A wide range of treatments is now available for nonmelanoma skin cancer, including 5-fluorouracil, ingenol mebutate, imiquimod, diclofenac, photodynamic therapy, methotrexate, cetuximab, vismodegib, and radiotherapy. All are associated with high clinical and histologic response rates. However, some tumors do not respond due to resistance, which may be primary or acquired. Study of the resistance processes is a broad area of research that aims to increase our understanding of the nature of each tumor and the biologic features that make it resistant, as well as to facilitate the design of new therapies directed against these tumors. In this second article, having covered the topical treatments of nonmelanoma skin cancer, we review resistance to other nonsurgical treatments, such as monoclonal antibodies against basal and squamous cell carcinomas, intralesional chemotherapy, photodynamic therapy, and radiotherapy.
En la actualidad existe una amplia variedad de tratamientos para el cáncer cutáneo no melanoma, como son 5-fluoracilo, mebutato de ingenol, imiquimod, diclofenaco, terapia fotodinámica, metotrexato, cetuximab, vismodegib, radioterapia, todos ellos con altas tasas de respuesta clínica e histológica. Sin embargo, algunos tumores no responden al tratamiento, debido a la aparición de resistencias, tanto primarias como adquiridas. El estudio de los procesos de resistencia es un campo extenso de investigación que conlleva a ampliar los conocimientos de la naturaleza de cada tumor, las características biológicas que lo hacen resistente y el diseño de nuevas terapias dirigidas contra los mismos. En este segundo trabajo se revisan las resistencias descritas a otros tratamientos no quirúrgicos frente al cáncer cutáneo no melanoma, diferentes a los tratamientos tópicos, como son diferentes anticuerpos monoclonales frente a CBC y CEC, la quimioterapia intralesional, la terapia fotodinámica y la radioterapia.
Skin cancer is the most common malignancy in the white population. The most common types include basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), followed at a distance by melanoma. According to statistical analyses, the incidence of these 3 skin cancers has increased worldwide in the last 20 years, although mortality rates have remained stable. An incidence of 2.53 cases per 1000 population per year has been reported for BCC.1 However, according to a recent study, the annual incidence of nonmelanoma skin cancer (NMSC) is underestimated, at 113 cases per 100000 population for BCC and 38 cases per 100000 population for SCC.2
Surgery is the most common treatment for NMSC, and in most cases it is curative. Nonetheless, 15.7% of NMSCs have positive margins3 and complete excision is associated with a recurrence rate of 4.2%, although lower rates have been described for excision with wide margins, albeit with large surgical defects.4
As mentioned in the first part of this review, a wide range of alternatives to surgery have emerged in recent years in the form of topical treatments and, even more recently, targeted therapies such as vismodegib and cetuximab, without forgetting of course other treatments that already existed, such as photodynamic therapy (PDT), radiotherapy, and even intralesional chemotherapy. One of the main reasons for treatment failure in cancer is resistance. The study of clinical, histologic, and biologic differences in responses to different treatments enhances our understanding of the mechanisms involved in resistance in NMSC and will help to achieve improved efficacy and efficiency by identifying patients who are more likely to respond favorably to a given treatment.
In this article, we discuss the main mechanisms that have been investigated in treatment resistance in NMSC. The mechanisms described for PDT, vismodegib, and cetuximab are summarized in Table 1.
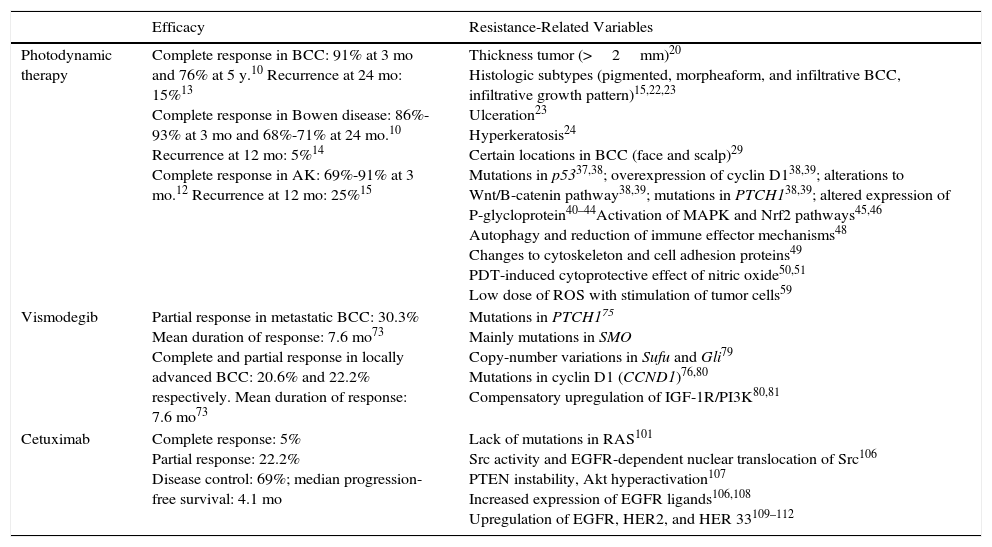
Summary of Efficacy Rates and Resistance Mechanisms Described for Photodynamic Therapy, Vismodegib, and Cetuximab.
