Congenital melanocytic nevi (CMN) are genetically determined benign proliferations of cells that arise from the neural crest and which are present from birth or the first weeks of life.1–3 They may remain unchanged or present a dynamic course.1,3 Regression is a rare process that causes progressive loss of pigment and may occur in different ways.1–5 It is frequently associated with the formation of distant achromic lesions.2,4
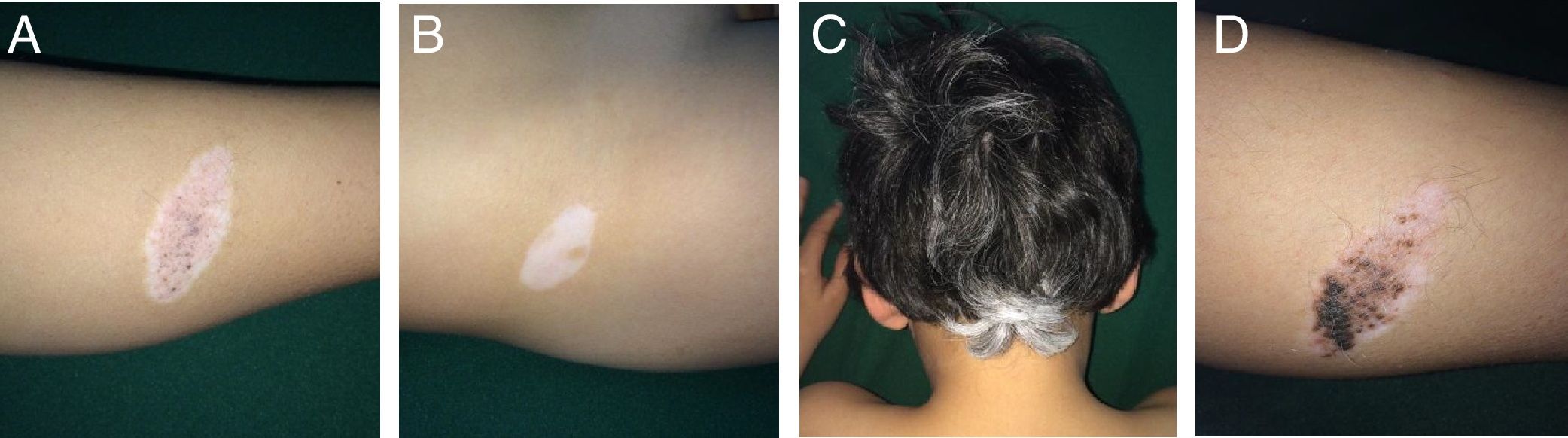
Case 1An 8-year-old boy was examined for changes in CMN lesions, which had appeared on his left leg 3 years earlier. These changes began with a halo and subsequent disappearance of pigment on the surface and of the hair covering the lesion. At the same time, the patient developed distant achromic lesions (in the region of the right iliac fossa and in the occipital region of the scalp) (Fig. 1A-C). Topical treatment was instated with mometasone furoate for 3 weeks, followed by tacrolimus 0.1% ointment, and repigmentation was achieved by sectors of both the nevus (Fig. 1D) and the distant achromic lesions, though without changes to the poliosis.
