In the second part of this review on the deep mycoses, we describe the main systemic mycoses—paracoccidioidomycosis, coccidioidomycosis, histoplasmosis, mucormycosis, and cryptococcosis—and their cutaneous manifestations. Skin lesions are only occasionally seen in deep systemic mycoses either directly, when the skin is the route of entry for the fungus, or indirectly, when the infection has spread from a deeper focus. These cutaneous signs are often the only clue to the presence of a potentially fatal infection. As with the subcutaneous mycoses, early diagnosis and treatment is important, but in this case, even more so.
En la segunda parte de este artículo se revisan las principales micosis sistémicas y sus manifestaciones cutáneas: paracoccidioidomicosis, coccidioidomicosis, histoplasmosis, mucormicosis y criptococosis. Las micosis sistémicas presentan lesiones en la piel solo en algunas ocasiones, ya sea por afectación directa de ella como puerta de entrada o tras la diseminación de la infección a partir de un foco profundo. Muchas veces estos signos cutáneos serán la única pista para el diagnóstico certero de patologías potencialmente fatales. Por lo mismo, y con mucho mayor énfasis que las micosis tratadas en la primera parte, es importante saber reconocer y tratar las micosis sistémicas.
The systemic mycoses are infections caused by fungi that enter the body through an internal organ or a deep focus, such as the lungs, the digestive tract, or the paranasal sinuses. Infections can spread through the blood, causing disseminated disease with frequent skin involvement. There are 2 types of systemic mycoses: opportunistic mycoses (systemic candidiasis, aspergillosis, and systemic mucormycosis) and endemic respiratory infections (histoplasmosis, blastomycosis, coccidioidomycosis, paracoccidioidomycosis, and cryptococcosis). In practice, however, it is difficult to distinguish between the 2 types as they tend to affect predisposed patients. Accordingly, they are generally studied together.
In the second part of this review, we discuss the main deep mycoses that produce cutaneous manifestations during the course of disease.
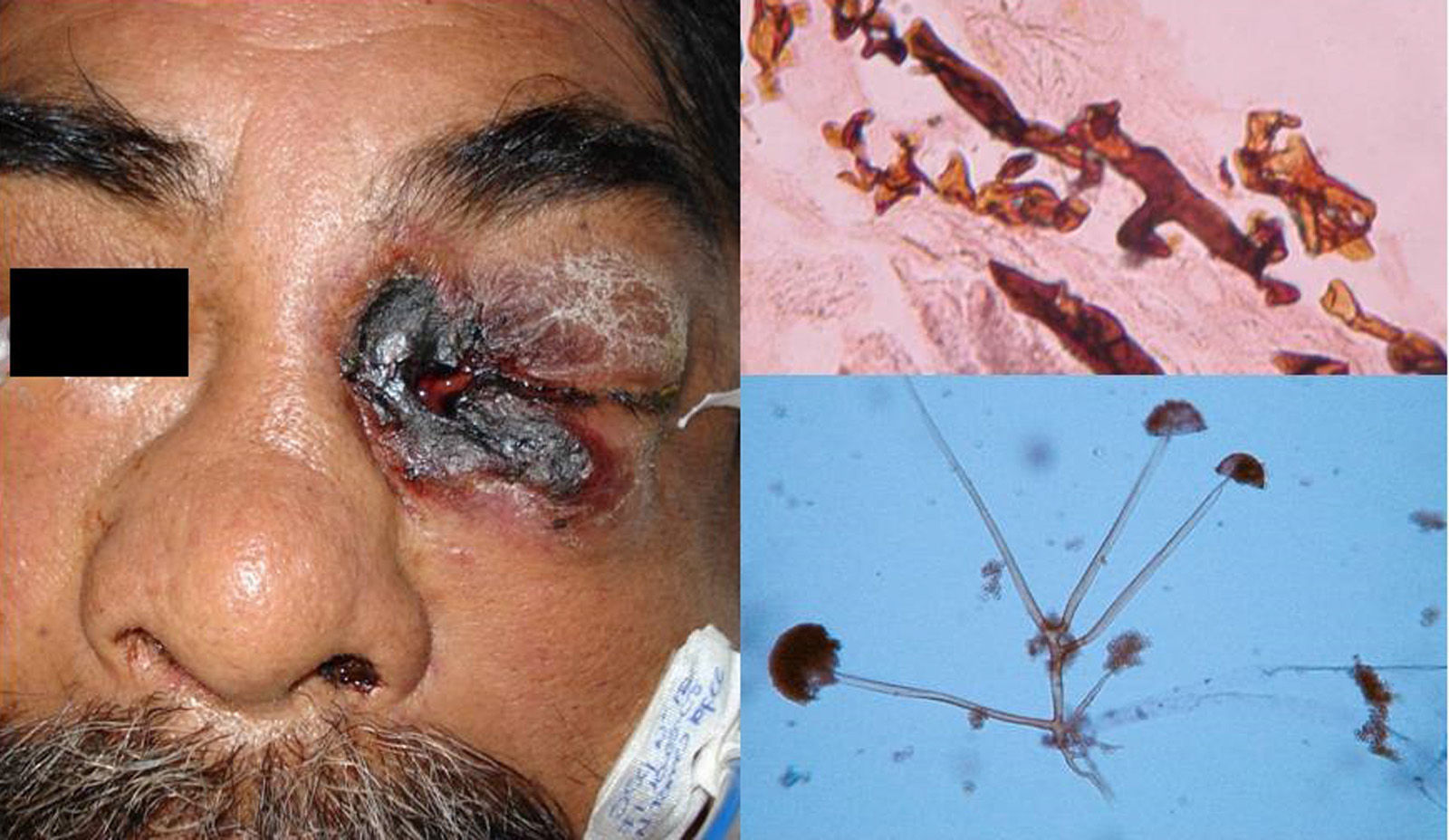