| Efficacy | Resistance-Related Variables | |
|---|---|---|
| Photodynamic therapy | Complete response in BCC: 91% at 3 mo and 76% at 5 y.10 Recurrence at 24 mo: 15%13 Complete response in Bowen disease: 86%-93% at 3 mo and 68%-71% at 24 mo.10 Recurrence at 12 mo: 5%14 Complete response in AK: 69%-91% at 3 mo.12 Recurrence at 12 mo: 25%15 | Thickness tumor (>2mm)20 Histologic subtypes (pigmented, morpheaform, and infiltrative BCC, infiltrative growth pattern)15,22,23 Ulceration23 Hyperkeratosis24 Certain locations in BCC (face and scalp)29 Mutations in p5337,38; overexpression of cyclin D138,39; alterations to Wnt/B-catenin pathway38,39; mutations in PTCH138,39; altered expression of P-glycloprotein40–44Activation of MAPK and Nrf2 pathways45,46 Autophagy and reduction of immune effector mechanisms48 Changes to cytoskeleton and cell adhesion proteins49 PDT-induced cytoprotective effect of nitric oxide50,51 Low dose of ROS with stimulation of tumor cells59 |
| Vismodegib | Partial response in metastatic BCC: 30.3% Mean duration of response: 7.6 mo73 Complete and partial response in locally advanced BCC: 20.6% and 22.2% respectively. Mean duration of response: 7.6 mo73 | Mutations in PTCH175 Mainly mutations in SMO Copy-number variations in Sufu and Gli79 Mutations in cyclin D1 (CCND1)76,80 Compensatory upregulation of IGF-1R/PI3K80,81 |
| Cetuximab | Complete response: 5% Partial response: 22.2% Disease control: 69%; median progression-free survival: 4.1 mo | Lack of mutations in RAS101 Src activity and EGFR-dependent nuclear translocation of Src106 PTEN instability, Akt hyperactivation107 Increased expression of EGFR ligands106,108 Upregulation of EGFR, HER2, and HER 33109–112 |
Abbreviations: AK, actinic keratosis; BCC, basal cell carcinoma; EGFR, epidermal growth factor receptor; PDT, photodynamic therapy; ROS, reactive oxygen species.
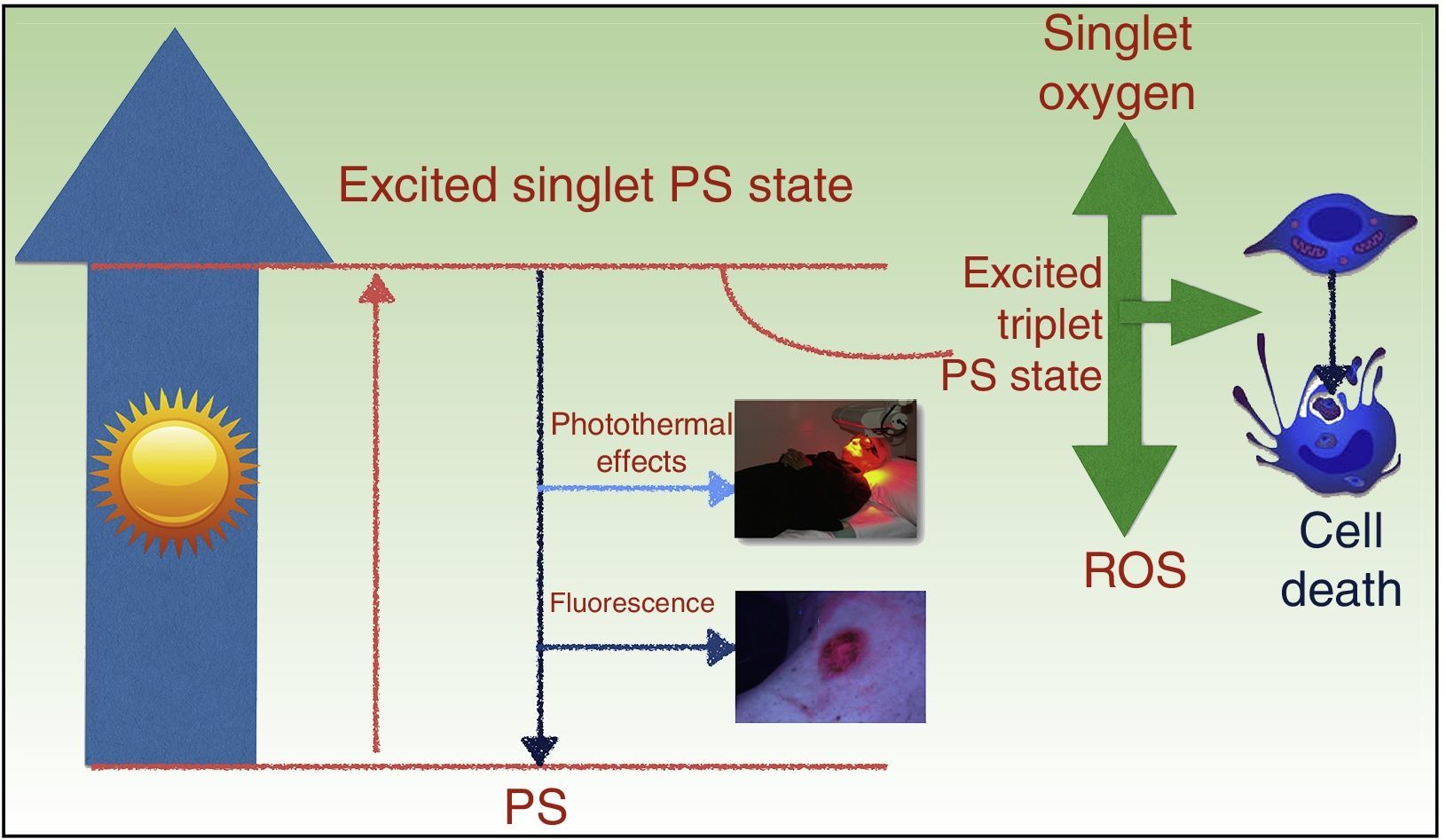
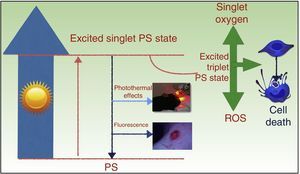
PDT takes advantage of the photosensitizing properties of certain compounds that are activated by light and that, in the presence of molecular oxygen (O2), generate reactive oxygen species (ROS) that induce cell death (Fig. 1). The treatment has 3 basic components: a photosensitizing agent, light, and 02.5,6
Mechanism of action of photodynamic therapy. The absorption of light by a photosensitizer (PS) promotes an electron to a higher-energy orbit (excited single PS state), and the spin angular momentum of this electron is then reversed, causing an excited triplet PS state. The longer lifetime of the triplet state favors the generation of free radicals and/or singlet oxygen, which damage key cell structures and ultimately cause cell death. ROS indicates reactive oxygen species.
The antitumor properties of PDT are the result of direct cytotoxic effects on the tumor cells and indirect effects on the tumor stroma. Treatment also produces inflammation and elicits an immune response against the tumor, which is essential to achieve long-term control.5,7,8
