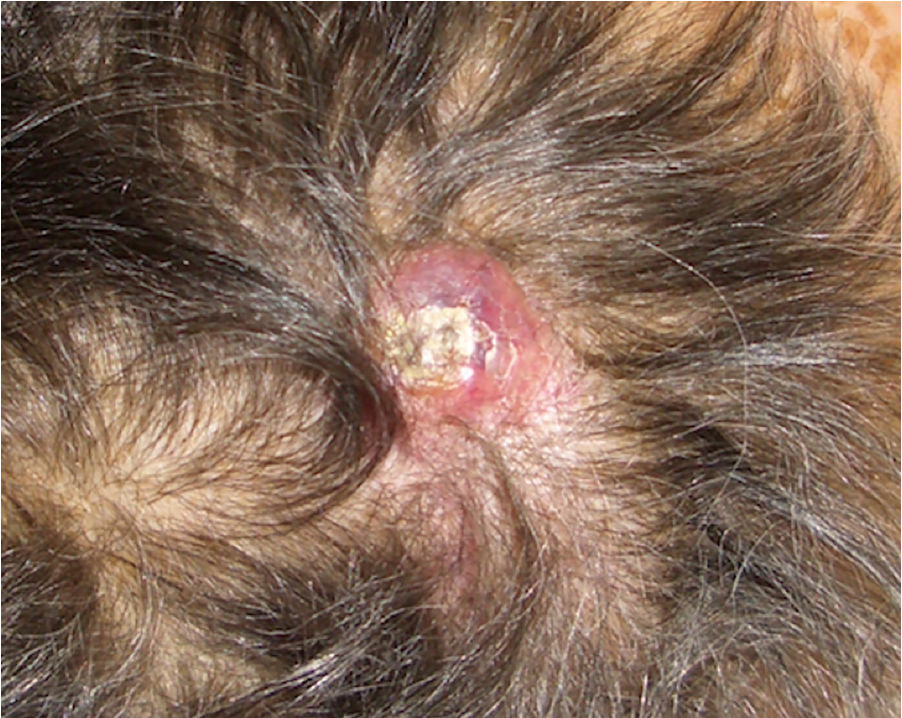
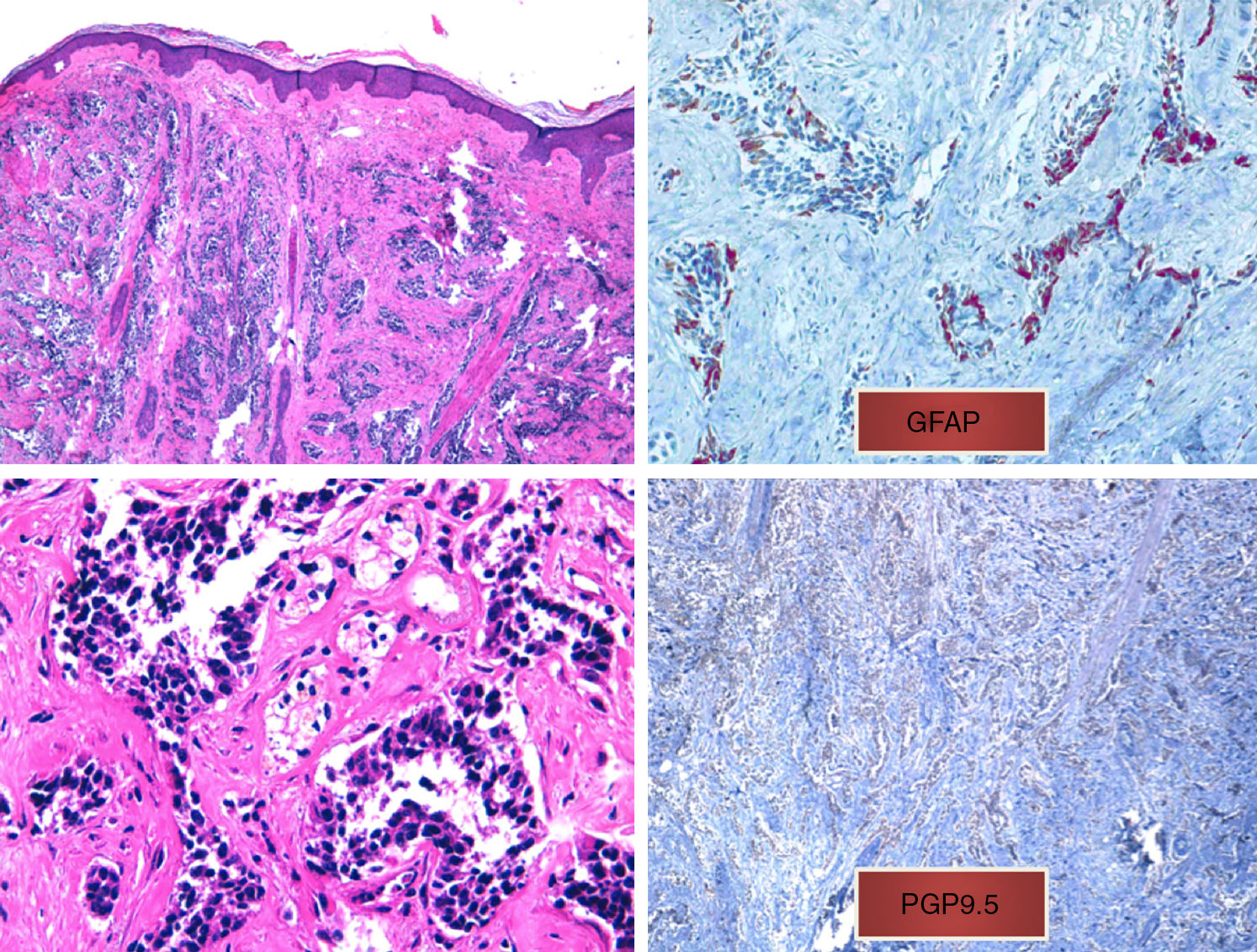
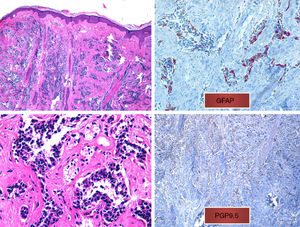
We present the case of a 75-year-old woman with a history of glioblastoma multiforme (GBM) who had undergone craniectomy and resection 7 months earlier and was participating in a clinical trial of nivolumab versus placebo. The patient had been referred from the oncology to the dermatology department for an erythematous mass (3 × 4 cm) with a crusted and ulcerated surface located in the right parietal region, close to the craniectomy scar (Fig. 1). The patient provided the results of a magnetic resonance imaging (MRI) study performed the previous day in which no continuity solution was evident in the bone. A biopsy was performed on suspicion of skin metastasis of GBM, and revealed marked diffuse dermal infiltrate consisting of mononuclear cells with hyperchromatic nuclei and marked pleomorphism. Immunohistochemistry revealed positive staining for CD10, protein gene product (PGP) 9.5, and glial fibrillary acid protein (GFAP), indicating a probable neural origin (Fig. 2). Staining for S100, HMB45, cytokeratin 20, and CD99 was negative. One month later the patient presented to the oncology department with absence seizures. Imaging tests revealed subacute hydrocephalus secondary to the initial surgical intervention. There was no possibility of placing a ventriculoperitoneal shunt. Interestingly, no changes in either the lesion or the perilesional edema were observed relative to the initial MRI findings. Given the patient's poor progression, it was decided to administer symptomatic palliative treatment.
