The Spanish standard patch test series, as recommended by the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC), has been updated for 2016. The new series replaces the 2012 version and contains the minimum set of allergens recommended for routine investigation of contact allergy in Spain from 2016 onwards.
Four haptens —clioquinol, thimerosal, mercury, and primin—have been eliminated owing to a low frequency of relevant allergic reactions, while 3 new allergens —methylisothiazolinone, diazolidinyl urea, and imidazolidinyl urea—have been added. GEIDAC has also modified the recommended aqueous solution concentrations for the 2 classic, major haptens methylchloroisothiazolinone/methylisothiazolinone mix, which is now to be tested at 200ppm in aqueous solution, and formaldehyde, which is now to be tested in a 2% aqueous solution.
Updating the Spanish standard series is one of the functions of GEIDAC, which is responsible for ensuring that the standard series is suited to the country's epidemiological profile and pattern of contact sensitization.
La serie estándar española de pruebas alérgicas de contacto recomendada por el Grupo Español de Investigación en Dermatitis de Contacto (GEIDAC) ha sido actualizada para 2016. La nueva serie sustituye a la que estaba vigente desde 2012, y el Grupo la recomienda utilizar a partir de ahora como herramienta básica de la consulta de eccema de contacto.
La nueva serie estándar elimina, por la falta de frecuencia de positividades relevantes, 4 haptenos: clioquinol, tiomersal, mercurio y primina, y añade 3 nuevos: metilisotiazolinona, diazolidinil urea e imidazolidinil urea. Modifica además la concentración en agua de 2 haptenos clásicos muy importantes: la mezcla metilcloroisotiazolinona/metilisotiazolinona, que pasa a 200ppm aq, y el formaldehído, que se parcheará a partir de ahora al 2% aq.
La actualización de la serie estándar española es una de las funciones del GEIDAC, que vela por su adecuación a la epidemiología y la casuística de nuestro entorno.
Scientific societies make recommendations in their individual areas of competence, and those specialized in contact allergy go to great lengths to design their own standard series in the knowledge that it will be the most widely used series in their setting and that it contains the minimum set of allergens necessary for the routine investigation of contact allergy. After a vote taken at the 60th meeting of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC) held on October 23-24, 2015, the members agreed on the new composition of the Spanish standard series for 2016.
Allergic contact dermatitis is caused by sensitization to substances that are often ubiquitous in the patient's environment, although the frequency and impact of potential sensitization to a specific allergen depend to a large extent on the circumstances in which the exposure occurs, the intensity of the contact, and environment-specific characteristics. Consequently, the frequency of sensitization to a specific allergen often differs considerably between neighboring countries or regions. Rates of sensitization reported in hospitals are often markedly affected by socioeconomic characteristics, geography, and climate. The most frequently detected allergens (preservatives, metals, perfumes, paraphenylenediamine, and rubber additives) are common to all of our neighboring countries. However, occasionally striking differences are found. In Spain, for example, allergy to leather shoes is still very common, mainly owing to considerable exposure and increased release of chrome to the skin as a result of heat and sweating in summer,1 as is the extremely high frequency of sensitization to nickel.2
The standard or baseline series is a minimum set of allergens included in the basic workup applied to all patients who attend a contact allergy clinic. It includes the most frequent allergens in the environment, and each scientific society proposes its own. For practical reasons, most recommended standard series contain around 30 allergens.