The main photosensitizing agents approved for use in skin cancer in Europe are 5-aminolevulinic acid (ALA, Ameluz) and its methylated derivative MAL (Metvix). Both are precursors of the endogenous photosensitizer protoporphyrin IX (PpIX), which is a metabolite in the heme pathway. This pathway is subject to negative feedback control, and therefore exogenously administered ALA or MAL results in the accumulation of PpIX in tumor cells. In Spain, MAL is approved for the treatment of actinic keratosis (AK), Bowen disease, superficial BCCs, and nodular BCCs with a depth of less than 2mm, while ALA is approved only for the treatment of AK.5,9
The advantages of PDT include high levels of complete response, with rates of 91% and 76% reported for BCC (at 3 months and 5 years, respectively) and of 86% to 93% and 68% to 71% for Bowen disease (at 3 and 24 months, respectively).10 In a study of AK, a single PDT session with ALA (Levulan and Kerastick) produced respective cure rates of 76% and 72% at 1 and 2 months posttreatment. These rates increased to 86% with the administration of a second session.11 In another study, PDT with MAL (Metvix) achieved a lesion response rate of between 69% and 91% at 3 months.12 Low recurrence has been reported for PDT with MAL, with reports of a 15% clearance rate for BCC at 24 months13 and a 5% and 25% rate for Bowen disease and AK, respectively, at 12 months.14,15
Other advantages of PDT are that it can be combined with other treatments16 and repeated as often as necessary, not to mention of course that it is associated with excellent cosmetic results and high satisfaction rates among patients.17 It does, however, have some disadvantages, such as pain during illumination, the limited penetration capacity of both the photosensitizing agent and the light, and the risk of resistance, with occasional reports of refractory tumors.18,19
Several clinical factors have been linked to resistance to PDT, but tumor thickness is the most widely studied factor, particularly in BCC. McKay et al.20 reported that the likelihood of recurrence was greater in tumors thicker than 0.4mm, and treatment guidelines do not recommend PDT for nodular BCCs with a thickness of over 2mm.21
Within the histological subtypes of BCC, pigmented tumors are the most difficult to treat, as the melanin absorbs the light, leaving the deepest part of the tumor without the light it needs to trigger the photodynamic reaction, possibly giving rise to resistance.22 Morpheaform and infiltrative BCCs are also more resistant to the penetration of the photosensitizer due to the increased density of collagen and cords of cells in the connective tissue; PDT is therefore not indicated in these tumors.15 Other factors that have been investigated include ulceration, an infiltrative growth pattern23 and hyperkeratosis,24 which can be a negative predictor of response.
According to some authors, giant tumors (>4cm in the case of BCCs) can predict poor response to PDT.25,26 However, other authors have found tumor diameter to have no effect on response.27–30 In a study of Bowen disease, Calzavara-Pinton et al.31 found that tumor diameter was not a predictor of final response, but they did observe an association with recurrence.