A, Diffuse infiltrate in the papillary and reticular dermis of tumor cells, sparing the epidermis (hematoxylin-eosin, original magnification ×10). B, Tumor cells with hyperchromatic nuclei and marked pleomorphism (hematoxylin-eosin, original magnification ×40). C, Positive immunohistochemistry (glial fibrillary acid protein, original magnification ×40). D, Positive immunohistochemistry (protein gene product 9.5, original magnification ×20).
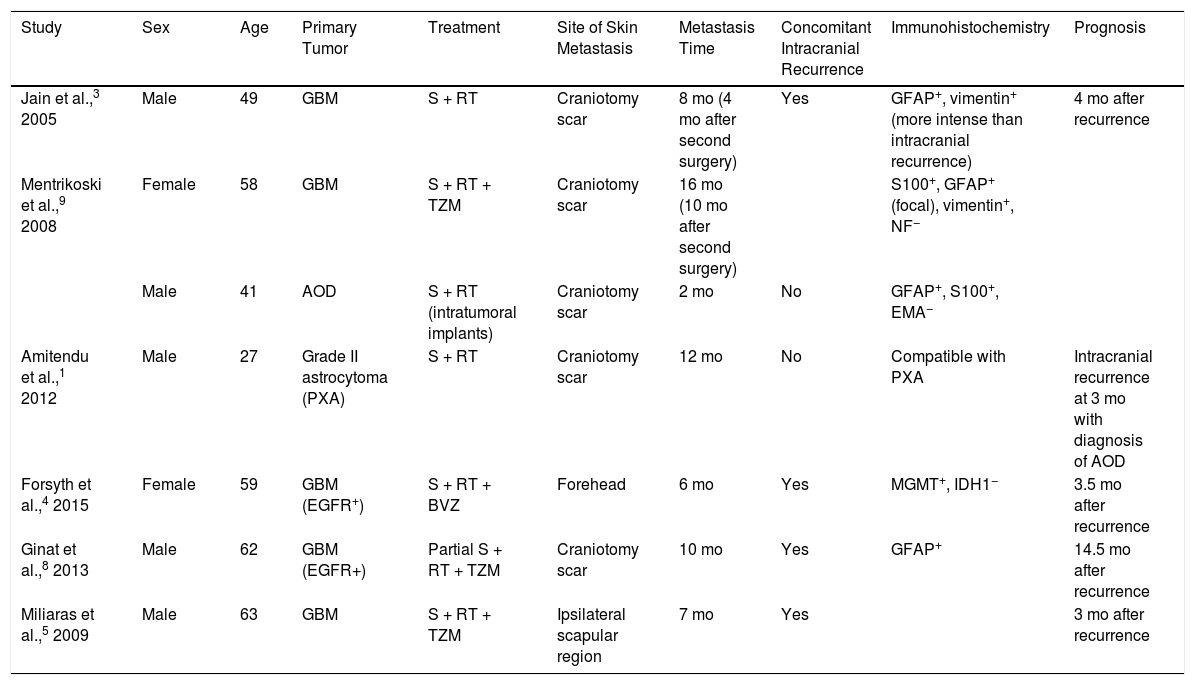
Although GBM is the most common malignant brain tumor, extracranial metastasis is uncommon, and has been reported in only 2.7% of 148 patients with a histological diagnosis of glioma who were followed up for 5 years.1 The most common sites of metastasis are the lung, pleura, and lymph nodes; skin metastasis is more rare.2 In the current literature there are 7 reports of skin metastasis of cerebral glioma grades III to IV (Table 1). Ours is the first reported case of skin metastasis of GBM without recurrence of intracranial disease,3 which is usually observed in patients with a history of invasive surgical procedures, suggesting a fundamental role of iatrogenic seeding. However, in 10% of patients extracranial metastasis occurs in the absence of previous surgical interventions4 or at a site distant from the intervention,5 suggesting the existence of other routes of dissemination (e.g. vascular, lymphatic, perineural, direct extension). Some studies suggest that mutation of the epidermal growth factor receptor (EGFR) gene is a predisposing factor for extracranial GBM growth,6 although our patient tested negative for EGFR mutations. Other theories propose that GBM promotes defective angiogenesis, giving rise to hypoxic areas in the blood-brain barrier, which is consequently disrupted.7 Another possibility is that this entity is underdiagnosed; the short survival time of these patients may be insufficient to allow the development of disseminated disease.8 Prophylactic irradiation immediately after surgery has also been proposed as a means of preventing metastasis secondary to iatrogenic tumor seeding.9
Skin Metastasis of High-Grade Glial Tumors Reported in the Literature.
