Dermatofibrosarcoma protuberans (DFSP) is a slow-growing, intermediate-grade fibrohistiocytic tumor characterized by high rates of local recurrence but low metastatic potential. Few cases of congenital forms have been reported, but this is probably because early lesions can go unnoticed or be confused with other entities. There is evidence that recurrence rates are lower with Mohs micrographic surgery (MMS) than with conventional surgery with wide margins.1 Nonetheless, in 3 recently published pediatric series, wide excision was used in the vast majority of patients (41/43).2–4 We present a case of congenital giant-cell fibroblastoma, a histologic subtype of DFSP, which was diagnosed and treated in an 8-year-old boy. In this report, we highlight the advantages of MMS and the use of negative-pressure wound therapy (NPWT) in children with DFSP.
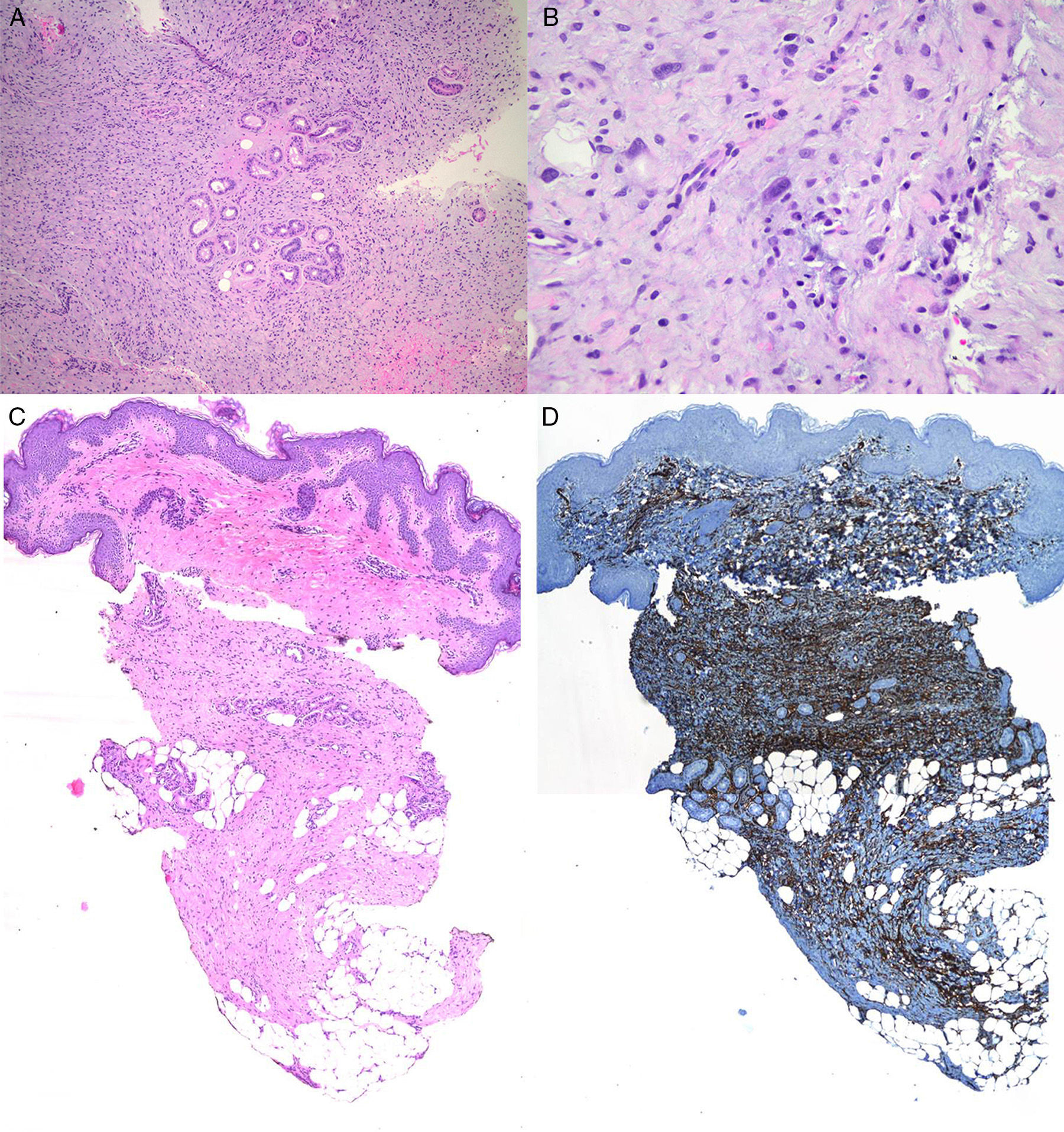
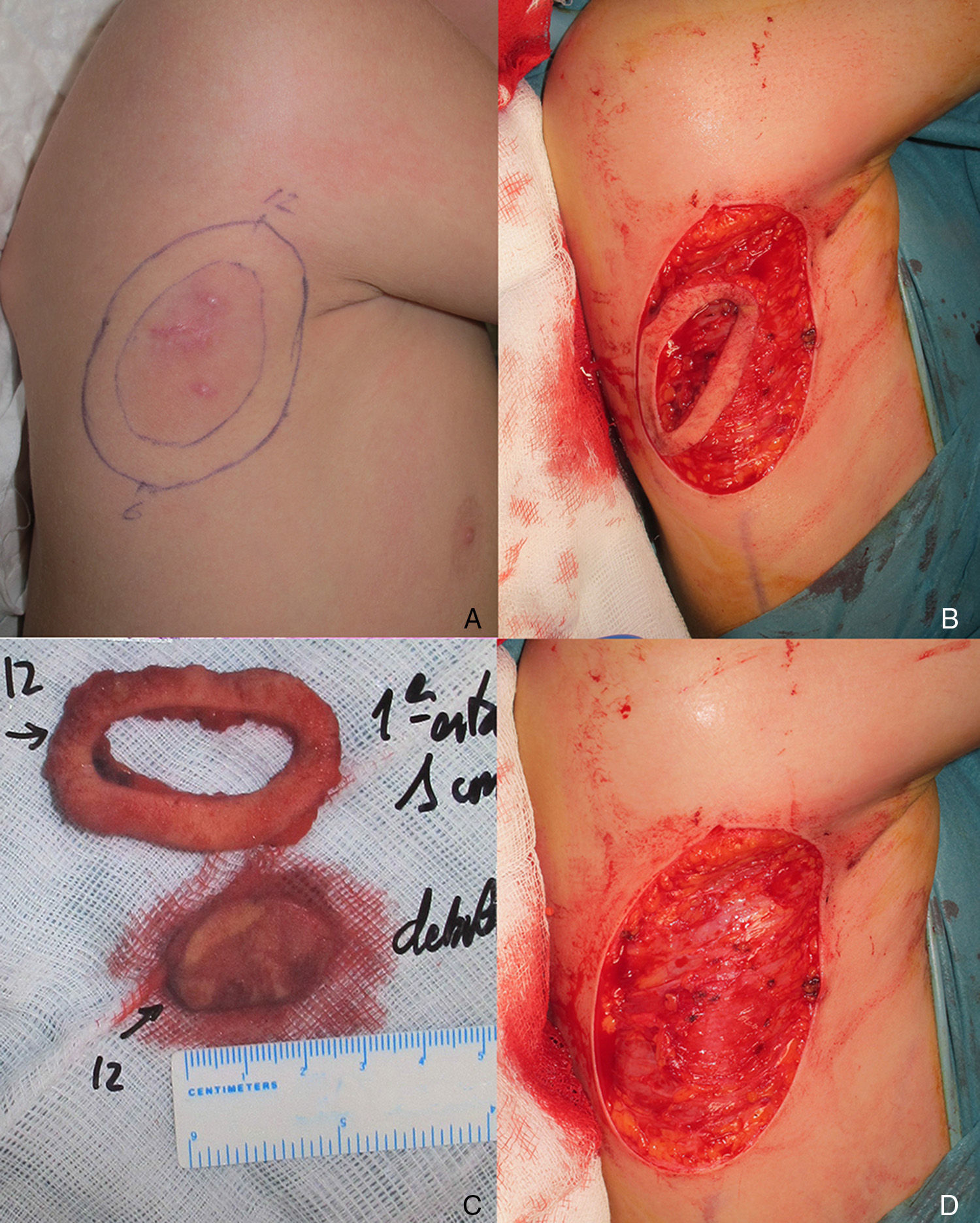
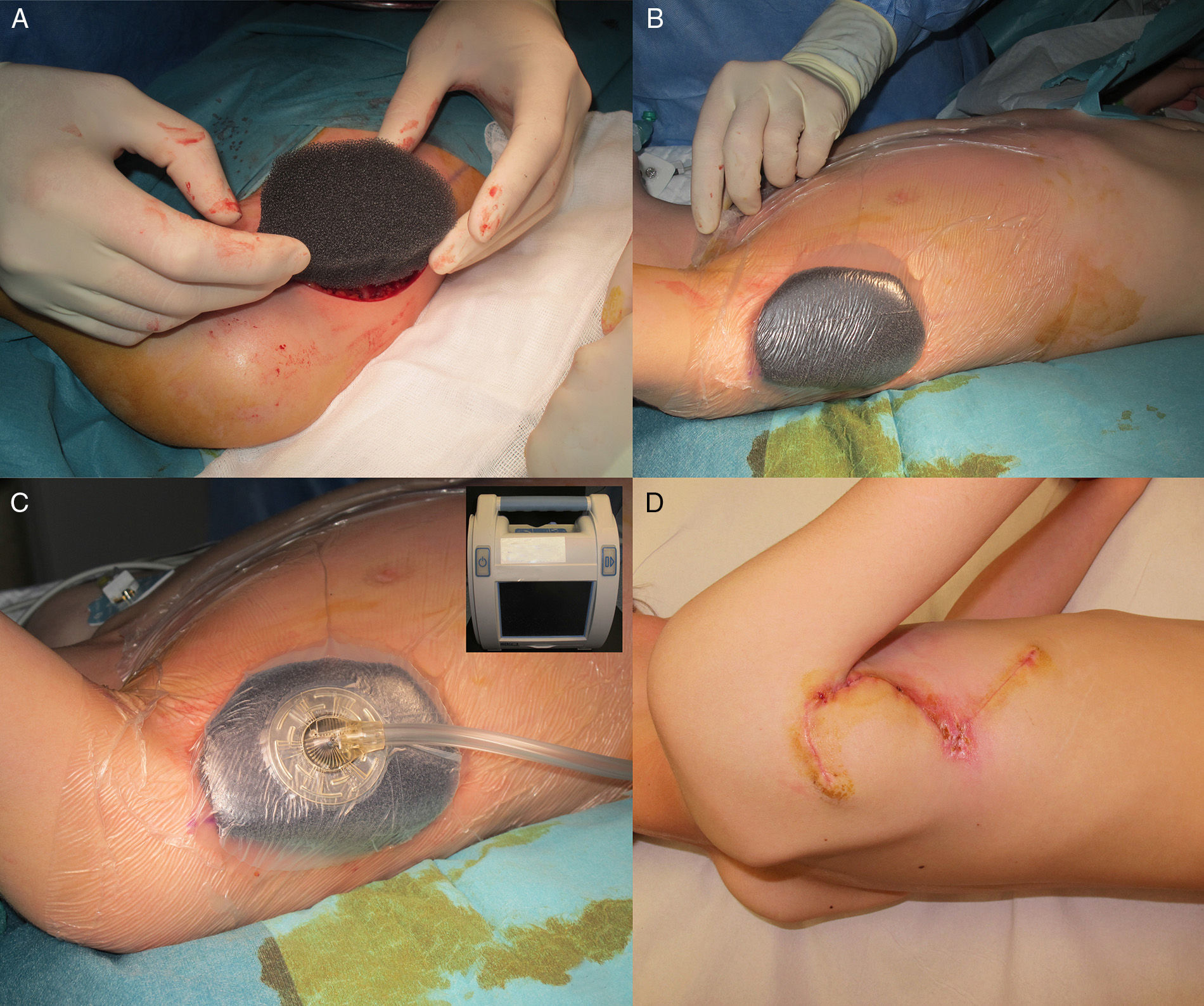
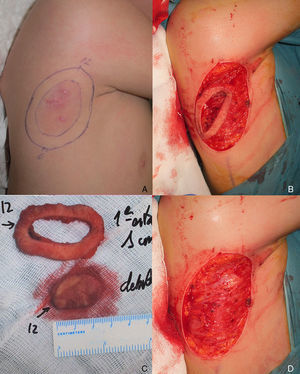
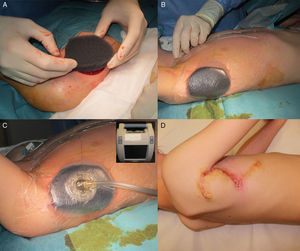
The patient was an 8-year-old boy with no relevant past history who was seen for a lesion in the right axilla that had grown and become progressively harder. The lesion, which was initially flat and reddish in color, had been present since birth. Examination revealed an indurated, mobile plaque measuring 4×2.5cm. The plaque was pink but had a purple, slightly elevated area. The regional lymph nodes were not enlarged. Biopsy showed a poorly delimited tumorous lesion composed of spindle and oval cells