MucormycosisMucormycosis affects the visceral organs in immunosuppressed patients.1,2 It is caused by mucoraceous Zygomycetes and can lead to rhinocerebral, cutaneous, or pulmonary manifestations, or disseminated disease. The most common presentation is rhinocerebral mucormycosis, which involves the nasal sinuses and causes palatal ulcers and extensive necrotic lesions involving the brain and skin. Most cases are associated with decompensated diabetes mellitus or neutropenic states (leukemia).3
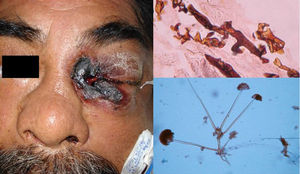
Clinical FormsMucormycosis typically follows an acute, rapidly progressive course associated with high mortality. Rhinocerebral manifestations are the most common clinical presentation, followed by pulmonary, intestinal, and cutaneous manifestations and dissemination.3 Primary cutaneous manifestations are extremely rare and occur mainly at venipuncture sites; they are therefore seen most often on the extremities. Lesions present as papules or nodules that progress to ulcers with a necrotic center and a foul-smelling or purulent exudate. Locally, primary infection can affect the deep tissues (muscle and bone) and is highly destructive.3 Secondary cutaneous manifestations are much more common in rhinocerebral mucormycosis, with 25% of patients developing palatal ulcers and extremely painful eyelid sinuses (Fig. 1).3
DiagnosisDirect mycological examination of necrotic material, sputum, bronchopulmonary lavage fluid, paranasal sinus aspirate, and skin scrapings shows long branching thin-walled cenocytic (nonseptate) hyphae (Fig. 1). Microorganisms grow quickly on Sabouraud dextrose agar (SDA) and can be identified on the basis of reproduction structures or by molecular biology techniques; the most widely used technique is polymerase chain reaction (PCR) analysis of internal transcribed spacer (ITS) regions of ribosomal DNA (rDNA).1,4
TreatmentTreatment is multifactorial and includes control of predisposing factors (ketoacidosis, neutropenia, etc.), systemic antifungals, and aggressive surgical debridement.4 Potassium iodide, ketoconazole, and fluconazole are used for subcutaneous lesions and can be combined with trimethoprim-sulfametoxazole.1 The antifungal of choice is amphotericin B and it can be used in association with caspofungin or posaconazole.