| Study | Sex | Age | Primary Tumor | Treatment | Site of Skin Metastasis | Metastasis Time | Concomitant Intracranial Recurrence | Immunohistochemistry | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| Jain et al.,3 2005 | Male | 49 | GBM | S + RT | Craniotomy scar | 8 mo (4 mo after second surgery) | Yes | GFAP+, vimentin+ (more intense than intracranial recurrence) | 4 mo after recurrence |
| Mentrikoski et al.,9 2008 | Female | 58 | GBM | S + RT + TZM | Craniotomy scar | 16 mo (10 mo after second surgery) | S100+, GFAP+ (focal), vimentin+, NF− | ||
| Male | 41 | AOD | S + RT (intratumoral implants) | Craniotomy scar | 2 mo | No | GFAP+, S100+, EMA− | ||
| Amitendu et al.,1 2012 | Male | 27 | Grade II astrocytoma (PXA) | S + RT | Craniotomy scar | 12 mo | No | Compatible with PXA | Intracranial recurrence at 3 mo with diagnosis of AOD |
| Forsyth et al.,4 2015 | Female | 59 | GBM (EGFR+) | S + RT + BVZ | Forehead | 6 mo | Yes | MGMT+, IDH1− | 3.5 mo after recurrence |
| Ginat et al.,8 2013 | Male | 62 | GBM (EGFR+) | Partial S + RT + TZM | Craniotomy scar | 10 mo | Yes | GFAP+ | 14.5 mo after recurrence |
| Miliaras et al.,5 2009 | Male | 63 | GBM | S + RT + TZM | Ipsilateral scapular region | 7 mo | Yes | 3 mo after recurrence |
Abbreviations: AOD, anaplastic oligodendroglioma; BVZ, bevazizumab; EGFR, epidermal growth factor receptor; EMA, epithelial membrane antigen; GBM, glioblastoma multiforme; GFAP, glial fibrillary acid protein; IDH1, isocitrate dehydrogenase 1; IHC, immunohistochemistry; MGMT, O6-methylguanine-DNA methyltransferase; PXA, pleomorphic xanthoastrocytoma; RT, radiation therapy; S, surgery; TZM, temozolamide.
Because this entity is particularly rare there are insufficient data to establish a definitive immunohistochemical staining profile. The pattern of GFAP staining, which is generally very intense in primary tumors, can vary in skin metastases, ranging from intense diffuse or focal staining to very fine staining of the cytoplasmic processes of neoplastic cells, as observed in our patient. There are also GBM subtypes (e.g. small-cell GBM) that may be negative for GFAP staining.10 Thus, while immunohistochemistry can help identify the cell type from which the tumor is derived, it is not diagnostically definitive. A clinical-pathological correlation must be established, taking into account the patient's medical history and ruling out other much more common entities.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors thank the pathological anatomy department of the Hospital General de Valencia, especially Dr. Ana Pérez for her excellent assistance with immunohistochemistry techniques that could not be performed in the dermatology department. Thanks also to our laboratory technician Sandra Mínguez for her help and dedication with each and every case.
Please cite this article as: Magdaleno-Tapial J, Valenzuela-Oñate C, Pérez-Pastor G, Alegre de Miquel V. Metástasis cutánea de glioblastoma multiforme: presentación de un caso y revisión de la literatura Eczema y urticaria en Portugal. 2019;110:780–783.