Standard series are renewed regularly, according to—as might be expected—regional variations in the frequency of sensitization. It therefore seems natural that the Spanish series is not a copy of series from other countries and that it is modified over time. Despite the availability of a standard European series recommended by the European Contact Dermatitis Research Group (ECDRG), within the European Society of Contact Dermatitis (ESCD), the position of the Spanish group is that of maintaining our own criteria in the choice of standard allergens. Consequently, our series differs from the European series in some aspects. The last European standard series was modified in 2014,3 and a proposal has been made to include a new allergen.4 It contains 30 allergens, 26 of which are in the Spanish series. The latest approved Spanish standard series comprises 32 allergens. It came into force in 2016 and replaces the last series, which was revised in 2012.5
Epidemiology of Contact Dermatitis in SpainEpidemiological changes take place year after year, although exhaustive epidemiological studies showing the exact figures for sensitization in Spain are lacking. The last national study to collect data on allergic sensitization to standard allergens was published in 2004.6
Several members of GEIDAC recently joined a European initiative, the European Surveillance System on Contact Allergies (ESSCA),7 which was formed as an ESCD working group, and founded the Spanish Network for Surveillance of Contact Allergy (REVAC). The network brings together sensitization data that each center reports to a national database and thus helps to determine the frequency and importance of various allergens in Spain (data are subsequently sent to the European network). The network can act as a sentinel, since by analyzing annual figures it can detect variations in the frequency of sensitization and the emergence of new allergens. Some of the results of the work carried out by REVAC have already been published.1,2
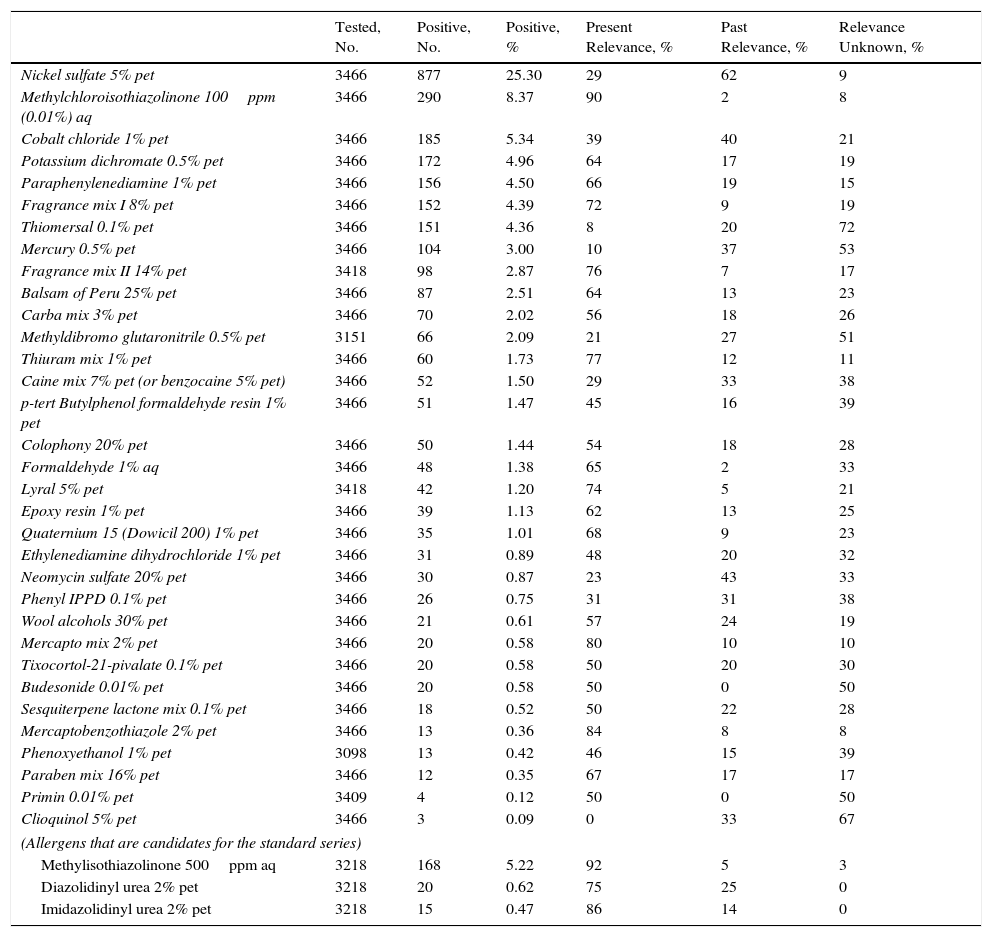
In 2012, GEIDAC participated in an epidemiological study that included 3466 patients who underwent patch testing in 17 centers throughout Spain and whose results were subsequently presented at the 12th congress of the European Society of Contact Dermatitis in 2014.8 The study population included all consecutive patients who underwent patch testing at each center during the natural year; the frequencies of sensitization to each standard allergen and its relevance are set out in Table 1. Interestingly, the study revealed that the 15 most prevalent allergens accounted for 85.3% of all positive test results, 82.7% of positive results with present relevance, and 92.5% of positive results with past relevance. The main allergens were nickel, which continues to be the most frequent of all, isothiazolinone mix, which has doubled in frequency, and fragrance mix II, which, despite being a “recent” allergen in the standard series, is the sixth most important allergen based on positive results with present relevance. Also noteworthy among the most common allergens in the study, albeit in a negative sense, were thiomersal and mercury, since, even though they often elicited reactions, less than 10% had present relevance. The allergens with the lowest rates of sensitization were primin and clioquinol. We can therefore deduce that it would be of little practical value to recommend a standard series of more than 30 allergens, since it would greatly increase workload with no appreciable improvement in detection capacity.