ParacoccidioidomycosisParacoccidioidomycosis is a chronic, subacute, or, in rare cases, acute mycosis caused by Paracoccidioides brasiliensis and Paracoccidioides lutzii. It affects the skin and visceral organs.5 This potentially fatal systemic granulomatous disease is considered endemic in Mexico, Argentina, Guyana, Brazil, Venezuela, and Colombia.6Pbrasiliensis is a dimorphic fungus that grows as mycelia in vegetation and soil in humid regions. It is not known whether human-to-human transmission occurs. The fungus enters the body through the respiratory system. Infections acquired through inhalation can remain latent for 1 or 2 decades and reactivation is dependent on immune status.6–8
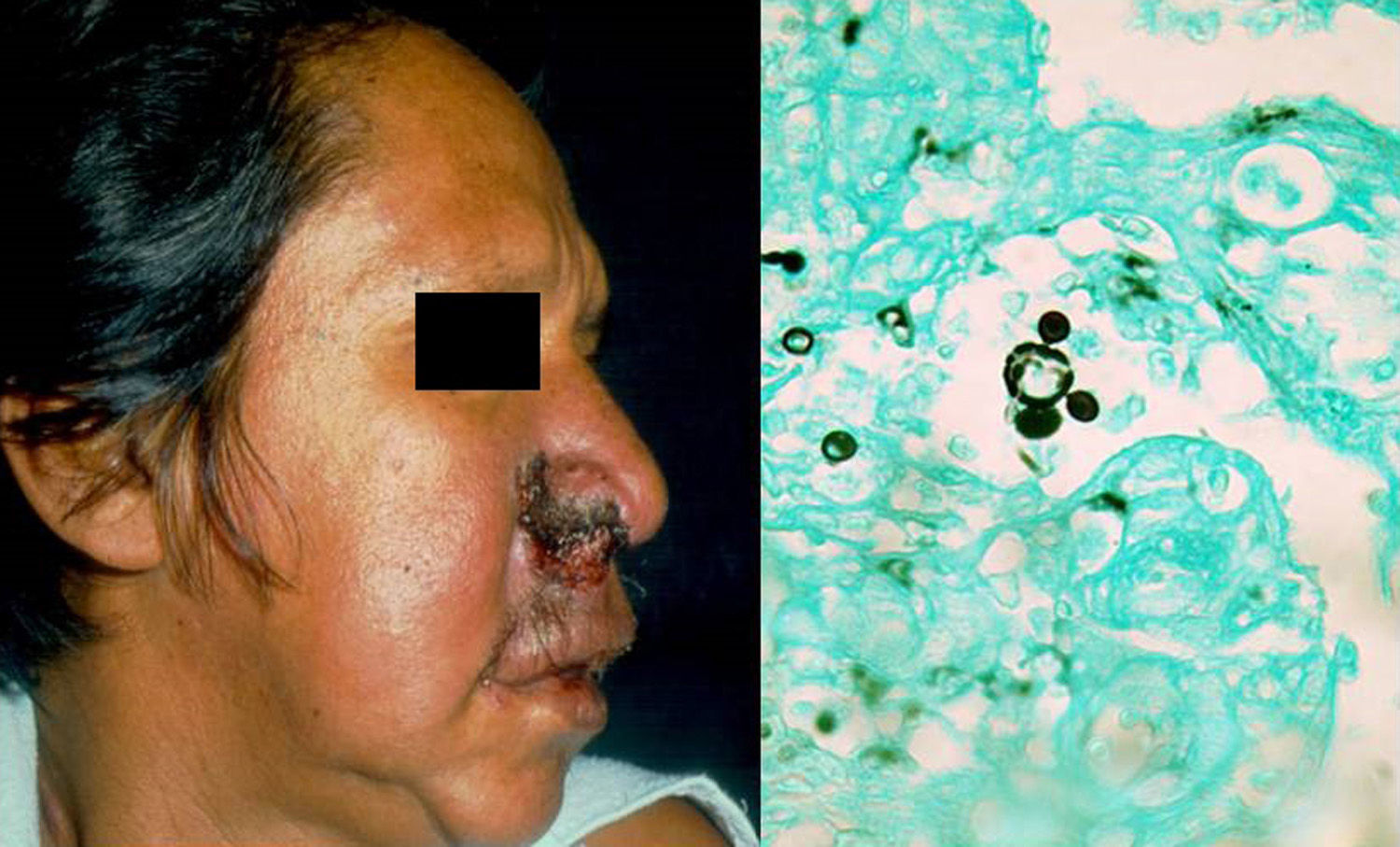
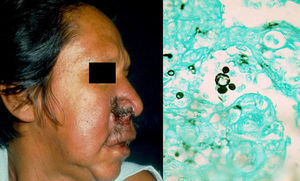
Clinical FormsPrimary infection tends to be asymptomatic and can resolve or leave a residual lesion (scar). Symptomatic disease is a result of the parasite-host relationship in which the virulence of each strain has an important role. Chronic paracoccidioidomycosis, which typically affects adults, is the most common form of disease (>90% of patients develop lung lesions and metastases in diverse organs). There is also an acute juvenile form characterized by pulmonary and reticuloendothelial involvement, with enlarged lymph nodes, hepatosplenomegaly, and bone involvement.6 Patients may also develop lesions in the oropharyngeal mucosa, mimicking chronic tonsillitis, periodontal and laryngeal lesions, and perioral ulcero-vegetative lesions. There may also be nodular cutaneous lesions that can become necrotic or result in subcutaneous cold abscesses (Fig. 2).6 There have been reports of extragenital lesions mimicking syphilis sores.8
The differential diagnosis should include mucocutaneous leishmaniasis, Wegener granulomatosis, syphilis, lymphoma, sporotrichosis, and scrofuloderma, among other entities.