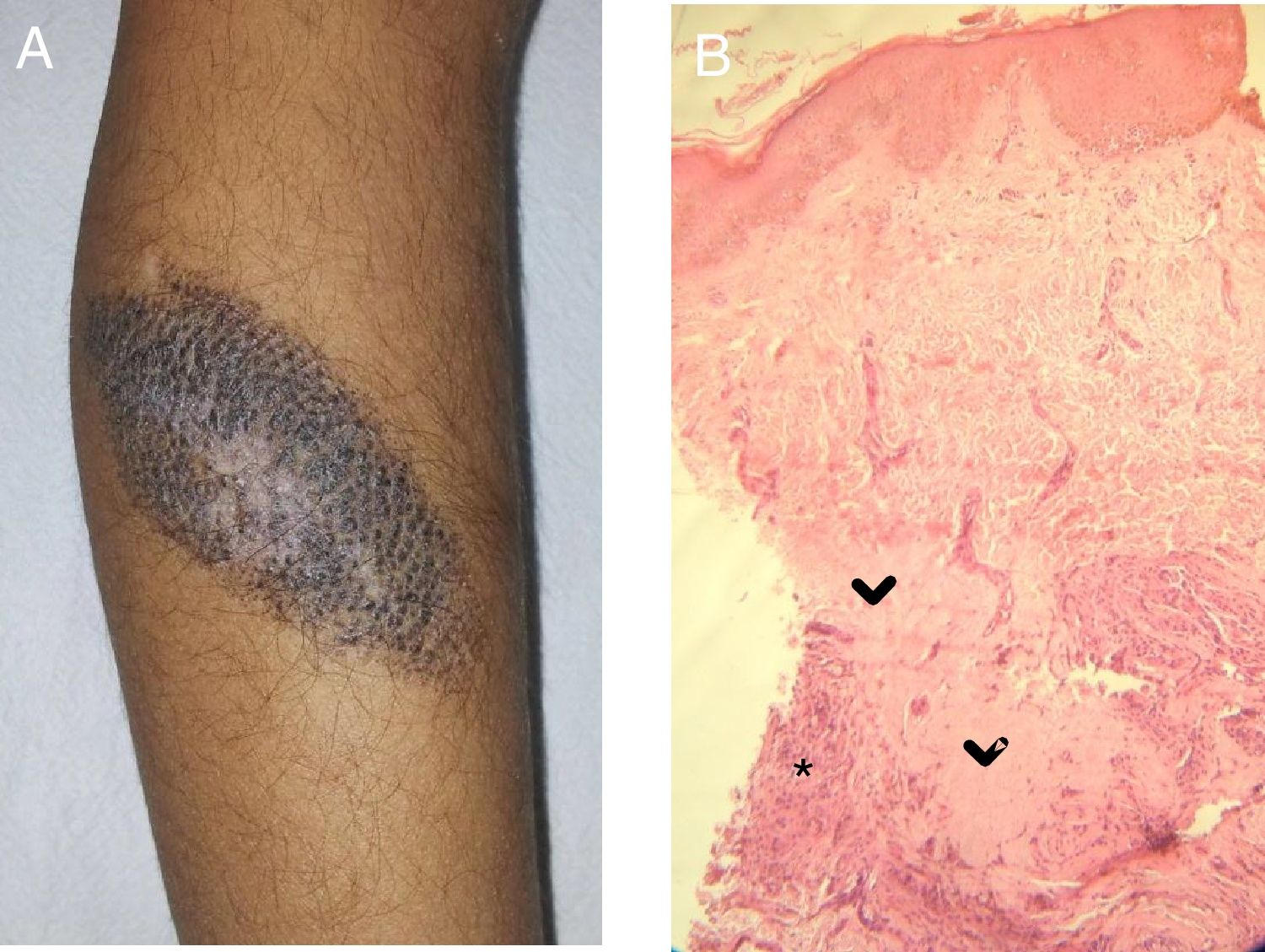
Case 2A 5-year-old girl was examined due to changes in CMN lesions, which had appeared a month earlier. Physical examination revealed a brown lesion measuring 9×5cm; the lesion was brown with a whitish central area, a corrugated surface, and a fibrous appearance, and had white hairs on the surface (Fig. 2A). At the same time, an achromic macule appeared on the upper left eyelid. Treatment was instated with tacrolimus 0.03% ointment for both lesions. The patient did not return.
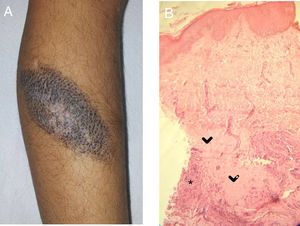
A, CMN with blackish-brown coloring and a whitish central area with a fibrotic appearance. Note the white hairs in this area. B, Histology: areas of collagen homogeneization (>) in the deep dermis, involving adnexa, surrounded by nevus cells and an inflammatory infiltrate (*). gr2.
