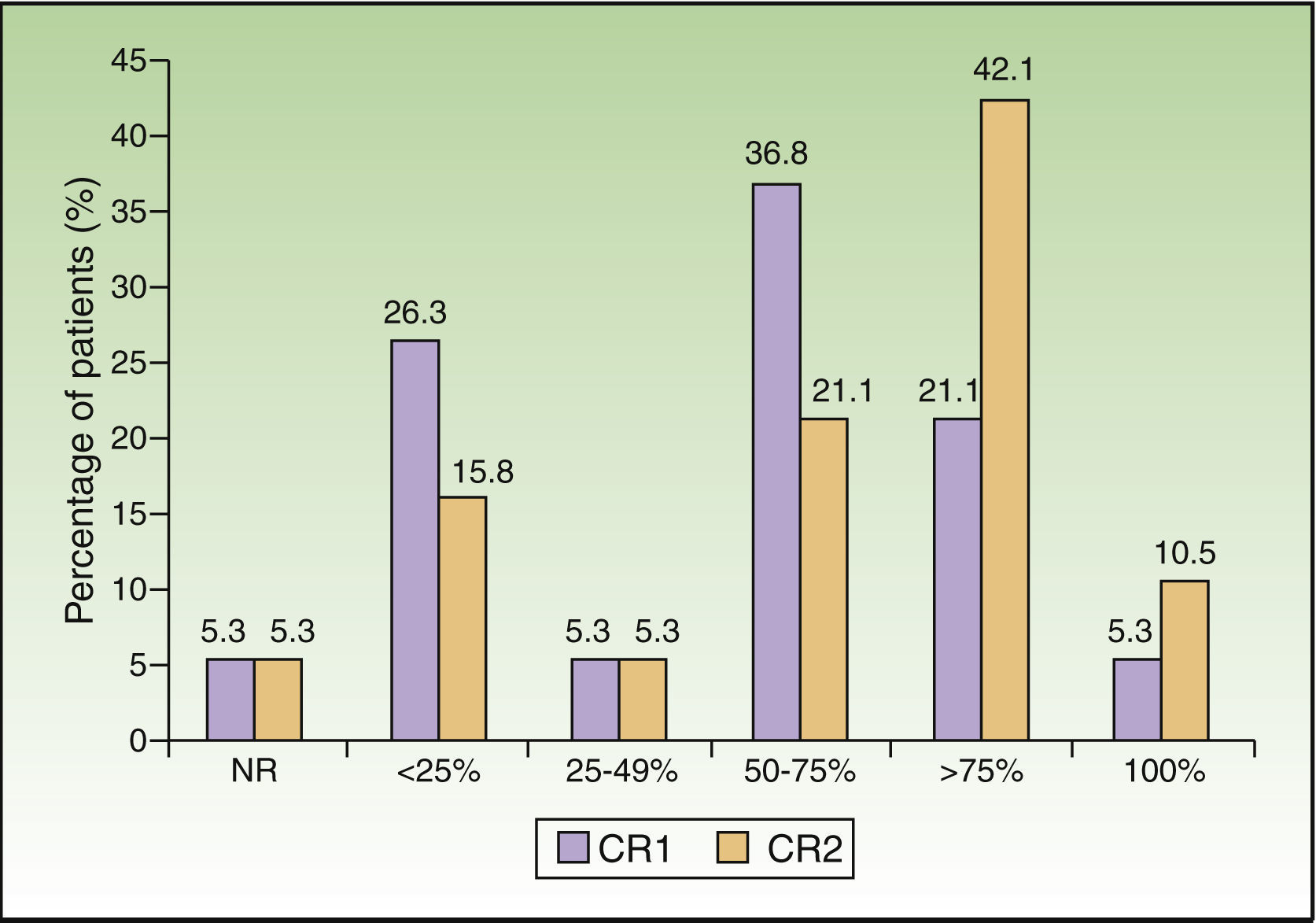
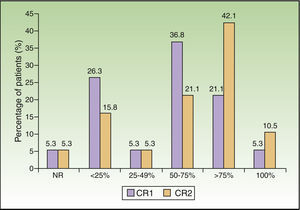
The percentage of patients with an improvement in clinical response of at least 75% was 26.4% at week 3 and had doubled by month 3 (52.6%). The difference in clinical response between these 2 periods was statistically significant (P=.008) (Fig. 1).
