The designation of a person as “transgender” or “trans” alludes to a mismatch between their gender identity and the sex assigned to them at birth.1 “Cisgender” is the term used for individuals whose sex assigned at birth aligns with their gender identity.2 The term “transmasculine” refers to a person whose gender identity is male and whose sex assigned at birth is female. Conversely, when the gender identity is female and the sex assigned at birth is male, the term used is “transfeminine.”
A total of 9% of the population worldwide identifies as transgender. This percentage is lower in Spain (about 4% of the population).
These individuals often undergo gender-affirming hormonal therapies to develop sexually characteristic traits socially recognized as female or male.3
These therapies may complicate the treatment of various pathological conditions, including androgenetic alopecia, a condition where the male sex hormone dihydrotestosterone plays a prominent pathogenic role.4
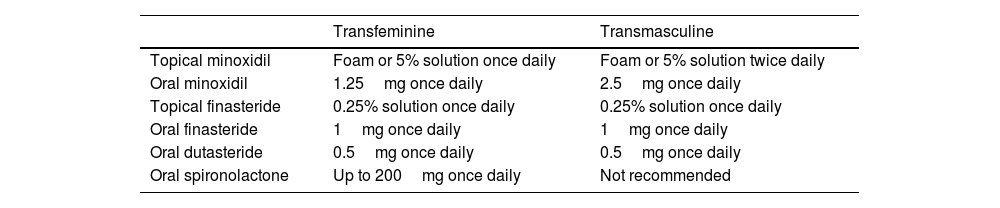
Recently, an article has been published to guide therapy (Table 1) for androgenetic alopecia based on the safety and efficacy profile of different drugs relative to the type of patient: transmasculine or transfeminine.5
Pharmacological recommendations for the treatment of androgenetic alopecia in transgender patients.
| Transfeminine | Transmasculine | |
|---|---|---|
| Topical minoxidil | Foam or 5% solution once daily | Foam or 5% solution twice daily |
| Oral minoxidil | 1.25mg once daily | 2.5mg once daily |
| Topical finasteride | 0.25% solution once daily | 0.25% solution once daily |
| Oral finasteride | 1mg once daily | 1mg once daily |
| Oral dutasteride | 0.5mg once daily | 0.5mg once daily |
| Oral spironolactone | Up to 200mg once daily | Not recommended |
First-line pharmacological therapies for transgender individuals include 5% topical minoxidil once daily for transfeminine patients and up to twice daily for transmasculine patients, where hypertrichosis is a beneficial side effect. Oral finasteride at a dose of 1mg per day is also included, as no clear variation in serum testosterone levels has been documented with its use, although its side effects must be discussed and agreed upon with the patient.
Second-line therapies include oral minoxidil at doses of 1.25mg or 2.5mg for transfeminine and transmasculine patients, respectively, and 0.25% topical finasteride as monotherapy or combined with 2% minoxidil for transfeminine patients. Oral dutasteride at a dose of 0.5mg daily is reserved for patients who have not responded to oral finasteride, while spironolactone is not recommended for transmasculine patients due to its potential to decrease serum testosterone levels and cause gynecomastia—this latter effect being desirable for transfeminine patients, where it is recommended at doses of up to 200mg daily.5
Other therapeutic alternatives commonly used in cisgender populations, such as oral contraceptives with antiandrogenic progestogens, bicalutamide, dutasteride injections, or hair transplantation, have not been included.
Given the likely increase in hormonal therapies among transgender patients and their bodily impact on areas such as the scalp, it is essential for dermatologists to remain updated so they can understand which drugs to use to help their patients according to their social, cultural, and sexual realities.
FundingNone declared.
Conflicts of interestNone declared.