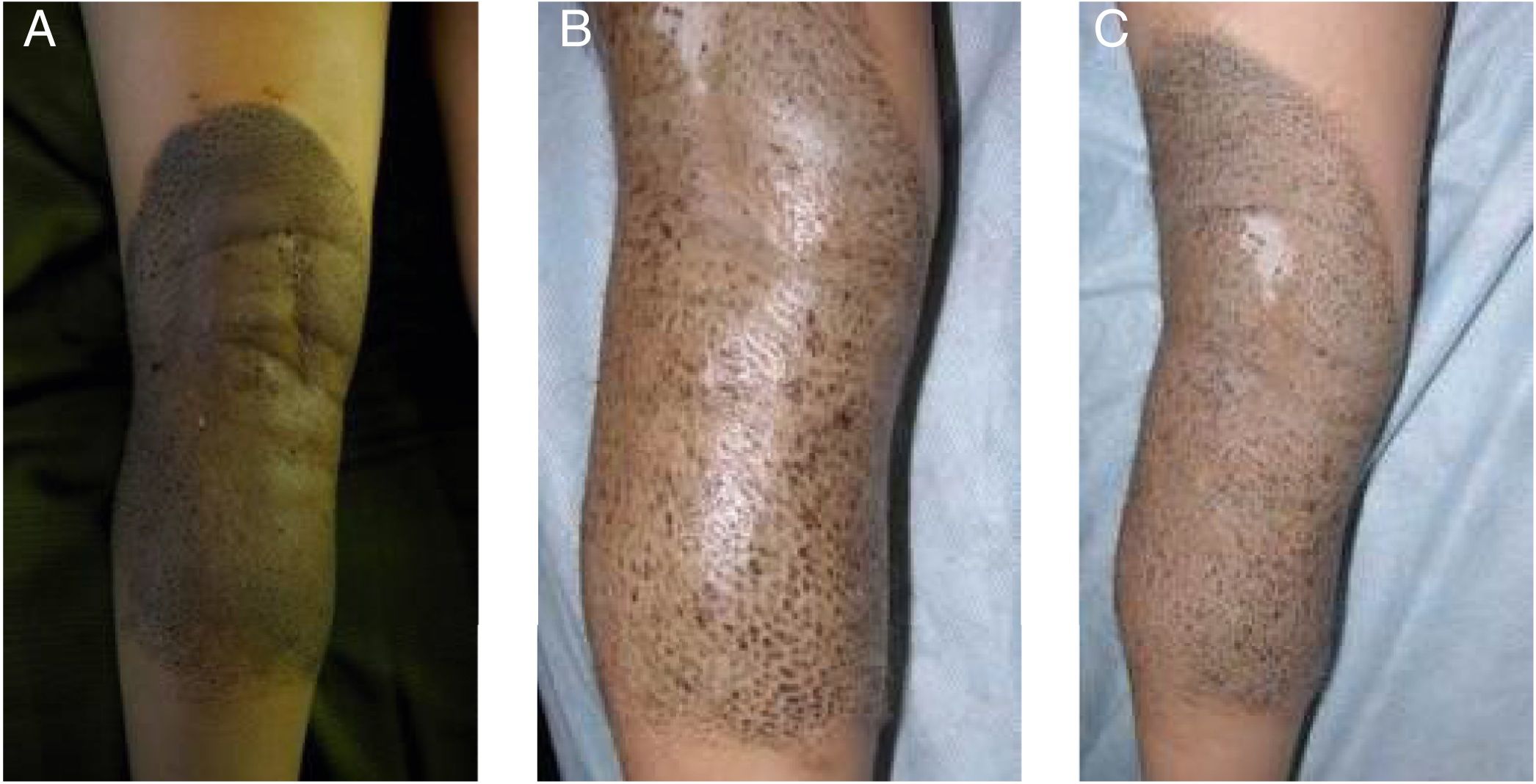
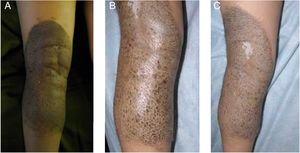
A 5-year-old girl was examined due to progressive depigmentation of her CMN lesions; this process had begun with a halo and gradual reduction of the pigment on the surface of the halo. At the same time, the patient developed achromic lesions on the upper eyelids. In the following years, she developed areas of fibrosis and follicular atrophy inside the CMN lesions. Treatment with pimecrolimus 1% was instated for the distant achromic lesions and no repigmentation was observed (Fig. 3A-C).