BCCs on the face or scalp are associated with lower rates of complete response than lesions on the trunk or neck (54% vs 88% at 24 months).29 Complete response rates are also lower in the H-zone of the face, regardless of tumor size.29 Nevertheless, in AK, studies comparing PDT and cryotherapy have found that lesions on the extremities appear to be more resistant to MAL-PDT than lesions on the face or scalp.32,33
Efficacy can also be affected by nonclinical factors. It is known, for example, that tumor cells do not always respond to the oxidative stress caused by PDT. These unresponsive cells are thought to be responsible for recurrent lesions that may become more aggressive. Resistance is generally thought to be due to intrinsic factors inherent to the tumor cells or extrinsic factors in the tumor environment, which has an important role in treatment response through the establishment of gradients in factors such as signaling, O2, and metabolites.34,35
Intrinsic resistance may be due to a complex set of factors, including aspects related to the expression of genes involved in the genesis of NMSC and other aspects related to the photodynamic process itself.36 UV light–induced DNA mutations in tumor suppressor genes, such as p53, are common and have been described as early events in the carcinogenesis of 50% of BCCs and AKs and in 90% of SCCs.37,38 There have also been reports of cyclin D1 overexpression in almost 50% of cases of AK, alterations of the Wnt/β-catenin signaling pathway (involved in SCC), and mutations in the PTCH1 gene (which codes for the Hedgehog [HH] protein receptor in BCC), possibly affecting response to PDT.38,39
Another mechanism of interest is intracellular drug concentration, which is regulated by transporters such as P-glycoprotein (Pgp) responsible for transporting the drug out of the cell. While some studies have reported Pgp overexpression in epithelial cell lines such as MA104 (a clonal population derived from the kidney of an African green monkey, Cercopithecus aethiops) and MCDK 60, and in K562 chronic myeloid leukemia cells made resistant to PDT by multiple treatment cycles, others have not.40–44
The oxidative stress caused by PDT activates signaling cascades involved in survival, proliferation, and inhibition of apoptosis mediated by protein kinase B (PKB/Akt), nuclear factor k (NF-kB), mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), and cyclooxygenase-2 (COX-2), as well as genes involved in the antioxidant response pathway, such as the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) gene. COX-2, which participates in prostaglandin (PGE) synthesis and inflammatory responses45,46 is overexpressed in NMSC and is considered an early marker of actinic damage. MAPK pathways can be activated by PDT and may have a relevant role in resistance to this treatment.36
PDT also results in the activation of the Nrf2 pathway, which is the main regulator of cellular responses to oxidative stress. Nrf2 activates the transcription of genes with an AU-rich element (ARE) in their promoter region, such as glutathione S-transferase (GSH), NAD(P)H quinone dehydrogenase 1 (NQO1), hem oxygenase 1, and superoxide dismutase, among others. The corresponding enzymes would have a key role in neutralizing ROS and promoting detoxification, possibly favoring resistance to PDT.47
Some studies have also reported an association between PDT resistance and autophagy, via the reduction of immune effector mechanisms,48 and cytoskeleton and cell adhesion proteins.49
Furthermore, it has been well established that nitric oxide mediates cell survival, growth, migration, and invasion in a wide variety of tumors. PDT induces nitric oxide production in the tumor and tumor microenvironment. Low levels of nitric oxide exert a cytoprotective effect by activating NF-kB/Snail YY1, enhancing survival/antiapoptosis and inhibiting the antisurvival/proapoptotic and metastasis suppressor Raf kinase inhibitory protein (RKIP).50,51
Few publications have analyzed the mechanisms that determine poor tumor response to PDT and even fewer have analyzed these in NMSC, although it is an area of growing research interest. Numerous studies have shown resistance to PDT in preclinical studies using cell and animal models,40–43,52–54 and also in patients.19,55,56 Our group observed resistance to MAL-PDT in murine keratinocyte cultures (Pam-212) with activation of the PI3K/Akt pathway.57 Activation of the PI3K/Akt58 and MAPK/ERK pathways has been identified in PDT-resistant cells isolated from the human SCC cell line SCC-13 and points to a possible relationship with the more aggressive nature of invasive SCCs in patients who have undergone PDT.19
Finally, PDT with low doses of MAL has been found to stimulate ROS-mediated growth of cultured cells,59 adding some strength to the hypothesis that low doses of ROS could stimulate the growth of tumor cells in areas that receive an insufficient PDT dose.
Key point: Diverse resistance mechanisms have been described for PDT. These include clinical factors, such as tumor thickness, size, location; histologic subtype; and molecular factors such as Pgp overexpression, MAPK pathway alterations, resistance secondary to Nrf2 and activation of the transcription of genes with an ARE in their promoter regions; autophagy and reduced immune effector mechanisms; and a PDT-induced cytoprotective effect of nitric oxide that activates NF-kB/Snail YY1 and inhibits RKIP, favoring tumor survival.
Resistance to MethotrexateIntralesional methotrexate has been shown to be an effective adjuvant to surgery in the treatment of keratoacanthoma60,61 and SCC.62
Methotrexate is a folic acid analog that binds to dihydrofolate reductase, inhibiting DNA synthesis and ultimately causing cell death. Keratoacanthoma is a fast-growing tumor that frequently reaches a considerable size and can leave considerable functional and cosmetic defects when removed surgically. Annest et al.63 reported a cure rate of 92% in a study of 38 patients with keratoacanthomas treated with a mean of 2.1 injections of methotrexate over an average of 18 days.