DiscussionApart from their association with malignancy, AK lesions also have a significant impact on patients’ QoL.11,22 The authors of a study of QoL in patients with NMSC reported that 43% of patients with moderate QoL impairment and 33% with severe QoL impairment had AK.11 Moreover, the long duration of treatment and the presence of side effects can also affect QoL.12 Three weeks after completing treatment with IM, the patients in the present study reported improvement in all 3 subscales of the Skindex-29 questionnaire (emotional state, symptom severity, and functional state) and in the overall score, as compared with the assessment of prior treatments made at baseline; these differences were statistically significant in the subscales relating to symptom severity and the patient's emotional state, and in the overall score. A pooled analysis of data from clinical trials in patients treated with IM in the US and Australia reported results in all Skindex subscales similar to those observed in the present study.15
An earlier study focusing on the effects of photodynamic therapy on QoL in patients with AK highlighted the impact of side effects on QoL.12 In that study the Dermatology Life Quality Index (DLQI) score rose from 1.6 before treatment to 7.3 after completion of photodynamic therapy as a result of side effects. However, as the intensity of side effects decreased after treatment, the DLQI decreased to 4.4 (at 2 weeks) and 0.1 (at 4 weeks). A recently published QoL questionnaire specifically designed for AK13 has just been validated in Spanish and could be used in future studies.23
In the case of side effects, our results showed that on the day after completion of treatment the median LSR score was 2 or less in all the LSR assessed (flaking or scaling, swelling, vesiculation or pustulation, erosion or ulceration, erythema, and crusting), and the composite LSR score was 8.0, which is considered to be low to intermediate intensity (≤12). All LSR had resolved by week 3, an important consideration because with other topical treatments, such as imiquimod, side effects tend to peak during the first 2 weeks of treatment and resolve 3–4 weeks after cessation of treatment at week 4.24 The results with respect to side effects in the present study are in line with those reported in clinical trials in the US and Australia, which showed that most patients treated with IM had a maximum composite LSR score of 12 or less.15 Moreover, similar results were reported previously in patients with AK treated with IM, with a mean maximum composite LSR of 9.1 for patients with AK on the face or scalp and 6.8 for patients with AK on the trunk or extremities.14 Other authors have reported that the composite score peaked on day 3 or 4 (the day after completion of treatment) and that side effects had begun to resolve by day 15, giving rise to a score lower than or similar to the baseline score at the end of follow-up (day 57).14,15 The maximum composite LSR in our study was slightly lower than that reported by Lebwohl et al.14 This may be because our patients distributed the dose over a larger area than that indicated in the Summary of Product Characteristics (SmPC), giving rise to a decrease in the concentration of IM and consequently of side effects. In addition, prior cycles of treatment, even if these were not enough to clear the lesions, may improve subclinical field cancerization, thereby decreasing the side effects associated with subsequent treatment.25 The short treatment period required with IM has been shown to be associated with relatively rapid resolution of LSR and very high adherence to therapy (>98%).14,15 A similar scale has recently been validated to measure the severity of topical 5-fluorouracil toxicity.26 Of particular note is the benefit associated with improved patient adherence due to the short duration of the IM treatment cycle, especially considering the high level of non-adherence observed with other topical AK treatments (around 90%), which has been attributed to the long duration of treatment cycles.10 A global study of physician perceptions of treatment for AK showed that 70% of physicians had specific concerns about the negative effect on adherence of long treatment durations, long-lasting LSRs, and low patient tolerance of the adverse effects of treatment.27 Since LSRs are treatment related, their duration tends to correlate with the duration of the therapy. This means that shorter therapies would be associated with a corresponding shortening in the duration of treatment-emergent LSRs.
Furthermore, treatment-related factors, such as duration of treatment, side effects, frequency of use, ease of use, and efficacy, can also affect patient satisfaction and consequently adherence to treatment.28
In a recent qualitative study, the main complaints of patients concerning the topical treatment of AK were the local inflammatory reaction, pain (especially with photodynamic therapy) as well as the lack of information about using the treatment at home, about alternative treatment options, and about the cost of the treatment.29 Nonetheless, patients in that study displayed a willingness to be compliant with treatment despite the adverse effects.29
The authors of clinical trials have reported significantly greater satisfaction with effectiveness, side effects, and the overall results of treatment (global satisfaction) at day 57 in patients treated with IM than in those treated with the vehicle alone.15 Our results showed patients to be more satisfied with IM than with their previous AK treatments in all domains (effectiveness, side effects, and convenience). A trend toward a higher global satisfaction with IM was observed, indicating a higher level of overall satisfaction in patients treated with IM compared to all the other prior treatments received.
Clinical trials evaluating topical treatments for AK have certain limitations that hinder the application of the results to clinical practice. In particular, they often exclude patients who have recently received other treatments and limit the number of lesional fields (normally to 1 or 2) or the global extent of the lesions to be treated (usually to 25cm2). This is paradoxical, since the aim of most of these studies is to assess the effect of these treatments on the cancerization field, which is usually larger than the treatment area approved in the SmPC (25m2). In this respect, it should be noted that the approach used in the present study was in line with clinical practice and that no restrictions were imposed regarding the size of the area of application or the number of sites. Consequently, the treatment areas in this study were larger than those recommended by the SmPC, with a mean lesion area of 97cm2, which was reduced to 42cm2 within 3 months of completion of treatment, a result that could be considered to indicate an effective action on the field cancerization. The treatment in this study of larger areas of skin does not appear to have had any impact on the results of Skindex or TSQM, which were similar to those reported in previously published in clinical trials.15 Complete clearance has been reported in 61.6% of lesions on face and scalp at week 8 following treatment with IM,16 but the relative frequency of lesions in different sites may affect overall efficiency. This effect is especially evident when using IM, which has 79% complete response for chest lesions, 35% for arm lesions, and only 23% on the scalp and 19% on the dorsum of the hands, and intermediate response rates in other sites.14 In our study, 42% of patients had AK on the scalp, which may have affected overall efficacy. Complete lesion clearance in our study was achieved in only 10% of patients at month 3. We considered the measurement of the affected area to be a more appropriate outcome for assessing improvement in the cancerization field. However, this choice makes it difficult to compare our results with those of studies in which the clinical lesion count was used as a measure of improvement.14–16 The lesion count appears to provide more reproducible results than area measurement.30 However, neither method is perfect for assessing subclinical field cancerization.30
One of the limitations of this study is the small sample size. Our findings could be seen as preliminary results that should be explored in a bigger sample; however, they are consistent with those obtained in clinical trials with IM.29
In conclusion, this pilot study indicates that QoL in patients with AK improves after treatment with IM. Improvement on a subjective patient scale is also observed. The presence of side effects affected neither QoL nor patients’ satisfaction with treatment probably because of their short duration.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
FundingThe writing of this manuscript was financially supported by Leo Pharma.
Conflict of interestNone declared.
We would like to thank Montserrat Sabaté from the Medical Writing Department at TFS Trial Form Support for her valuable writing assistance.