Anti-p200 pemphigoid is a rare autoimmune subepidermal blistering disease characterized by the presence of circulating immunoglobulin G antibodies directed against laminin gamma-1, a 200-kDa protein located in the lamina lucida of the basement membrane. We review the clinical, histopathological and immunological characteristics of the first 2 cases described in Spain. Anti-p200 pemphigoid shares histopathological and immunopathological findings with epidermolysis bullosa acquisita, the main entity in the differential diagnosis. However, its management follows the same guidelines as those used for bullous pemphigoid. The diagnosis is confirmed by immunoblotting, which is a complex technique available in few centers. We propose the immunohistochemical detection of collagen type IV on the floor of the blister, combined with standard immunofluorescence techniques, as a simple, accessible alternative to differentiate anti-p200 pemphigoid from epidermolysis bullosa acquisita.
El penfigoide anti-p200 es una enfermedad ampollosa subepidérmica autoinmune infrecuente, asociada a la presencia de anticuerpos circulantes de tipo IgG dirigidos frente a la laminina gamma-1, una proteína de 200kDa localizada en la lámina lúcida de la membrana basal. Revisamos las características clínicas, histopatológicas e inmunológicas de los 2 primeros casos descritos en España. El penfigoide anti-p200 comparte hallazgos histopatológicos e inmunopatológicos con la epidermólisis ampollosa adquirida, su principal diagnóstico diferencial. Sin embargo, su manejo sigue las mismas pautas descritas para el penfigoide ampolloso. El diagnóstico se confirma mediante inmunoblot, una técnica compleja y accesible en pocos centros. Proponemos la detección mediante inmunohistoquímica del colágeno iv en el suelo de la ampolla, combinándola con las técnicas habituales de inmunofluorescencia, como alternativa sencilla y disponible, para diferenciarlo de la epidermólisis ampollosa adquirida.
