Dermatofibrosarcoma protuberans is a locally aggressive skin tumor that affects young and middle-aged adults. A number of histological variants have been described, the myxoid type being one of the least common. Microscopically it is formed of a neoplastic growth that is located in the dermis and hypodermis and has a predominant myxoid component. Peripherally there are infiltrating bundles of spindle-shaped cells that are diffusely positive for the CD34 immunohistochemical marker. We report a case of myxoid dermatofibrosarcoma protuberans on a finger of the left hand of a 14-year-old girl. The tumor had been present for at least 10 years. This is the first pediatric case of myxoid dermatofibrosarcoma protuberans at this site. This histological subtype has mainly been described on the extremities in adults and is very rare in children. We discuss the differential diagnosis with other CD34+ myxoid mesenchymal tumors.
El dermatofibrosarcoma protuberans (DFSP) es un tumor cutáneo, localmente agresivo, que afecta a adultos jóvenes o de edad media. Se han descrito diferentes formas histológicas, siendo la mixoide una de las más infrecuentes. Microscópicamente está constituido por una neoformación que ocupa la dermis e hipodermis, de predominio mixoide, con áreas periféricas conformadas por haces de células fusiformes, de crecimiento infiltrativo, que expresan el marcador inmunohistoquímico CD34 de forma difusa. Presentamos por primera vez un DFSP mixoide en un dedo de la mano izquierda de una niña de 14 años, de más de 10 años de evolución. Se trata del primer caso infantil de DFSP mixoide en dicha localización. Este subtipo histológico se ha descrito fundamentalmente en las extremidades de adultos, siendo excepcional en niños. Se comenta el diagnóstico diferencial con otros tumores mesenquimales mixoides CD34 positivos.
Dermatofibrosarcoma protuberans is a mesenchymal tumor with fibroblast and myofibroblast differentiation that occurs in the dermis and subcutaneous tissue of young or middle-aged adults.1,2 Incidence peaks in individuals in their 30s.3 The tumor grows slowly, is locally aggressive and of intermediate malignancy, and recurs in more than a third of cases. Metastasis is rare but spread is usually hematogenous when it does occur. Between 10% and 15% of recurrences are found in areas of fibrosarcoma, neurofibrosarcoma, or myxofibrosarcoma.3–5
Different types of dermatofibrosarcoma protuberans have been described, including the classic, pigmented (Bernard tumor), fibrosarcomatous, granular cell, flat atrophic, and myxoid variants, as well as variants with myogenic differentiation.1,2 The fibrosarcomatous subtype is associated with a worse prognosis, a risk of metastasis of 10% to 15%, and a rate of tumor-related death of 5.8%.6,7 One of the least common types is the myxoid variant, which presents on the limbs, head, neck, and trunk in adults. It is very rare in children and only 3 cases have been reported in the literature.3 We present the first report of myxoid dermatofibrosarcoma protuberans on the finger of 14-year-old girl.
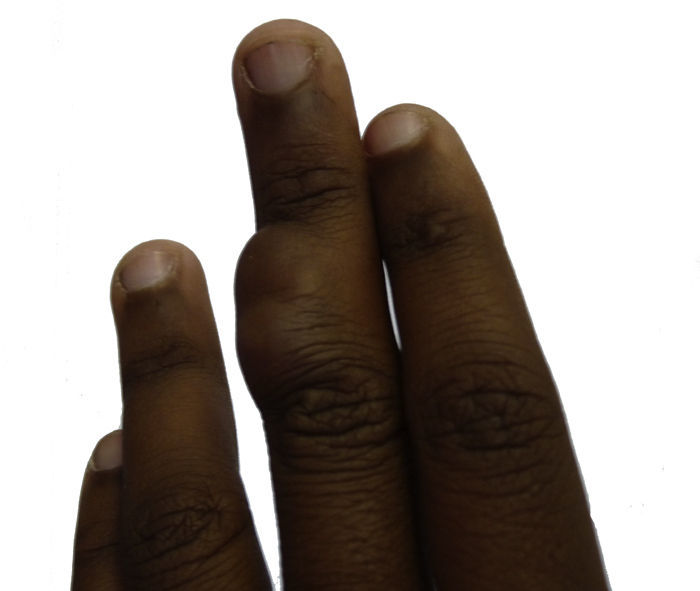
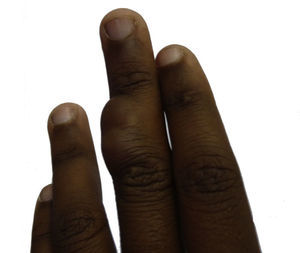
Case DescriptionThe 14-year-old patient attended the dermatology department of our hospital with a lobulated mass measuring 2.5cm across at the widest point. The tumor was on the dorsal aspect of the middle phalanx of the middle finger of the left hand (Fig. 1). According to the patient, the lesion had been present for 10 years. It had been growing slowly and progressively but had become painful in the last few months prior to the visit. It was impossible to ascertain whether the lesion had been present from birth. Physical examination showed that the lesion had a variable consistency and extended to the deep layers. It did not move with the tendons and did not hinder movement of the patient's finger.