Results of the GEIDAC 2012 Epidemiological Studya
| Tested, No. | Positive, No. | Positive, % | Present Relevance, % | Past Relevance, % | Relevance Unknown, % | |
|---|---|---|---|---|---|---|
| Nickel sulfate 5% pet | 3466 | 877 | 25.30 | 29 | 62 | 9 |
| Methylchloroisothiazolinone 100ppm (0.01%) aq | 3466 | 290 | 8.37 | 90 | 2 | 8 |
| Cobalt chloride 1% pet | 3466 | 185 | 5.34 | 39 | 40 | 21 |
| Potassium dichromate 0.5% pet | 3466 | 172 | 4.96 | 64 | 17 | 19 |
| Paraphenylenediamine 1% pet | 3466 | 156 | 4.50 | 66 | 19 | 15 |
| Fragrance mix I 8% pet | 3466 | 152 | 4.39 | 72 | 9 | 19 |
| Thiomersal 0.1% pet | 3466 | 151 | 4.36 | 8 | 20 | 72 |
| Mercury 0.5% pet | 3466 | 104 | 3.00 | 10 | 37 | 53 |
| Fragrance mix II 14% pet | 3418 | 98 | 2.87 | 76 | 7 | 17 |
| Balsam of Peru 25% pet | 3466 | 87 | 2.51 | 64 | 13 | 23 |
| Carba mix 3% pet | 3466 | 70 | 2.02 | 56 | 18 | 26 |
| Methyldibromo glutaronitrile 0.5% pet | 3151 | 66 | 2.09 | 21 | 27 | 51 |
| Thiuram mix 1% pet | 3466 | 60 | 1.73 | 77 | 12 | 11 |
| Caine mix 7% pet (or benzocaine 5% pet) | 3466 | 52 | 1.50 | 29 | 33 | 38 |
| p-tert Butylphenol formaldehyde resin 1% pet | 3466 | 51 | 1.47 | 45 | 16 | 39 |
| Colophony 20% pet | 3466 | 50 | 1.44 | 54 | 18 | 28 |
| Formaldehyde 1% aq | 3466 | 48 | 1.38 | 65 | 2 | 33 |
| Lyral 5% pet | 3418 | 42 | 1.20 | 74 | 5 | 21 |
| Epoxy resin 1% pet | 3466 | 39 | 1.13 | 62 | 13 | 25 |
| Quaternium 15 (Dowicil 200) 1% pet | 3466 | 35 | 1.01 | 68 | 9 | 23 |
| Ethylenediamine dihydrochloride 1% pet | 3466 | 31 | 0.89 | 48 | 20 | 32 |
| Neomycin sulfate 20% pet | 3466 | 30 | 0.87 | 23 | 43 | 33 |
| Phenyl IPPD 0.1% pet | 3466 | 26 | 0.75 | 31 | 31 | 38 |
| Wool alcohols 30% pet | 3466 | 21 | 0.61 | 57 | 24 | 19 |
| Mercapto mix 2% pet | 3466 | 20 | 0.58 | 80 | 10 | 10 |
| Tixocortol-21-pivalate 0.1% pet | 3466 | 20 | 0.58 | 50 | 20 | 30 |
| Budesonide 0.01% pet | 3466 | 20 | 0.58 | 50 | 0 | 50 |
| Sesquiterpene lactone mix 0.1% pet | 3466 | 18 | 0.52 | 50 | 22 | 28 |
| Mercaptobenzothiazole 2% pet | 3466 | 13 | 0.36 | 84 | 8 | 8 |
| Phenoxyethanol 1% pet | 3098 | 13 | 0.42 | 46 | 15 | 39 |
| Paraben mix 16% pet | 3466 | 12 | 0.35 | 67 | 17 | 17 |
| Primin 0.01% pet | 3409 | 4 | 0.12 | 50 | 0 | 50 |
| Clioquinol 5% pet | 3466 | 3 | 0.09 | 0 | 33 | 67 |
| (Allergens that are candidates for the standard series) | ||||||
| Methylisothiazolinone 500ppm aq | 3218 | 168 | 5.22 | 92 | 5 | 3 |
| Diazolidinyl urea 2% pet | 3218 | 20 | 0.62 | 75 | 25 | 0 |
| Imidazolidinyl urea 2% pet | 3218 | 15 | 0.47 | 86 | 14 | 0 |
Abbreviations: aq, aqueous solution; GEIDAC, Spanish Contact Dermatitis and Skin Allergy Research Group; IPPD, N-isopropyl-N-phenyl–paraphenylenediamine; pet, petrolatum.