DiagnosisA diagnosis can be made by direct visualization of multiple budding of yeast cells forming a structure similar to a ship's wheel (Fig. 2) in sputum samples, skin scrapings, or pus. The microorganisms grow over a period of 15 to 20 days on SDA with or without antibiotics, producing white filamentous colonies with visible mycelia. PCR analysis of tissue or serum targeting ITS9 or other regions10 can also be used for diagnosis. Skin biopsy shows a suppurative cutaneous granulomatous inflammation and pseudoepitheliomatous hyperplasia.6
TreatmentTreatment includes long-term administration of amphotericinB, systemic triazoles, and sulfonamides.
CoccidioidomycosisCoccidioidomycosis is caused by 2 dimorphic fungi: Coccidioides immitis and Coccidioides posadasii. The reservoir of the fungi is dry soil with an alkaline pH. Coccidioidomycosis affects both humans and animals and the infection is acquired by inhalation of Cimmitis arthroconidia from soil in endemic regions (United States, Mexico, Argentina, Paraguay, Colombia, Venezuela, and Brazil). The incubation period is 1 to 4 weeks.5,11,12
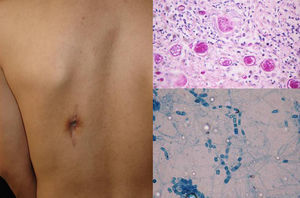
Clinical FormsPulmonary involvement is the predominant clinical form of coccidioidomycosis, but the infection can also affect the skin, larynx, bones, joints, and meninges. Skin lesions are diverse and can present as papules, pustules, plaques, abscesses, sinus tracts (Fig. 3), ulcers, diffuse macular rash, erythema multiforme, or erythema nodosum.11,12
The differential diagnosis should include tuberculosis, paracoccidioidomycosis, sporotrichosis, histoplasmosis, and even neoplasms.