Anti-p200 pemphigoid is an autoimmune subepidermal blistering disease first described by Zillikens et al.1 in 1996 in a 54-year-old man. The patient presented with generalized bullae associated with IgG antibodies directed against a 200kDa protein located in the lamina lucida of the basement membrane. Subsequently, 91 additional cases have been reported and the actual incidence is unknown.2
We report the first 2 cases in Spain.
Case HistoriesCase 1A 65-year-old man presented with a history of Horton arteritis treated with low-dose oral corticosteroids and bullous pemphigoid (BP) diagnosed 3 years earlier in another hospital. He attended our department with a new episode of pruriginous lesions. Physical examination showed tense bullae and urticarial plaques predominantly on the legs, with isolated lesions on the trunk. There was no mucosal involvement.
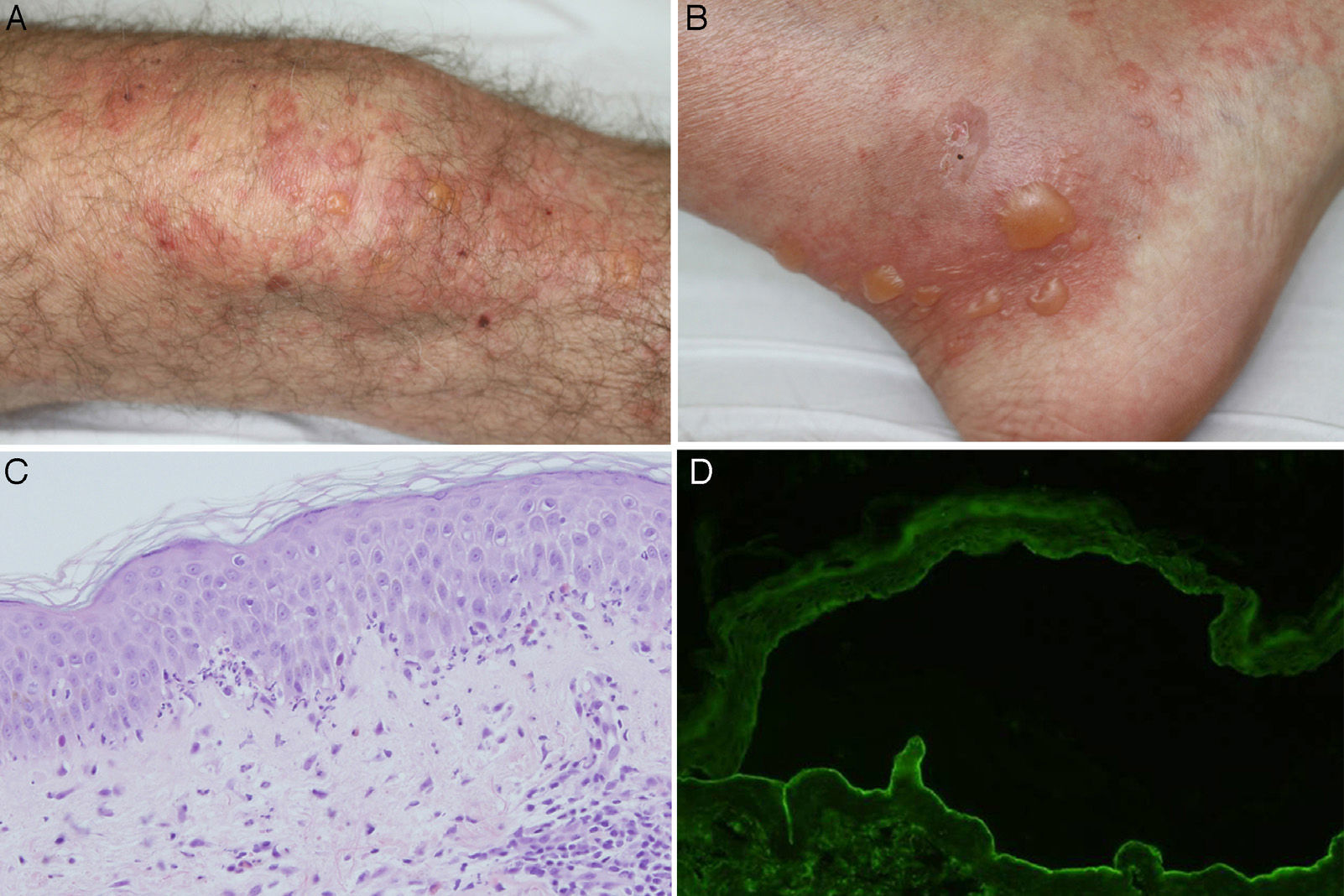
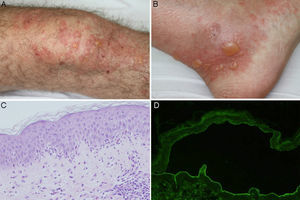
Biopsy of the lesioned skin showed subepidermal bullae and an inflammatory infiltrate composed of neutrophils throughout the basement membrane (BM), with papillary microabscesses of neutrophils and some foci of eosinophilic spongiosis. Direct immunofluorescence (IF) revealed linear deposits of C3 and IgG in the BM. Circulating immunoglobulin (Ig) G antibodies directed against the dermal part of the BM were detected in indirect IF of salt-split skin (sodium chloride 1M) (Figure 1). Anti-BP180 and anti-collagen vii antibodies were negative with enzyme-linked immunosorbent assay (ELISA). An immunoblot of extracts of human skin, performed according to previously described methods, detected IgG antibodies directed against a 200kDa protein (Figure 2).3 Treatment was initiated with topical clobetasol according to a regimen for moderate to severe BP,4 and the prednisone dose (10mg/d) was maintained for arteritis, with good response. The patient had a new episode of blistering on reducing the dose of oral corticosteroids to 5mg/d. The prednisone dose was increased to 20mg/d and dapsone 50mg/d was added. Clinical improvement was seen. Finally, the patient remained free of lesions with dapsone 75mg/d and prednisone 7.5mg/d.