An initial incisional biopsy was taken and, after histopathologic diagnosis, it was decided to fully excise the lesion. Simple surgical excision was performed with primary wound closure. The excision extended down to the tendon and margins exceeded 1cm. During the operation, the tumor appeared to be of variable consistency, with soft areas of myxoid appearance and no invasion of the underlying tendons. Six months after excision, there had been no recurrence.
Histopathologic StudyThe material from the incisional biopsy and the surgical excision were both fixed in 10% buffered formalin and embedded in their entirety in paraffin. Sections 3μm thick were taken and stained with hematoxylin-eosin. Subsequently, immunohistochemical staining was performed with the following antibodies using the avidin-biotin complex method and with positive and negative controls: CD34 (DAKO, 1:50), protein S-100 (DAKO, 1:5000), factor XIIIa (Behring, 1:1000), desmin (DAKO, 1:100), actin 1A4 (DAKO, 1:50), Ki-67 (DAKO, 1:300), epithelial membrane antigen (EMA) (DAKO, 1:100), and B-cell lymphoma (bcl-2) (DAKO, 1:80). A fluorescence in situ hybridization (FISH) analysis was performed on the paraffin-embedded tumor tissue to detect the translocation t(17;22)(q22;q13).
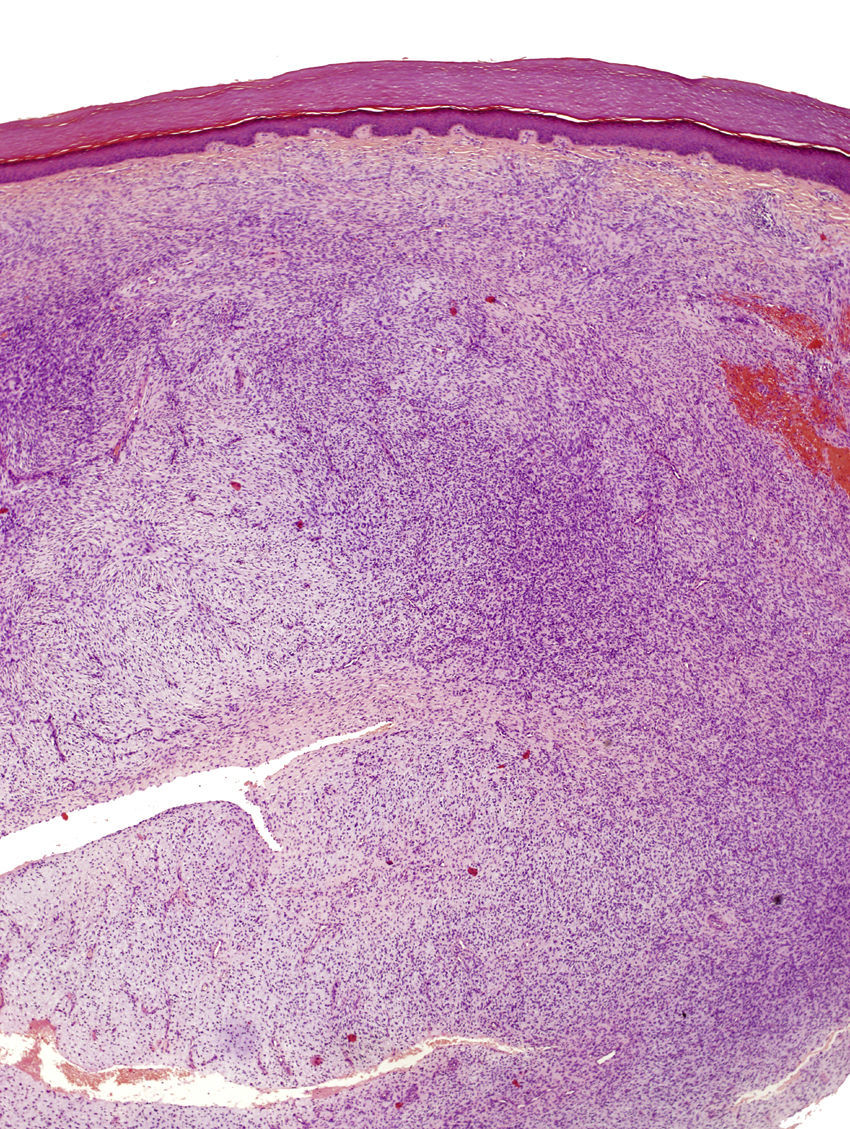
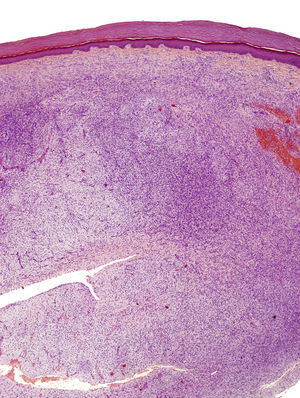