Growth rate can determine sensitivity or resistance to methotrexate in keratoacanthoma. Sensitivity, for instance, is high in the early stages of growth but is of little consequence at later stages, when growth is slower.64
Although there are no standardized protocols for the use of methotrexate in the treatment of keratoacanthoma, drug concentration (generally 12.5-25mg/mL) and/or frequency of injections (1-4 weekly) appear to be related to tumor response.65
Key point: The growth rate of keratoacanthomas appears to influence response to treatment, with higher resistance rates seen in slow-growing tumors.
Resistance to VismodegibVismodegib (GDC-0449) is an oral molecule that selectively inhibits the HH signaling pathway. It is the first drug available for the treatment of locally advanced and metastatic BCC.66,67
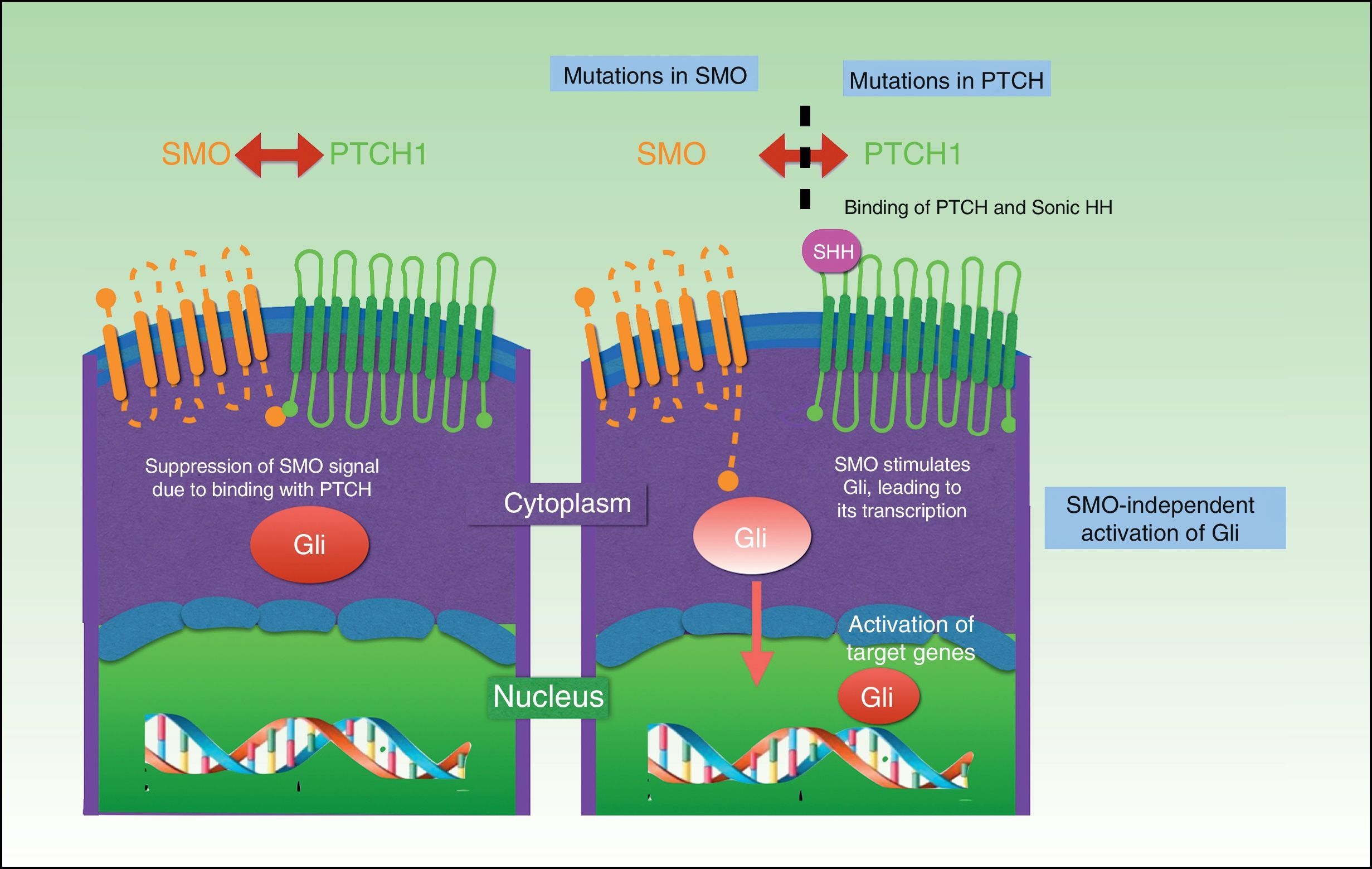
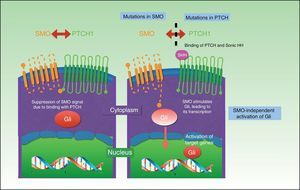
Three HH signaling molecules have been described in vertebrates: Indian HH (expressed in the intestine and chondrocytes), Desert HH (expressed in Sertoli cells), and the better-known Sonic HH, which has been linked to many processes. The HH signaling pathway is composed of 3 basic elements: HH ligands, the inhibitory receptor Patched (PTCH), and the signaling receptor Smoothened (SMO).68
The PTCH protein is the Sonic HH receptor and negatively regulates this pathway. The SMO protein, by contrast, positively regulates the pathway and is permanently activated in the absence of PTCH. SMO also activates Gli transcription factors, which penetrate the nucleus, activating the transcription of genes involved in cell growth, which, in turn control PTCH and Gli via a negative feedback mechanism. The result is activation of the pathway, with proliferation, apoptosis, and epidermal differentiation (Fig. 2). Proteolyzed Gli factors act as transcription inhibitors and their proteolysis depends on binding to microtubules and to suppressor of fused (Sufu) proteins that, when bound to Gli, prevent the activation of the target genes in the HH pathway.69–71
UV radiation–induced mutations can be found in the HH pathway in sporadic BCC; 80% are caused by inactivation of PTCH1, 10% by SMO gain-of-function mutations, and just 1% by Sufu mutations.68
Vismodegib binds to and specifically inactivates SMO, preventing the activation of Gli, and thereby halting cell proliferation and tumor growth. In a phase I clinical trial, Von Hoff et al.72 observed respective response rates of 60% and 50% for locally advanced and metastatic BCCs treated with vismodegib. Phase II studies have shown that a daily dose of vismodegib 150mg produces a partial response rate of 30.3% in metastatic BCC and 22.2% in locally advanced BCC and a complete response rate of 20.6% in locally advanced BCC.73
Resistance to treatment, however, has also been reported since the approval of vismodegib. The first case corresponded to a patient with medulloblastoma due to a PTCH1 mutation (PTCH1-W844C) and mutations in other genes involved in the HH pathway, leading to rapid disease progression. Since then, there have been reports of isolated, mostly heterozygous, SMO mutations.74–76
Brinkhuizen et al77 investigated acquired resistance to vismodegib, i.e., resistance related to mutations present in the resistant tumor but not in the primary, untreated, tumor. They found 2 SMO mutations: c.842G[T (p.Trp281Leu) in exon 4 and c.961G[A(p.Val321Met) in exon 5.