In all of the patients, histology revealed nevus cells and a predominantly lymphocytic inflammatory infiltrate in the deep dermis. Replacement of the nevus cells with fibrous tissue was observed in Cases 2 and 3 (Fig. 2B).
In all 3 cases, dermatoscopy revealed perifollicular hyperpigmentation, a blue-gray pigment associated with the deep component, globules, and pink areas without structures.
DiscussionCMN are classified by size into small (less than 1.5cm), medium (1.5 to 10cm), large (10 to 20cm), and giant (greater than 20cm). They may be located on any part of the skin surface and may appear in isolation or as part of more complex syndromes.1,3
Torrelo et al.6 suggest that nevi reflect mosaicisms and show that they follow certain skin patterns and phenotypic characteristics. Postzygotic mutations of the H-Ras gene have also been shown in these nevi.3,6,7
The course of CMN is variable and they may remain stable throughout life, increase in size, change color and thickness, become more hairy, become malignant, and even regress.1,3,4 To date, multiple forms have been reported in which these nevi show signs of regression.1,2,4,5 The most frequent form starts with a halo phenomenon that spontaneously regresses from the periphery to the center. Another more frequent form lacks this halo phenomenon.6 In exceptional cases, regression occurs by means fibrosis of the lesions.5
The halo phenomenon is an area of depigmentation that may occur around CMN and other entities.2 It affects 1% of the general population and incidence is higher in patients with pediatric vitiligo.2,8 There are many theories regarding its pathophysiology, including cellular and/or humoral immune responses to nevus cells or melanocytic nevi, which may present associated antigens or nonspecific abnormalities, even leading to cross-reactivity with distant melanocytes.8 The latter would explain the appearance of distant achromic lesions. Drakensjö et al.1 believed that nevus cells remained in the dermis despite the regression.
Both vitiligo and the halo phenomenon share immunologic mechanisms and it has been shown that, when both are present, they are associated with some subtypes of the human leukocyte antigen HLA complex.4,8
Regression of CMN without the halo phenomenon is very rare and alternative regression mechanisms have been proposed, such as reduced melanin synthesis and/or nevus cell apoptosis. In Case 2, the CMN regressed without a prior halo phenomenon but with distant achromic lesions.
Regression by means of fibrosis has been reported very rarely and the underlying mechanism is unknown. One variant of this form of regression by fibrosis, described by Ruiz-Maldonado et al.,9 is desmoplastic nevus. In that case, the authors proposed the immune system as a trigger.9,10 Histology shows replacement of nevus cells with connective tissue, and the most salient clinical finding is induration of the lesion. The principal differential diagnosis is malignant transformation and clinical and histologic follow-up is therefore essential.9,10
The immune response to neoplastic cells would act concomitantly against the melanocytic nevus cells. Although an association has been described between the halo phenomenon and melanoma, it is rarely associated with CMN.5
We believe that these clinical cases are of interest as they provide examples of the different forms of regression. Knowledge of this process will make it possible to anticipate the potential development of vitiligo and follow-up with therefore allow for dearly diagnosis and treatment. Case 2 is notable, as regression occurred without a prior halo phenomenon, despite the appearance of distant achromic lesions, suggesting that the immune system played an important role in the process.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Alperovich R, Fiandrino MJ, Asial R, Boente MC. Spontaneous Regression of Medium-Sized Congenital Melanocytic Nevi: Report of 3 New Cases. Actas Dermosifiliogr. 2019;110:414–416.