Members of GEIDAC have performed other multicenter studies on specific allergens from the standard series or on allergens that are candidates for the standard series,9–11 and several studies are ongoing.
In addition to data from multicenter studies or national studies on the frequency of sensitization to patch test allergens, members of GEIDAC have their own databases, which were often the fruit of careful annual recording of the results of the work carried out in their units and clinics. This very valuable information, some of which has been published,12 enables professionals to have their own highly qualified criteria with respect to the frequency and importance of the allergens in their setting.
Methods. How Was the Standard Series Chosen?During a scientific session at the latest GEIDAC meeting, participants presented and discussed the epidemiologic data analyzed by REVAC between 2008 and 2014, as well as several relevant epidemiological studies on allergy to rubbers, corticosteroids, nickel, methylisothiazolinone, and occupational allergens. This session was followed by a specific session dedicated to updating the Spanish standard series.
The meeting was attended by 26 of the 28 members of GEIDAC, and a voting system was established to maintain each hapten in the standard series or to exclude it. The members agreed that at least a solid majority would be necessary (two-thirds of the votes) to make changes with respect to the allergen. Participants were given the opportunity to defend conflicting opinions when doubts arose. The vote was then repeated, and a majority of half plus one (absolute majority) was considered valid for accepting the change proposed.
Consensus was very broad, and agreement was unanimous for almost all the allergens. A second round of voting was necessary for ethylenediamine, paraben mix, carbamate mix, methyldibromo glutaronitrile, and phenoxyethanol, which were finally maintained. A second vote was also necessary for 4 haptens that were conclusively eliminated from the series with a wide majority (more than two-thirds), namely, clioquinol, thiomersal, mercury, and primin.
Of note, allergens that were relatively uncommon according to the statistics of GEIDAC were maintained by general approval. These included epoxy resin, which is relevant in the workplace, and markers of sensitization to topical corticosteroids (tixocortol and budesonide), which are important and not usually suspected by physicians.
The most controversial allergens were as follows: carbamate mix, which is problematic (frequent irritant, doubtful, or weak reactions) and was eliminated from the European series; ethylenediamine, which was eliminated from the European series and has fallen into disuse, although it is still found in some topical medicines that are widely used in Spain (eg, Positon cream); and methyldibromo glutaronitrile, which has been prohibited in cosmetics13 since 2007, but which may still be present in the workplace. The prevalence of contact allergy to parabens and phenoxyethanol is very low. Phenoxyethanol in particular was the subject of intense debate, although eventually it was decided to maintain both allergens in the standard series, since they are the most commonly used preservatives in cosmetics (phenoxyethanol will be reevaluated in future updates, depending on its frequency of sensitization).
GEIDAC has undertaken to review the standard series every 2 years with the aim of adapting it to future needs and changes.
The 2016 Spanish Standard SeriesThe modifications agreed upon affect the 2012 standard series. The recommendations set out herein came into force at the beginning of the year 2016, and GEIDAC recommends they be used from now onward. The updated series is shown in Table 2.