The incisional biopsy showed diffuse infiltration of the reticular dermis by a neoformation consisting of short bundles of spindle cells interlaced in different directions and an evident Grenz zone. The tumor engulfed the skin appendages but neither invaded nor destroyed them (Fig. 2). The proliferative cells had an eosinophilic cytoplasm with blurred edges and elongated oval nuclei showing fine chromatin and no nucleoli. Only 2 nonatypical mitotic figures were identified per 10 high-magnification fields. Cellularity was less marked in the deep part of the lesion, which had stromal areas with a myxoid appearance.
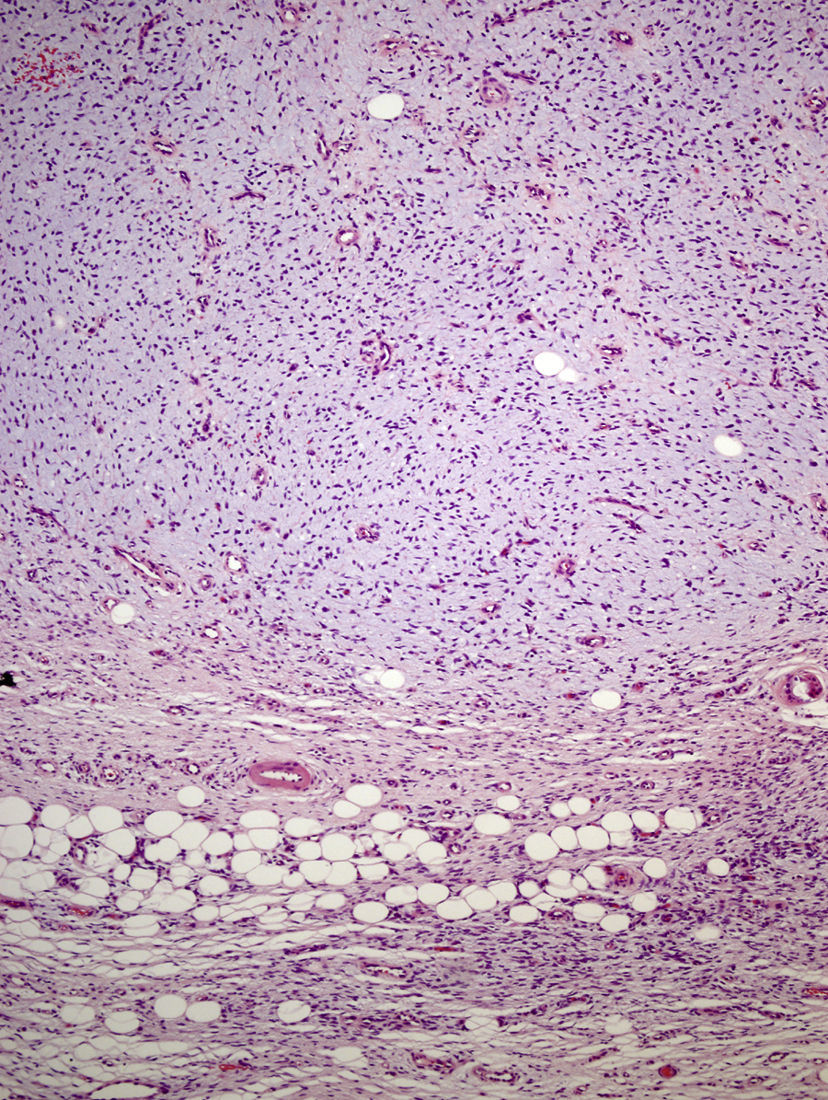
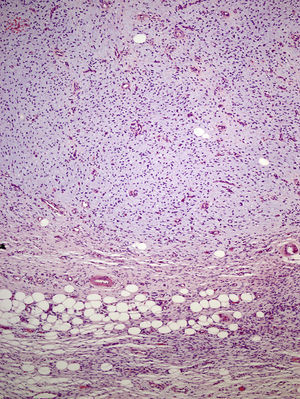
The excised tissue consisted of an ellipse of skin with a nodule measuring 2.4cm along the longest axis. Histologically, it comprised a mesenchymal neoformation with a diffuse growth pattern and infiltrative borders. The tumor occupied the entire reticular dermis and extended to the subcutaneous cell tissue. It displayed a honeycomb pattern, in which individual adipocytes were isolated (Fig. 3). However, 90% of the neoformation comprised hypocellular areas with a myxoid appearance. The cells that were present were fusiform and stellate, without atypia or mitosis. Thin-walled capillaries were seen in abundance and inflammatory cells were few. Focally, a dense fibrous stroma was present. At the edge of the lesion, both in the superficial and deep layers, there were focal areas with the same characteristics as those seen in the incisional biopsy material. The surgical margins at the sides and bottom were extensively involved.
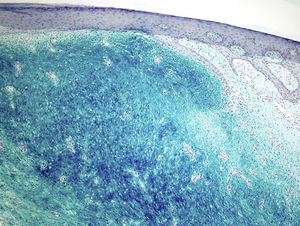