A and B, Tense bullae on an urticarial base located on the knee and ankle. C, Neutrophilic infiltrate with some eosinophils throughout the basement membrane, with formation of papillary microabscesses (hematoxylin-eosin ×200). D, Presence of IgG class antibodies directed against the dermal side of the blister in indirect immunofluorescence of salt-split skin (sodium chloride 1M) (1/40 antibody dilution).
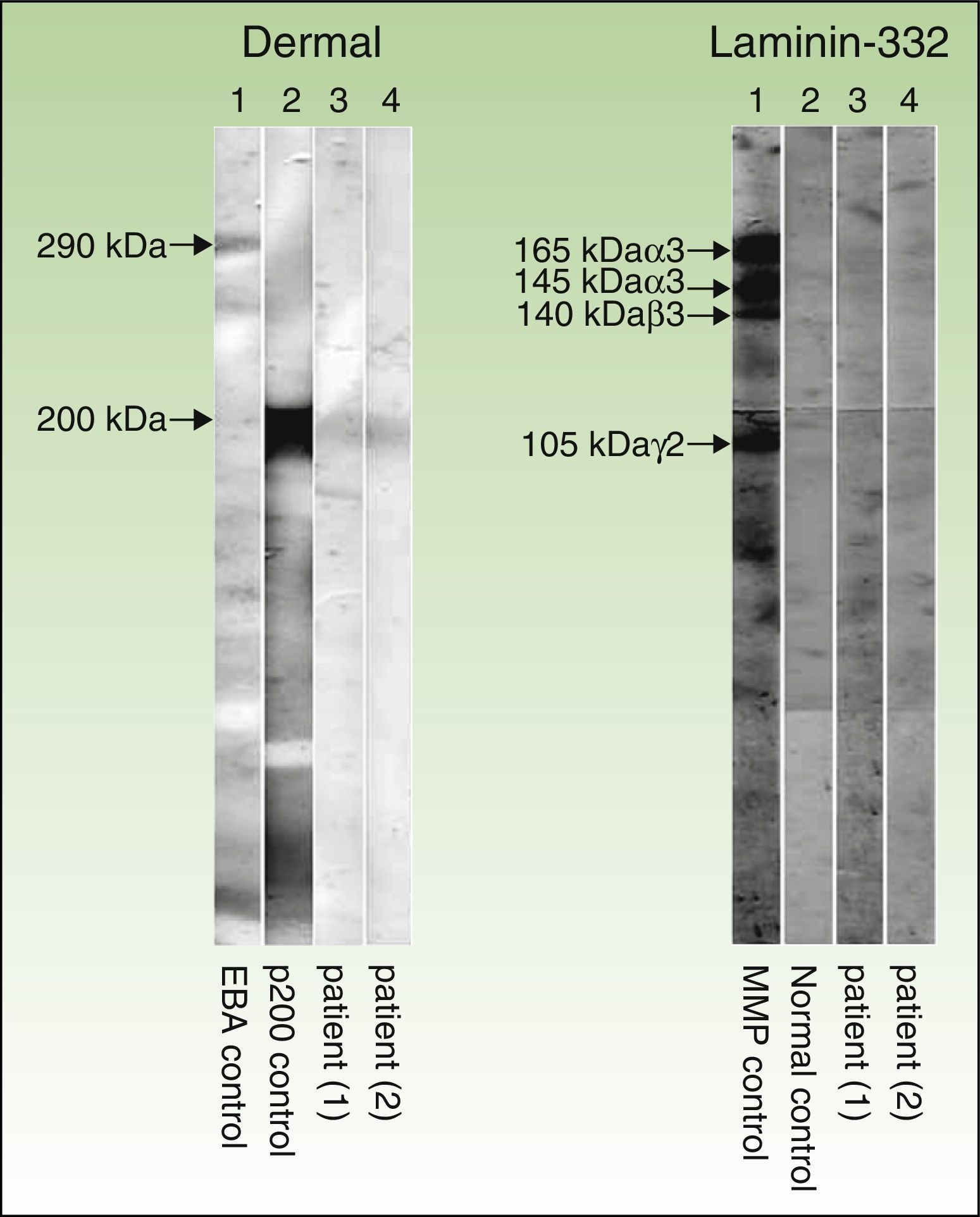
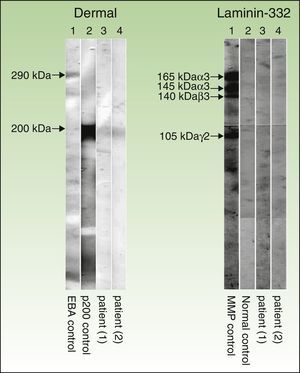
Immunoblot technique performed with dermal extracts of human skin, obtained by splitting skin with ethylenediamine tetraacetic acid and submitting to electrophoresis on SDS-polyacrylamide gel, as per the Laemmli method (left) as well as immunoblotting with recombinant laminin 332 (right). As shown in the left panel (dermal extracts of human skin), sera of patients 1 and 2 (corresponding to columns 3 and 4, respectively) show a band at 200kDa, corresponding to the same band present in the serum of another patient with anti-p200 pemphigoid (column 2). This band is not present in the serum of a patient with epidermolysis bullosa acquisita (column 1), but a band is present at 290kDa, corresponding to collagen vii. In the right panel (recombinant laminin 332), the recombinant protein is not detected in the sera of patients 1 and 2 (columns 3 and 4, respectively) and the serum