GEIDAC Standard Series 2016.
| 1. | Nickel sulfate 5% pet |
| 2. | Wool alcohols 30% pet |
| 3. | Neomycin sulfate 20% pet |
| 4. | Potassium dichromate 0.5% pet |
| 5. | Caine mix 7% pet |
| 6. | Fragrance mix I 8% pet |
| 7. | Colophony 20% pet |
| 8. | Epoxy resin, bisphenol A 1% pet |
| 9. | Methylisothiazolinone 0.2% aq (2000ppm) |
| 10. | Balsam of Peru 25% pet |
| 11. | Ethylenediamine dihydrochloride 1% pet |
| 12. | Cobalt chloride 1% pet |
| 13. | p-tert Butylphenol formaldehyde resin 1% pet |
| 14. | Parabens mix 16% pet |
| 15. | Carba mix 3% pet |
| 16. | N-Isopropyl-N-phenyl–paraphenylenediamine 0.1% pet |
| 17. | Methylchloroisothiazolinone/methylisothiazolinone 0.02% aq (200ppm) |
| 18. | Quaternium 15 1% pet |
| 19. | Mercaptobenzothiazole 2% pet |
| 20. | Paraphenylenediamine 1% pet |
| 21. | Formaldehyde 2% aq |
| 22. | Mercapto mix 2% pet |
| 23. | Imidazolidinyl urea 2% pet |
| 24. | Thiuram mix 1% pet |
| 25. | Diazolidinyl urea 2% pet |
| 26. | Sesquiterpene lactone mix 0.1% pet |
| 27. | Tixocortol-21-pivalate 0.1% pet |
| 28. | Budesonide 0.01% pet |
| 29. | Methyldibromo glutaronitrile 0.5% pet |
| 30. | Fragrance mix II 14% pet |
| 31. | Lyral 5% pet |
| 32. | 2-Phenoxyethanol 1% pet |
Abbreviations: aq, aqueous solution; GEIDAC, Spanish Contact Dermatitis and Skin Allergy Research Group; pet, petrolatum.
The main changes are as follows:
Elimination of 4 HaptensClioquinol: Clioquinol was eliminated because of its low frequency of sensitization (1 per 1000 patients who undergo patch testing), its uncommon clinical relevance, and the fact that it has fallen into disuse as a fungicide and amebicide, even though it is a traditional allergen that is still present in the European standard series.
Thiomersal and mercury: Thiomersal and mercury were eliminated owing to obsolescence, because mercury was prohibited in Europe in 2005 (mercury-based antiseptics including thiomersal are restricted to concentrations of <0.007% of total mercury and are only found in eye makeup and makeup remover).14–16 Sensitization to these classic haptens in the patch tests was very frequent (3%-4% of patients tested), although without clinical relevance in the vast majority of cases. Mercury and thiomersal were not in the European standard series, but they were maintained in the Spanish series because of their historical relevance: mercury is used in dental amalgams, contact allergy to merbromin was frequent in the past, and systemic contact dermatitis due to inhalation of mercury from broken thermometers was occasionally detected, although this is increasingly unlikely today, since the ban on the use of mercury came into force in the European Union in 2007.15 Thiomersal was often used in the past as a preservative in vaccines and antitoxins and as an antiseptic in contact lens fluid and eye cosmetics. However, sensitization to thiomersal is rarely the cause of symptoms today, and its involvement has been relegated to potential cross-reactivity with piroxicam owing to its thiol group.
Primin, or 2-methoxy-6-n-pentyl-parabenzoquinone: Primin is the main allergen from the plant Primula obconica and is relatively common in the Baltic states and Benelux. However, it is extremely uncommon in Spain, with such a low prevalence among patients undergoing patch testing that its presence in the standard series is no longer justified. This is because of the reduced availability of and exposure to the sensitizing plant in Spain.
Other ModificationsCaine mix 7% in petrolatum (pet) is the only allergen that has been accepted as a marker of allergy to ester-type local anesthetic drugs (para-aminobenzoate group). The previous series allowed patch testing both with benzocaine mix and with benzocaine 5% pet alone, although this is no longer accepted.
The test concentrations of 2 major classic allergens have also been modified in the new standard series, as follows:
- -
Methylchloroisothiazolinone/methylisothiazolinone (Kathon CG): the concentration has increased from 100 to 200ppm in aqueous solution (aq) (200ppm [0.02%] aq, which is equivalent to 0.006mg/cm2), in accordance with the latest scientific evidence.3
- -
Formaldehyde: the concentration has increased from 1% to 2% aq (from 0.30 to 0.60mg/cm2), as recommended by Ponten et al.17 in 2013 on behalf of the ESCD.