Staining with colloidal iron was observed (Fig. 4). On immunohistochemical staining, cytoplasmic expression of CD34 was strong in the areas of the lesion with a classic pattern but weak in the myxoid areas. The other markers used—protein S-100, factor XIIIa, actin 1A4, EMA, and bcl-2—were negative. The Ki-67 cell proliferation index was 5% in areas with the classic pattern and 20% in myxoid areas. Diagnosis of dermatofibrosarcoma protuberans was confirmed on demonstrating translocation t(17;22)(q22;q13) by FISH.
DiscussionThe myxoid subtype is a very rare dermatofibrosarcoma protuberans variant first described in 1983 by Frierson et al.9 In one of the largest published series of dermatofibrosarcoma protuberans, only 4.3% of the lesions were of this type.3 Myxoid lesions differ from the classic variant by presenting mainly on the limbs; the next most frequent sites are the head and neck, followed by the trunk and the anogenital region.3 Myxoid dermatofibrosarcoma protuberans is very rare in children. Only 3 childhood cases have been reported; these occurred on the eyelid, scalp, and elbow, and all were in boys.3,4 We report the first case of myxoid dermatofibrosarcoma protuberans on the finger of a girl.
Clinically, the lesion presented as a nodular dermal or subcutaneous, firm, raised mass that was slow-growing and progressive.3 The most common potential diagnoses for such a lesion are epidermal, mucinous, or synovial cyst; lipoma; pyogenic granuloma; and lymphoma.3 In view of the site, our case was initially considered to be a giant-cell tumor of the tendon sheath.