that occupied practically the entire dermis and extended down into the subcutaneous tissue. Pseudovascular spaces surrounded by multinucleated giant cells were also visible. The spindle cells expressed CD34 and were negative for S-100 protein and smooth muscle actin (Fig. 1). A diagnosis of giant cell fibroblastoma was established, and extracutaneous invasion was ruled out by magnetic resonance imaging. The tumor was excised under general anesthesia. The technique chosen was slow MMS (Breuninger technique), with excision of 1-cm margins in the first stage (Fig. 2). This technique consists of the en bloc resection of the tumor at a 90° angle to the surface and the achievement of a uniform wound bed. In our case, the depth of excision reached the fascia. The resected specimen was fixed in formol and embedded in paraffin. Because the slides with samples from the deep and peripheral margins were to be reviewed at a later date, it was decided to cover the surgical defect with a NPWT system with a negative continuous pressure of 125 mmHg (Kinetic Concepts Inc.) (Fig. 3 A-C). On day 9, with histologic confirmation of tumor-free margins, the surgical defect was reconstructed using a Limberg flap over the granulation tissue formed during NPWT; no specific preparation was required (Fig. 3D). There have been no recurrences in 4 years of follow-up.
A. Proliferation of spindle cells arranged in fascicles (original magnification, hematoxylin-eosin×100). B, Irregular pseudovascular spaces surrounded by multinucleated giant cells (original magnification, hematoxylin-eosin×100).C, Involvement of full thickness of the dermis with extension into the subcutaneous tissue (original magnification, hematoxylin-eosin×20). D, Spindle cells expressing CD34 (×20).
DFSP is a rare tumor in children, with an estimated incidence of 1 case per 1 000 000 population under the age of 20 years.5 Its prevalence could, however, be underestimated as time to diagnosis ranges from 5 to 15 years. Congenital forms of DFSP are even rarer. In 1 series of 152 cases of childhood DFSP, for example, only 20 tumors were congenital.6
Childhood and adult forms are similar in terms of immunohistochemical characteristics, the translocation of genetic material between chromosomes 17 and 22, and the association with the COL1A1-PDGFB fusion gene. Congenital variants of DFSP present more frequently as a stain or an atrophic plaque than as a tumorous lesion. The differential diagnosis is broad and includes vascular anomalies, aplasia cutis congenita, atrophoderma, myofibromatosis, childhood fibromatosis, and fibrosarcomas.
Compared with adults, in children, tumors are more frequently located on the lower extremities and at acral sites. Other differences in children include lower recurrence rates, very few cases of metastasis, and little use of MMS.6
The treatment of choice for DFSP is surgery,7 with imatinib8 and radiation therapy reserved for recurrent, unresectable, or metastatic tumors. In a report of 74 patients, mostly adults, with DFSP, and a review of the literature, Serra-Guillen et al.1 concluded that MMS was associated