The new standard series includes 3 new haptens:
Methylisothiazolinone, which is recommended for patch testing at a concentration of 2000ppm aq (2000ppm [0.2%] aq),18 has been the main emergent allergen in contact allergy clinics in recent years. The frequency of sensitization in our study was 5% (although the allergen was tested at a lower concentration than currently recommended; see Table 1). In most centers today, we see a positive test result in 1 of every 8-10 patients who undergo patch testing, often independently of the classic mix of methylchloroisothiazolinone/methylisothiazolinone (ie, a positive reaction to methylisothiazolinone only and not to the mix). Furthermore, to date, a high percentage of present relevance has been observed with this allergen. The geometric growth in the number of cases of allergy to this preservative is undoubtedly due to the authorization of its use at concentrations of up to 100ppm in cosmetics in the European Union.19,20 Unless the situation of methylisothiazolinone allergen is resolved soon through regulation, the allergen will continue to be one of the main causes of contact allergy in the future.21
Diazodinyl urea 2% pet and imidazolidinyl urea 2% pet are both formaldehyde-releasing preservatives that have become increasingly used in cosmetics and topical drugs during the last 10 years. The frequency of sensitization to these drugs in Spain is around 0.5% to 1% of all patients undergoing patch tests,8,11 which is similar to frequencies reported in the rest of Europe but lower than that reported in the United States of America.22 The importance of these findings lies in the fact that patients who are allergic to these preservatives often do not test positive to formaldehyde alone; therefore, the preservative is of no use as a marker in screening for allergies to its releasers.
The main suppliers of patches and patch tests in Spain—Chemotechnique, Vellinge (Sweden), and Martí Tor Alergia, Barcelona (Spain)—have been notified of our recommendations and are now marketing the GEIDAC 2016 standard series presented here.
Appendix B (see supplementary material) clarifies various aspects of the allergens and mixes, as well as the differences in some marketed allergens.
What to test when TRUE test is used?The prepared allergen system known as the Thin-layer Rapid Use Epicutaneous Patch Test (TRUE test, SmartPractice) does not coincide exactly with either the European or the Spanish standard series. The main differences between the TRUE test and the Spanish standard series are as follows23:
- -
The TRUE-TEST lacks 5 allergens: methylisothiazolinone, sesquiterpene lactone mix, fragrance mix II, lyral, and phenoxyethanol; therefore, dermatologists who use this test in their daily practice will have to test the 5 individual allergens separately.
- -
The concentration of some allergens is lower and does not meet recommendations. This is particularly important in the case of methylchloroisothiazolinone/methylisothiazolinone (0.004mg/cm2) and formaldehyde (0.18mg/cm2)
- -
The patch with N-isopropyl-N-phenyl-paraphenylenediamine also includes black rubber mix. The paraben mix includes a fifth paraben (benzylparaben). The mercapto mix contains 3 components, not 4.
- -
The TRUE test includes 8 allergens that are no longer included in the Spanish standard series: thiomersal, quinoline, gold sodium thiosulfate, hydrocortisone-17-butyrate, bacitracin, parthenolide, disperse blue 106, and bronopol.
Since there is no clear evidence to support the traditional testing system (syringes and Finn chambers) over the TRUE test or evidence to the contrary,24,25 the choice of one or the other is based on personal criteria and on the experience of the dermatologist.
How Can the Dermatologist Benefit From the Standard Series?Data show that, in all countries, the standard series enables the detection a variable yet very substantial percentage of contact allergies, even in highly specialized units.26 Therefore, testing with a standard series should be the common minimum requirement in workups for confirmation of a reasonable suspicion of contact allergy and should offer a guarantee to the dermatologist who uses it that the initial study is relatively complete.
The standard series is not exhaustive, although it does contain mixes and a very varied sample of haptens, thus making it possible to test patients with the chemical structures that most commonly cause sensitization (electrophilic metals, short-chain alcohols and aldehydes, amines, peroxides, terpenes, and radical groups in alfa beta unsaturated rings) and that are therefore present in the most common contact allergens. All of the experts agree that it should be the starting point of any study. Of course, it can never replace other tests, which will always be performed based on clinical suspicion, history, and occupational or domestic exposure.27 The patient's own products are particularly important and play a key role in diagnosis, although appropriate application often requires experience and sound judgment.28
Experience and good sense will sometimes lead the dermatologist to refer the patient to a reference clinic, especially when further tests are complex or unavailable and when the case is particularly severe or has an unusual presentation or when the patient's condition does not improve after the initial basic workup.
Future PerspectivesGEIDAC is currently participating in several international studies on contact allergy to fragrances, hydroperoxides, and textile dyes and in a Spanish study on allergy to corticosteroids.