Pricl et al.78 studied primary and acquired resistance to vismodegib in 2 patients. The first patient had metastatic BCC in which disease progression (primary resistance) was confirmed by computed tomography 2 months after treatment with vismodegib 150mg/d. The second patient had an unresectable large (12cm) ulcerated locally advanced BCC in the suprascapular region, also treated with vismodegib 150mg/d. Although the treatment resulted in a complete clinical response at 5 months, the tumor returned 6 months later (acquired resistance). The authors detected a new mutation, SMO G497W, in the first case, and in the second case, they found a SMO D473Y mutation only in the biopsy sample of the pretreatment tumor. The first mutation gave rise to a conformational rearrangement of the protein that resulted in a partial obstruction of the drug entry site, while the second mutation induced a direct effect on binding site geometry, giving rise to compete disruption of hydrogen bond formation. The mutations may therefore reflect different mechanisms.
In a recent whole-exome sequencing study of BCC, Sharpe et al.79 observed intratumoral heterogeneity, without mutations in the pretreatment biopsies, providing evidence that the mutations were all acquired and led to hyperactivation of the HH pathway. These mutations affected 2 regions of the SMO gene: the drug binding pocket and a distal location, suggesting possible cross-resistance. Copy number variations were also observed for Sufi and Gli2, but to a lesser extent.
Other mechanisms of resistance observed include mutations in the target HH gene cyclin D1 (CCND1)76,80 and compensatory upregulation of IGF-1R/PI3K as a possible pathway involved in the development of resistance to SMO inhibitors. This mechanism has been observed in LDE-225-resistant tumors.80,81
Current research efforts are focused on new therapeutic targets and the development of second-generation inhibitors that remain active in the presence of mutations. Among the strategies being contemplated is inhibition of the HH pathway through itraconazole or arsenic oxide, which are both HH signaling pathway antagonists with a different mechanism of action to vismodegib.82,83
An alternative approach might consist of blocking other pathways that interact with the HH signaling pathway in BCC and give rise to SMO-independent activation of Gli1, such as EGFR,84 atypical protein kinase C ι/λ,85 protein kinase A,86 and the RAS/MAPK and PI3K pathways.70,81,87
Despite an initial favorable response, patients treated with SMO inhibitors develop resistance due to compensatory mechanisms generated over time. Furthermore, SMO inhibitors are not efficient in tumors where HH hyperactivation is due to mutations of components along the distal SMO pathway, or in the case of noncanonical activation, explaining why SMO-independent Gli transcription factors are activated. For all these reasons, intensive research efforts have been focused on HH inhibitors that act downstream of SMO,88 such as GANT61, which blocks Gli function,89 and on a new class of drugs targeting extra C-terminal (BET) bromodomain proteins and the bromodomain proteins BRD2-4 and BRDT, which recognize acetylated lysine residues such as histone N-terminal lysine residues, giving rise to a protein-histone association and subsequent chromatin remodeling. BRD4 regulates Gli transcription by acting on Gli1 and Gli2 promoters, evading all SMO resistance mechanisms described to date, including SMO and Sufu mutations and Gli2 amplifications.90,91
Key point: Resistance to vismodegib is due to somatic mutations in PTCH and in SMO in particular, to mutations located distally to this transmembrane receptor, and to SMO-independent Gli activation and compensatory upregulation of IGF-1R/PI3K.