from healthy control (column 2), whereas the serum of a patient with anti-laminin 332 pemphigoid (column 1) has several bands at 165, 145, 140, and 105 140kDa, corresponding to the α3, β3, and γ2 chains of laminin 332, respectively.
The patient was a 43-year-old man with history of plaque psoriasis who, 18 months earlier, coinciding with a stressful event, had experienced an episode of tense bullae on the face, scalp, genitals, and groin, with no mucosal involvement. He was treated with prednisone at a dose of 0.5mg/kg/d, with remission of the lesions. Subsequently, he presented with a new episode of lesions, for which he received treatment with ciclosporin (without success) in another center. He finally responded to high doses of dapsone (200mg/d).
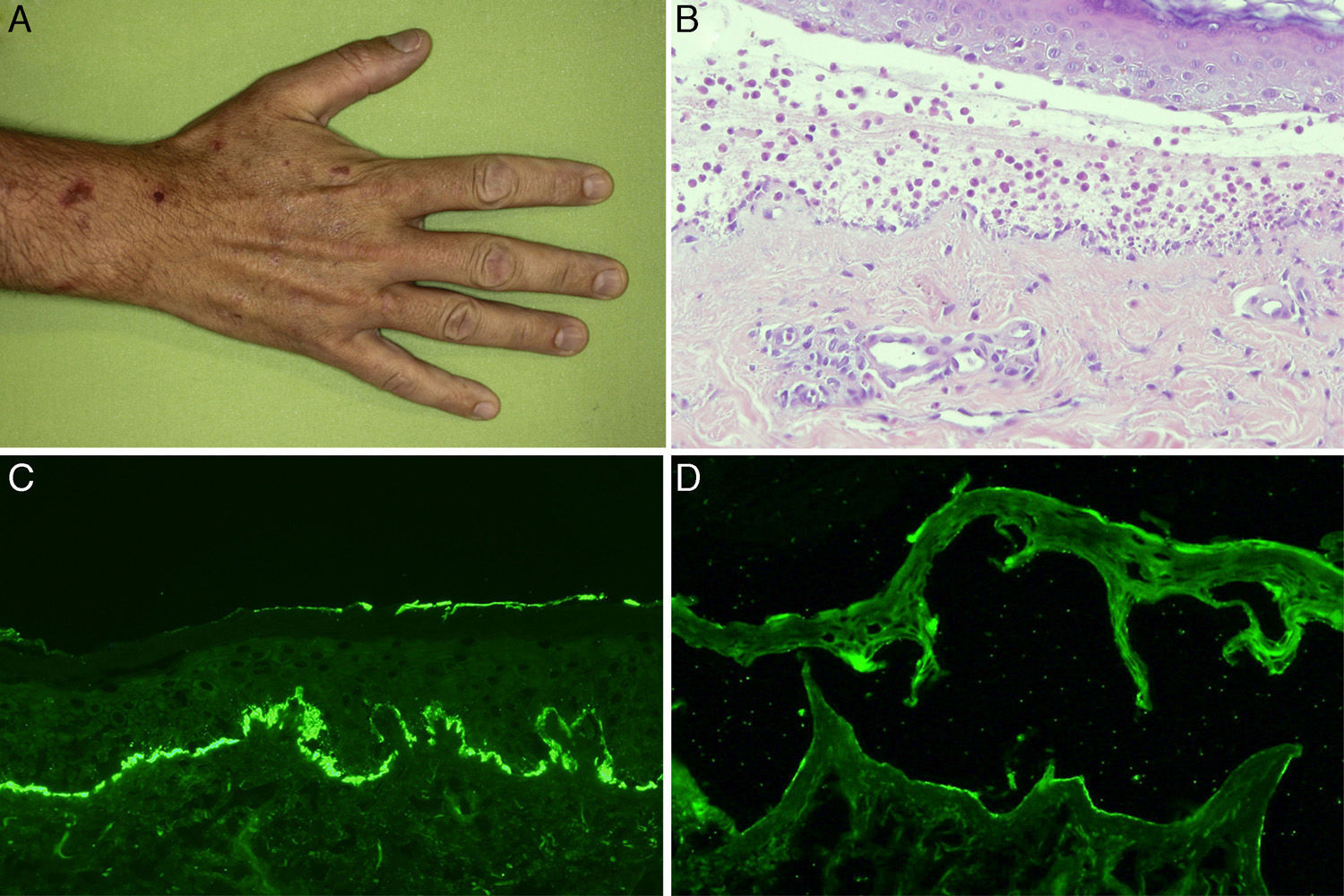
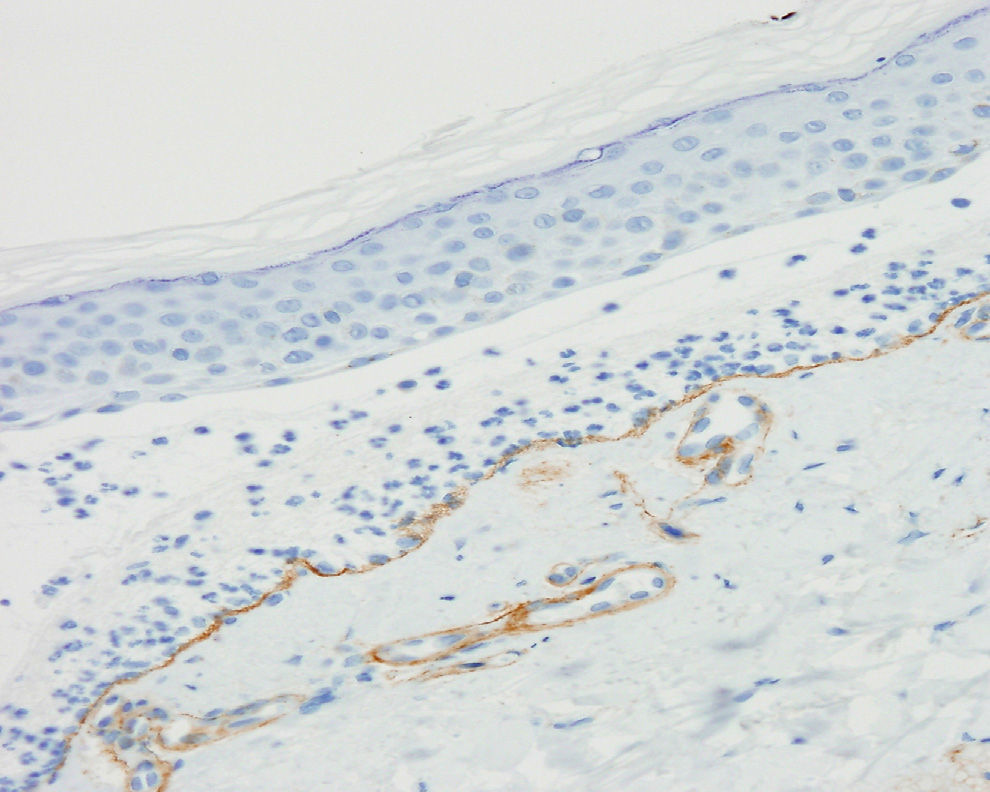
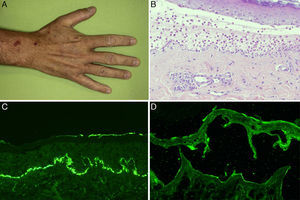
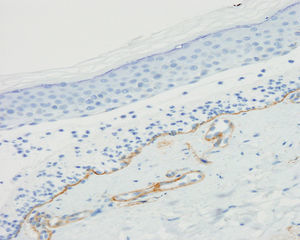
The physical examination on his first visit showed multiple erythematous scaling plaques on the trunk and limbs, corresponding to psoriasis lesions, along with macules and hyperpigmented plaques predominantly on the arms. Skin biopsy showed subepidermal bullae with abundant polymorphonuclear cells and microabscesses of neutrophils in dermal papillae. The results of direct and indirect IF and anti-BP180 and anti-collagen vii ELISA were identical to