GEIDAC has undertaken to step up its involvement in multicenter studies and to continue to play an active part in epidemiological surveillance in Spain. In the coming years, GEIDAC also aims to integrate data collected annually by all its members into a national database, which will lend consistency to future epidemiological studies by GEIDAC and make it possible to detect trends among emerging allergens. This valuable information will in turn provide better criteria that can help to perfect and update the Spanish standard series so that dermatologists can apply it in their own in daily practice.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
M. Hervella-Garcés. Servicio de Dermatología, Complejo Hospitalario de Navarra, Pamplona.
J. García-Gavín. Clínica Pérez & Gavín Dermatólogos, Vigo.
J.C. Armario-Hita. Servicio de Dermatología, Hospital Universitario de Puerto Real, Cádiz.
L. Borrego-Hernando. Servicio de Dermatología, Complejo Hospitalario Universitario Insular Materno Infantil, Las Palmas de Gran Canaria.
J.M. Carrascosa-Carrillo. Servicio de Dermatología, Hospital Universitari Germans Trias i Pujol, Badalona.
S. Córdoba-Guijarro. Servicio de Dermatología, Hospital Universitario de Fuenlabrada, Madrid.
V. Fernández-Redondo. Servicio de Dermatología, Complejo Hospitalario Universitario de Santiago de Compostela.
B. García-Bravo. Departamento de Dermatología, Hospital Virgen Macarena, Sevilla.
M.E. Gatica-Ortega. Servicio de Dermatología, Hospital Virgen de la Salud, Toledo.
A. Giménez-Arnau. Servicio de Dermatología, Hospital del Mar-Parc de Salut Mar, Barcelona.
E. Giménez-Arnau. Dermatochemistry Laboratory, University of Strasbourg-CNRS, Estrasburgo, Francia.
E. Gómez-de la Fuente. Servicio de Dermatología, Hospital Universitario Fundación Alcorcón, Madrid.
R. González-Pérez. Servicio de Dermatología, Hospital Universitario de Araba, Vitoria-Gasteiz.
D. Guimaraens-Juanena. Dermatóloga, Madrid.
F. Heras-Mendaza. Servicio Dermatología, Hospital Fundación Jiménez Díaz, Madrid.
P. Manrique-Martínez. Servicio de Dermatología, Hospital Galdakao, Bilbao.
P. Mercader-García. Servicio de Dermatología, Hospital General Universitario Morales Meseguer, Murcia.
A. Miranda-Romero. Servicio de Dermatología, Hospital Clínico Universitario de Valladolid.
F.J. Ortiz-de Frutos. Servicio de Dermatología, Hospital Universitario 12 de Octubre, Madrid.
M.A. Pastor-Nieto. Servicio de Dermatología, Hospital Universitario de Guadalajara.
M. Rodríguez-Serna. Servicio de Dermatología, Hospital Universitario La Fe, Valencia.
I. Ruiz-González. Servicio de Dermatología, Complejo Asistencial Universitario de León.
P. Sánchez-Pedreño. Servicio de Dermatología, Hospital Clínico Universitario Virgen de la Arrixaca, Murcia.
J. Sánchez-Pérez. Servicio de Dermatología, Hospital Universitario de la Princesa, Madrid.
T. Sanz-Sánchez. Servicio de Dermatología, Hospital Universitario Infanta Sofía, San Sebastián de los Reyes, Madrid.
E. Serra-Baldrich. Servicio de Dermatología, Hospital de la Santa Creu i Sant Pau, Barcelona
J. Vilaplana-Vilaplana. Dermatólogo. Tarragona.
J.F. Silvestre-Salvador. Servicio de Dermatologia, Hospital General Universitario de Alicante.
Los miembros del Grupo Español de Investigación en Dermatitis de Contacto y Alergia Cutánea (GEIDAC) se presentan en el Anexo A.
Please cite this article as: Hervella-Garcés M, García-Gavín J, Silvestre-Salvador JF, en representación del Grupo Español de Investigación en Dermatitis de Contacto y Alergia Cutánea (GEIDAC). Actualización de la serie estándar española de pruebas alérgicas de contacto por el Grupo Español de Investigación en Dermatitis de Contacto y Alergia Cutánea (GEIDAC) para 2016. Actas Dermosifiliogr. 2016;107:559–566.