Resistance to EGF Receptor Antibodies (Cetuximab)The relatively recent approval of cetuximab, a monoclonal antibody that inhibits EGF receptor (EGFR) activity, has opened up new horizons for the systemic treatment of patients with unresectable or metastatic SCC. EGFR belongs to the ErbB receptor family, which has 4 members: ErbB1 (EGFR), ErbB2 (neu), ErbB3, and ErbB4.92
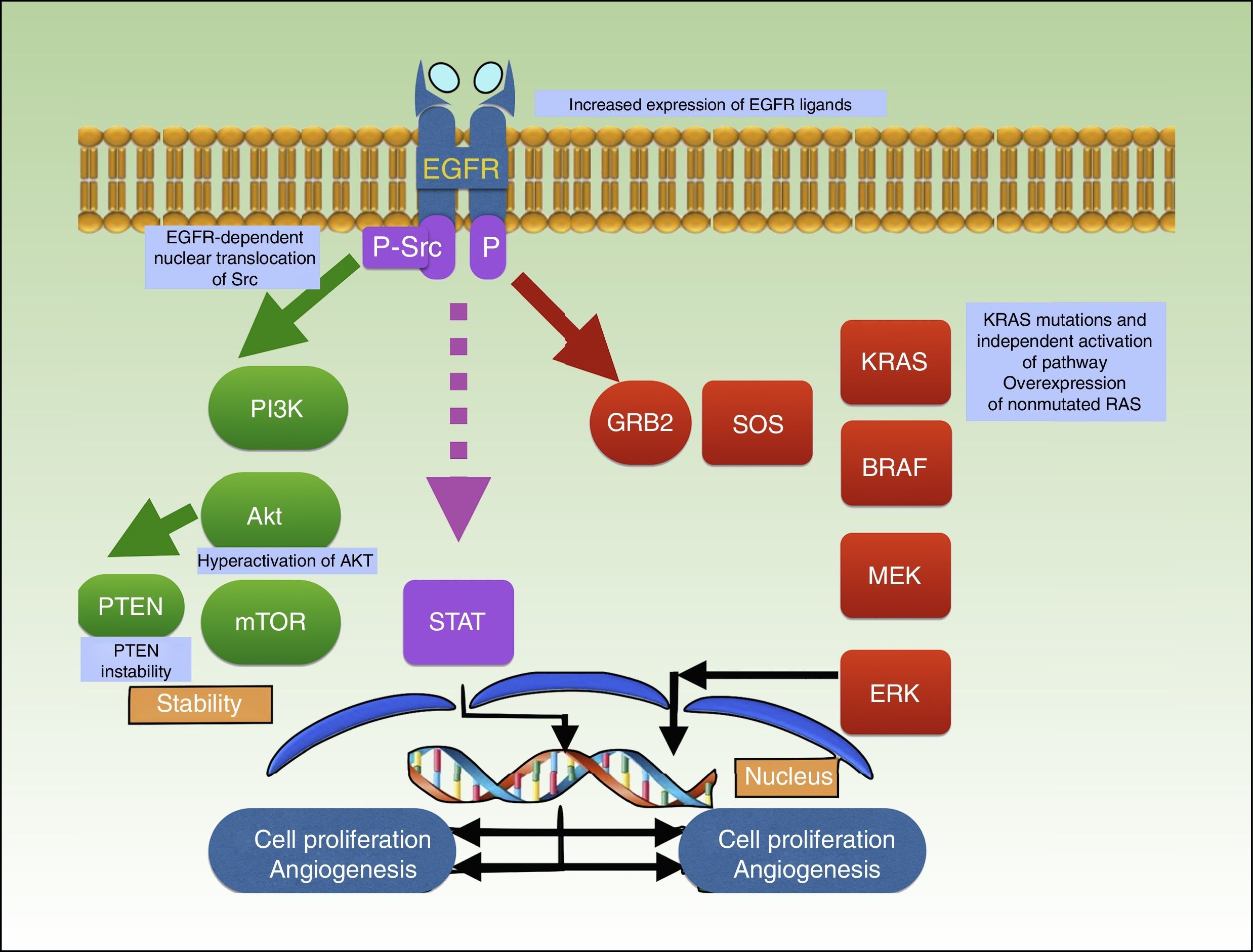
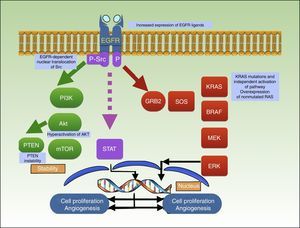
Binding of a ligand to the extracellular domain of the receptor activates several intracellular signaling pathways, including the PI3K/Akt pathway, the signal transducer and activator of transcription (STAT) pathway, and the RAS-MAPK pathway, which are all involved in cell growth, proliferation, and/or survival (Fig. 3).92–94
Mechanism of action of cetuximab, which blocks EGFR, preventing the activation of several intracellular signaling pathways, including PI3K/Akt, STAT, and RAS-MAPK. The graph shows the different mutations described to date in resistance to cetuximab in squamous cell carcinoma. EGFR indicates epidermal growth factor receptor; P, phosphorylation; P-Src, phosphorylated Src; STAT, signal transducer and activator of transcription.