those described for the first patient (Figure 3). Immunohistochemical study for collagen iv of the paraffin block was also performed, and in contrast to the first patient, intense marking of the blister floor was observed (Figure 4). Immunoblotting confirmed diagnosis of anti-p200 pemphigoid (Figure 2).
A, Macules and hyperpigmented plaques in the hand. B, Subepidermal blister containing abundant polymorphonuclear cells (hematoxylin-eosin, ×200).C, Linear deposit in the basement membrane of C3 in direct immunofluorescence. D, Weak marking of IgG class antibodies directed against the dermal side of the blister in indirect immunofluorescence of salt-split skin (sodium chloride 1M) (antibody dilution 1/10).
Given that the lesions had practically remitted with dapsone 200mg/d and that the patient tolerated treatment poorly due to asthenia, the dose was tapered progressively until discontinuation. During this time, the isolated appearance of blisters resolved with potent topical corticosteroids.
DiscussionAnti-p200 pemphigoid is a recently described blistering disease.1,2,5,6 Patients are usually middle-aged adults (<65years) with tense bullae and generalized urticarial pruriginous plaques, with clinical characteristics similar to BP or the inflammatory form of epidermolysis bullosa acquisita (EBA).2,5,6 Mucosal lesions are observed in approximately 20% of cases.2,5,6 The bullae usually resolve without leaving scars or milia.2,5 A high prevalence of psoriasis has been reported.2,5–7 Some cases have been associated with drugs (penicillin) or psoralen and ultraviolet A radiation phototherapy.6,8
Characteristic histopathological findings include presence of subepidermal blisters, accompanied by an inflammatory infiltrate in the superficial dermis, which is usually neutrophilic and less often eosinophilic.2,5,6,9 Occasionally, microabscesses of neutrophils in dermal papillae and neutrophilic or eosinophilic spongiosis can be observed.2,5,6,9
Direct immunofluorescence (IF) reveals linear deposits of C3 and IgG in the BM. Indirect IF of salt-split skin (sodium chloride 1M) shows circulating IgG antibodies directed against the dermal side of the blister, although deposits have occasionally been observed on both sides.1,2,5,6,9
Diagnosis is established with an immunoblot of extracts of human skin, in which the serum of patients reacts to a 200kDa protein.1,2,5 In 25% of cases, weaker reactivity has been observed with other antigens, such as BP180, BP230, or laminin 332. This observation may be explained by intermolecular expansion of epitopes.2,5,6,10 Immunoblotting is a complex technique that is available in only a few laboratories. This has probably limited the diagnosis in some cases of anti-p200 pemphigoid.