Some authors suggest that the myxoid variant has a slightly better prognosis than the classic dermatofibrosarcoma protuberans variant, although others disagree.1,3 No metastases were reported in a recent series of 23 cases of myxoid dermatofibrosarcoma protuberans. In that same series, 2 patients had local recurrence, one after incomplete resection and the other after leaving margins less than 2mm.3 Metastases have not been reported in children.4 In our patient, the lesion had grown slowly and progressively for more than 10 years. There was no recurrence or metastasis 6 months after excision.
Microscopically, the tumor is located in the reticular dermis and subcutaneous cell tissue and shows a Grenz zone, although it occasionally affects the epidermis and forms an ulcer.5 The areas with myxoid, basophilic, or pale stroma predominate, due to the high hyaluronan content, as indicated by staining with colloidal iron. It is likely that the myxoid transformation is due to an increased synthesis of glycosaminoglycans, which inhibit polymerization of the collagen.8 It is not uncommon for traditional dermatofibrosarcoma protuberans to have focal areas with a myxoid appearance, particularly in recurrence, although the presence of such areas does not necessarily point to a myxoid variant. The myxoid subtype is diagnosed when more than 50% of the stroma of the lesion is myxoid.3,8 In the present case, 70% of the tumor was myxoid. A myxoid stroma has few nonatypical cells. These cells have a fusiform and stellate morphology, with mildly eosinophilic or pale cytoplasm, undefined borders, oval nuclei with fine chromatin and no nucleoli, and occasional or no mitotic figures.1,5 In addition, the tumor contains randomly distributed fine-walled capillaries, which rarely contain mast cells. As in a Bernard tumor, pigmented dendritic cells can also be identified.3,5,9 One of the keys to diagnosis is the presence of areas with the characteristic features of classic dermatofibrosarcoma protuberans at the edge of the lesions; these features are spindle cells arranged in interlaced strands with scant collagen between them and wagon-wheel areas. The lesion engulfs skin appendages without invading them, and on invading subcutaneous adipose tissue, a characteristic honeycomb pattern is formed when individual adipocytes are isolated.1,3,5,8,9 In our case, 30% of the tumor had the features of the classic variant and there were areas at the edge of the lesion in which invasion of the underlying fatty tissue had occurred.
The most specific immunohistochemical marker is CD34, for which tumors show strong diffuse staining in 50% to 100% of cases; the sensitivity of CD34 positivity is 84% to 100%. In myxoid and fibrosarcomatous areas, staining is weak and focal, or negative.3,5,6 In our case, the lesion showed diffuse positive staining which was stronger in areas of low cellularity. In addition, these tumors are positive for vimentin and CD99. Some are focally positive for factor XIIIa.5 EMA expression might occur in myxoid areas, possibly indicating perineural differentiation,10 but S-100, actin, and desmin are not expressed.5 Ki-67 cell proliferation indices have been reported to be greater in myxoid areas (19.8%) and fibrosarcomatous areas (11.8%) than in areas with classic histology (2.2%).11 In fact, in our case, the Ki-67 proliferative index in myxoid areas was 20% and as low as 5% in the areas of highest cellularity.
Dermatofibrosarcoma protuberans is associated with reciprocal translocation t(17;22)(q22;q13) or a supernumerary ring chromosome r(17;22). Both give rise to a chimeric gene COL1A1-PDGFB through fusion of the collagen type 1α gene on chromosome 17 and the platelet growth factor-β chain gene on chromosome 22. This leads to continual stimulation of cell growth.1,6 Translocation is found most frequently in children, whereas the ring chromosome predominates in adults, possibly indicating that this change is a late event in the pathogenesis of dermatofibrosarcoma protuberans.4 This genetic abnormality has also been found in the myxoid variant and in giant-cell fibroblastoma.5 Currently, treatment of dermatofibrosarcoma protuberans with selective tyrosine kinase inhibitors is reserved for unresectable disease, recurrences, or metastatic disease.6 A positive immunoreaction with the dermatofibrosarcoma protuberans receptor (PDGFR) B antibody indicates constitutional activation of PDGFR, thereby providing an alternative indirect method for confirming the presence of the dysregulated PDGF gene involved in this translocation.6
Faced with a cutaneous/subcutaneous lesion with predominantly myxoid areas and staining for CD34, the pathologist should consider differential diagnosis with benign and malignant mesenchymal lesions with different biological behaviors and different treatment approaches,1 especially when only superficial or small biopsy samples are available. The presence of classic areas of dermatofibrosarcoma protuberans, even if focal, is usually of great help in the diagnosis.3 In the present case, incisional biopsy showed classic areas of dermatofibrosarcoma protuberans, which helped the initial diagnosis. However, this diagnosis had to be reconsidered when the excised specimen was found to be predominantly of myxoid type.