with considerably fewer recurrences than conventional surgery with wide margins, and also achieved greater preservation of healthy tissue. The most common MMS technique used is slow MMS, which requires coverage of the surgical defect with biosynthetic skin substitutes or other dressings. NPWT, however, has been proposed as an alternative to skin substitutes for temporary wound coverage.9 This system reduces the need for analgesia and sedation and also requires less frequent wound dressing. In our case, it additionally allowed the patient to walk around and move his arm. Portable vacuum systems can be used on an outpatient basis, but because of the geographic distribution of our community (our hospital is on an island), we were not able to use a portable NPWT system.
In 2014, there were 3 reports of childhood DFSP in hospitals in Taiwan,2 Canada,3 and Spain.4 The series included, respectively, 13, 17, and 13 patients under the age of 18 years, but surprisingly, MMS was used in just 2 of these 43 patients. If MMS has been shown to achieve better therapeutic, functional, and cosmetic results, why is it underused in children with DFSP? Finally, we would like to stress the importance of working closely with multidisciplinary teams to ensure that children with DFSP are diagnosed and treated correctly.
Please cite this article as: Jubert E, del Pozo LJ, Saus C, Martín-Santiago A. ¿Por qué la cirugía micrográfica de Mohs está infrautilizada en el tratamiento del dermatofibrosarcoma protuberans infantil?. Actas Dermosifiliogr. 2016;107:158–162.