Recognition of the important role played by EGFR in human tumor cell proliferation, survival, and progression has led to the development of therapeutic targets, representing an important advance in oncology.95–99
Expression of EGFR has been correlated with worse prognosis in SCC.100 Blockage of EGFR with cetuximab has proven to be useful in SCC of the head and neck and, also, although there are fewer studies available, in locally advanced, unresectable, and metastatic cutaneous SCC. In one phase II clinical trial of 36 patients, cetuximab produced 8 partial responses, 2 complete responses, and a disease control rate of 69% at 6 weeks, with a median progression-free survival of 4.1 months. EGFR overexpression was an inclusion criterion in the trial, which showed an association between treatment efficacy and EGFR tumor expression levels. The authors also studied other mutations and concluded that a lack of RAS mutations increased tumor sensitivity to cetuximab.101
Molecular mechanisms possibly involved in treatment resistance in colorectal cancer and SCC of the head and neck include K-RAS mutations and constitutive activation of EGFR and its distal pathway.102–105
Li et al.106 described an association between acquired resistance to cetuximab and Src activity and EGFR-dependent nuclear translocation of Src. Other resistance mechanisms reported in the literature are PTEN instability, Akt hyperactivation,107 increased expression of EGFR ligands,106,108 and positive regulation of EGFR, HER2, and HER3.109–112
Key point: Mechanisms described for resistance to cetuximab include certain K-RAS mutations, constitutive EGFR activation, overexpression of nonmutated RAS, EGFR-dependent nuclear translocation of Src, PTEN instability, Akt hyperactivation, increased expression of EGFR ligands, and positive regulation of EGFR, HER2, and HER3.
Resistance to RadiotherapyRadiotherapy uses ionizing radiation to shrink or kill tumors. The radiation can damage cells either directly or indirectly through the production of ROS; DNA is the main target. There are 2 main types of ionizing radiation: photon radiation (x-rays and gamma rays), which is the most widely used, and particle radiation (electrons and protons, among others). In the first case, the effect are exerted through the production of free radicals, while in the second case, direct DNA damage is more important. Exposure to radiation can induce cell-cycle arrest, damage repair, or apoptosis, and in some cases it can promote cell division and generate resistance.113,114
Radiotherapy is an effective first-line treatment for BCC and SCC, and it is associated with a 5-year cure rate of around 90%. It is also effective in skin cancers that have been surgically treated. It has been used for decades to treat NMSCs that have failed to respond to or were not suitable for other treatments. The adverse effects of ionizing radiation, however, are well-known and therefore the amount of radiation that can be applied is limited and recurrences may appear.115,116
Most studies of radiotherapy in BCC have reported 5-year cure rates of over 90%, with very few complications. Radiotherapy can also be used in recurrent tumors, tumors with perineural invasion, and tumors with positive margins after SCC. It is also beneficial in certain anatomic locations, such as the lower eyelid, the lip, the tip of the nose, and the ear, where it is associated with an efficacy of between 70% and 95%. Recurrence rates are around 3% to 11%, although certain histologic differentiation grades and tumor sites are associated with higher rates.116–120
Inhibition of EGFR and STAT3, which both have an important role in the transmission of growth signals initiated by EGFR, has been found to improve the effects of conventional treatments, such as radiotherapy in SCC. Accordingly, the constitutive activation of these factors can also give rise to less radiosensitive, i.e., more resistant, tumors.121
Key point: Activation of EGFR and STAT3 affect the radiosensitivity of SCC cells.
Resistance to ChemotherapyVarious chemotherapy agents have been used in the treatment of SCC and advanced or unresectable BCC. In the first case, cisplatin has been used either alone or combined with other agents, and it has produced variable results. It has been mostly combined with bleomycin, doxorubicin, and 5-fluorouracil, and has also been used in combination with fluorouracil-based chemoradiation. However, its efficacy has not been demonstrated in randomized clinical trials and most of the data available are based on findings from small series. The most effective options for BCC appear to be cisplatin, used alone or in combination, and a regimen such as vincristine, bleomycin, and prednisolone. Nevertheless, with very few exceptions, long-term response rates have not been demonstrated for traditional chemotherapy, and it is therefore mainly used as a palliative treatment.122
ConclusionsA wide variety of mechanisms have been described for resistance of NMSC to nonsurgical treatments. They include mechanisms related to intracellular drug transport (e.g., Ppg in PDT), mutations in target genes such as SMO with vismodegib, alterations to molecular pathways such as MAPK in PDT and EGFR inhibitors and even intrinsic cell characteristics such as adhesion molecules, cytoskeleton components, size, and tumor progression. Analysis of primary and acquired resistance mechanisms will help to extend knowledge of the biology of different types of tumor and open new avenues for the investigation of new targets and alternative pathways to treat recurrences and overcome lack of response.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Gracia-Cazaña T. Resistencias al tratamiento no quirúrgico en cáncer cutáneo no melanoma. Parte II: terapia fotodinámica, vismodegib, cetuximab, metotrexato intralesional y radioterapia. Actas Dermosifiliogr. 2016;107:740–750.