The main differential diagnosis in this case is superficial acral fibromyxoma, which usually affects patients between 14 and 72 years of age and grows for between 3 months and 30 years as a solitary mass that reaches a size of 0.6 to 5 cm across. Acral fibromyxoma has a predilection for fingers and toes and tends to involve the nails. Recurrences are frequent after partial resections.12 Both tumors are located in the dermis and subcutaneous tissue and are composed of stellate and spindle cells, have a fascicular growth pattern which can either be random or form a wagon-wheel pattern in a myxoid or collagenous matrix. Both tumors have moderate vascularization, slight atypia, and limited mytosis. Both also express CD34, EMA, and CD99, and are negative for S-100, actin, desmin, keratin, and HMB-45.5,12 Clinically, our case appears to correspond more to a superficial acral fibromyxoma. However, subcutaneous tissue infiltration with a honeycomb pattern, the presence of translocation t(17;22)(q22;q13) in the FISH analysis, and the absence of giant multinucleated cells that are present in superficial acral fibromyxoma ruled out this possibility.5,12
Other tumors such as cellular digital fibroma can be readily ruled out because clinically they present as a papule and not as a lobulated mass and are rare in children, and a predominantly myxoid histology has not been reported.13 Cellular digital fibromas are positive for CD34 and factor XIIIa, whereas our patient's tumor was negative for these antibodies. According to some, a cellular digital fibroma can be thought of as a cellular variant of a superficial acral fibromyxoma.13
Superficial angiomyxoma is another tumor that often recurs locally, although it does not metastasize. It grows slowly and is located in subcutaneous tissue, although it can extend to the dermis. It follows a lobulated growth pattern, however, without the diffuse honeycomb infiltation1 that was present in our case. A superficial angiomyxoma is composed of spindle and stellate cells in an abundant and highly vascularized basophilic stroma. Neutrophils surround thin-walled vascular structures and epithelial elements (epithelial cords or keratin cysts) are observed in up to half the cases; none of these features were observed in our case.1,3 These tumors are also positive for CD34, vimentin, and actin, and negative for desmin, S-100, and factor XIIIa.5 Our lesion was negative for actin.
Giant cell fibroblastoma is a tumor that affects children, with a predilection for boys. Its morphologic spectrum overlaps that of dermatofibrosarcoma protuberans, and the 2 tumors share a histologic, immunohistochemical, and genetic profile. Areas of giant cell fibroblastoma are found in almost 15% of cases of dermatofibrosarcoma protuberans. These mixed tumors affect the dermis and subcutaneous cellular tissue, engulfing the appendages.4,6 The lesions are hypocellular, with loose bundles of wavy spindle cells in an abundant highly-vascularized collagenous or myxoid stroma. They have a characteristic cell population of pleomorphic cells with a single nucleus or giant multinucleated cells (absent in our case), are positive for CD34, and have translocation t(17;22)(q22;q13). They can recur as dermatofibrosarcoma protuberans and vice versa.4
Extraneural spindle cell perineuriomas may have a prominent myxoid stroma, but these tumors have well defined borders (whereas the border in our case was infiltrative) and are composed of spindle cells arranged in a wagon wheel pattern, sheets, or bundles. Half the cases are positive for CD34, but they are also positive for perineural markers such as Glut-1 and EMA.1 The lesion we describe was negative for these markers. Finally, we note that although solitary fibrous tumors are also positive for CD34 and CD99 and can have a prominent myxoid stroma, they are well delimited, express bcl-2, and have a characteristic hemangiopericytoma-like vascular pattern, unlike the lesion in our case.1
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Campos M, et al. Dermatofibrosarcoma protuberans mixoide infantile. Actas Dermosifiliogr.2012;103:422-6.