Immunohistochemical study of the components of the BM in biopsies of blisters recently fixed in paraffin can usually locate collagen iv (which labels the dense lamina) on the dermal side of the blister, thereby assisting diagnosis if immunoblotting is not available.9
Currently, large gaps still remain in our knowledge of the pathogenesis of anti-p200 pemphigoid.2 The p200 antigen is a noncollagen protein localized at the interface between the lamina lucida and the lamina densa of the BM. Recently, laminin gamma-1 has been identified as the autoantigen in 90% of cases, with an epitope localized in residue 246 of the carboxy terminal domain.5,7,11 However, all attempts to demonstrate the pathogenicity of antibodies directed against the carboxy terminal domain of laminin gamma-1 have failed.12–14
Differential diagnosis should include other subepidermal blistering diseases with C3 deposits and/or linear IgG in direct IF, mainly BP and EBA.2,5 Indirect IF of salt-split skin (or direct IF of split skin when circulating antibodies are not detected) enables us to distinguish the entity from BP but not from EBA. In these cases, immunohistochemistry for collagen iv is a simple technique that enables us to differentiate anti-p200 pemphigoid from EBA: collagen iv would be present in the blister floor in the former case and in the roof of the blister in the latter case. However, this technique may not be informative in cases of intense inflammatory infiltrate.9 These findings are not pathognomic and to establish an unequivocal diagnosis, immunoblotting or even more complex techniques such as immunoprecipitation are required.2,5,7
With regard to treatment, the regimens proposed for BP are as follows: potent topical corticosteroids, prednisone 0.5mg/kg as monotherapy or in combination with dapsone (1.5mg/kg).2,5,15 The outcome after treatment is variable, but response is usually rapid and favorable to immunosuppressants.2,5
In conclusion, we present the first 2 cases of anti-p200 pemphigoid described in Spain and have characterized the lesions clinically, pathologically, and immunologically. Given the difficult techniques required for diagnosis, it is likely that anti-p200 pemphigoid is underdiagnosed, with cases erroneously classified as BP or EBA. Differential diagnosis with respect to EBA is particularly important as both entities differ substantially in terms of management and prognosis. We propose immunohistochemistry with collagen iv of lesioned skin, combining it with the usual IF techniques, as a simple and accessible tool for differential diagnosis with EAA.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed their hospital's protocol on the publication of data concerning patients.
Right to privacy and informed consentThe authors obtained the informed consent of patients and/or subjects mentioned in this article. The informed consent form is located in the archives of the corresponding author.
FundingThe study was partially funded with Research Project PI 09/1410 (J. Herrero), with cofunding from FEDER.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We would like to express our gratitude to Dr. María Teresa Fernández Figueras and Dr. Josep Palou Aymerich, for their valuable collaboration by donating blocks of paraffin from patients for new sections to enable immunohistochemical study with collagen iv.
Please cite this article as: García-Díez I, Martínez-Escala ME, Ishii N, Hashimoto T, Galy JMM, Pujol RM, et al. Descripción de 2 casos de penfigoide anti-p200. Utilidad de una técnica inmunohistoquímica sencilla en el diagnóstico diferencial con otras enfermedades ampollosas autoinmunes. Actas Dermosifiliogr. 2017;108:e1–e5.