DiagnosisCulture and direct mycological examination show double-membrane spherules containing spores (Fig. 3). The fungi can be cultivated on SDA with or without antibiotics, although the procedure is dangerous due to the high risk of infection. Colonies will show hyaline arthroconidia separated from each other by a disjunctor cell following lysis. Histology is useful as it can detect spherules with endospores (Fig. 3) Serology with specific immunoglobulin (Ig) M antibodies in acute infections is diagnostic only after 4 weeks. Coccidioidin skin tests and complement levels are also important diagnostic aids.11,12 Finally, coccidioidomycosis can be diagnosed by PCR analysis of the 28S region of rDNA.13
TreatmentTreatment consists of deoxycholate or liposomal amphotericin, itraconazole, or fluconazole for 6 to 12 months.12
HistoplasmosisAmerican histoplasmosis or Darling disease is a systemic mycosis that is caused by the dimorphic fungus Histoplasma capsulatum var. capsulatum14 and primarily affects the reticuloendothelial system.5
The lung is the most common site of primary infection. The fungus may subsequently spread to various organs, including the skin.14 Histoplasmosis is the most common pulmonary fungal infection and it occurs worldwide, with cases reported in over 60 countries. The pathogen is particularly prevalent in regions with tropical climates, such as Central and South America, Eastern United States, and South Mexico. It is found in soil, decomposing organic matter, and in the droppings of bats (typically in caves)15 and some birds such as chickens, turkeys, pigeons, and geese.16
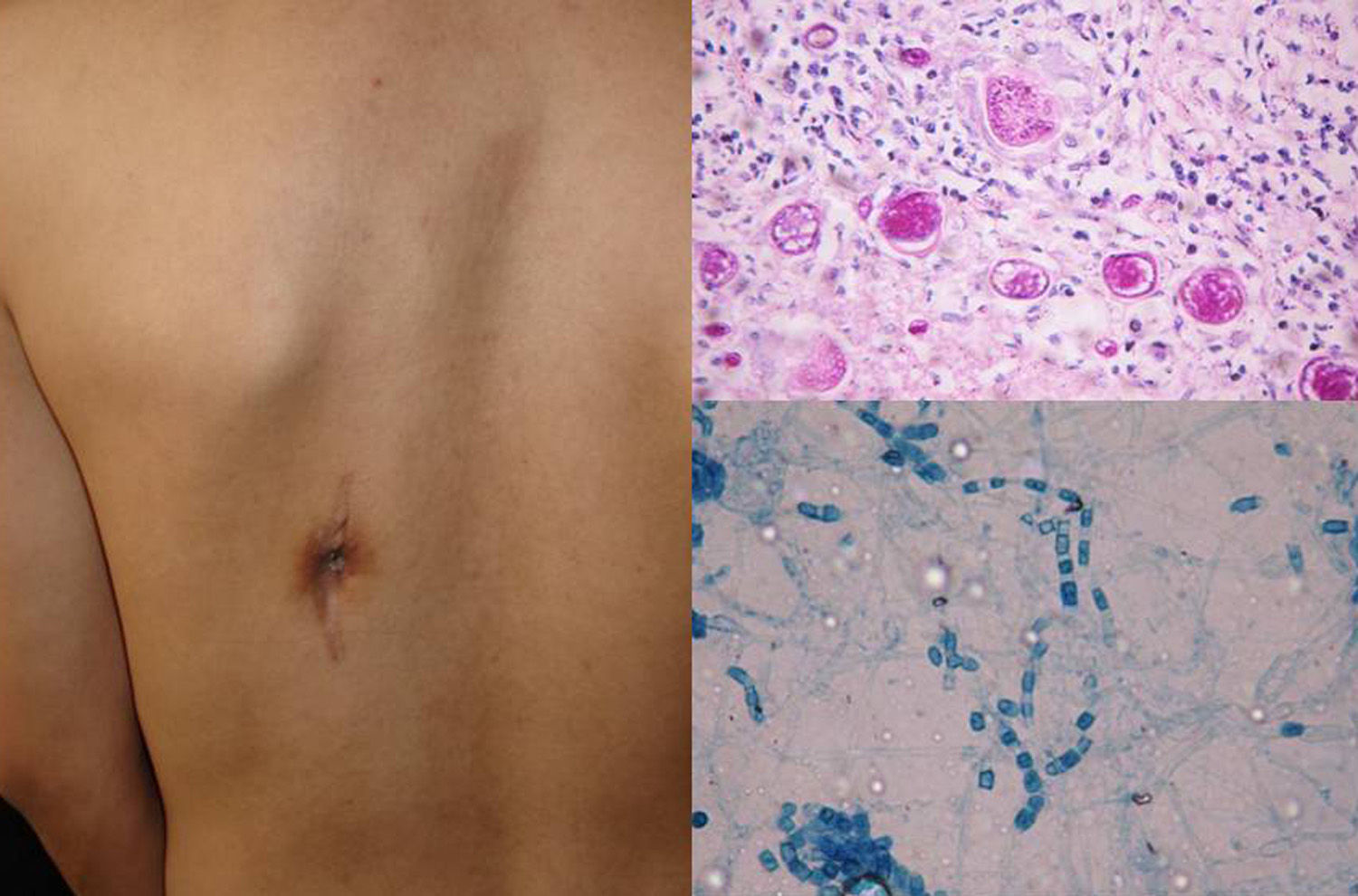
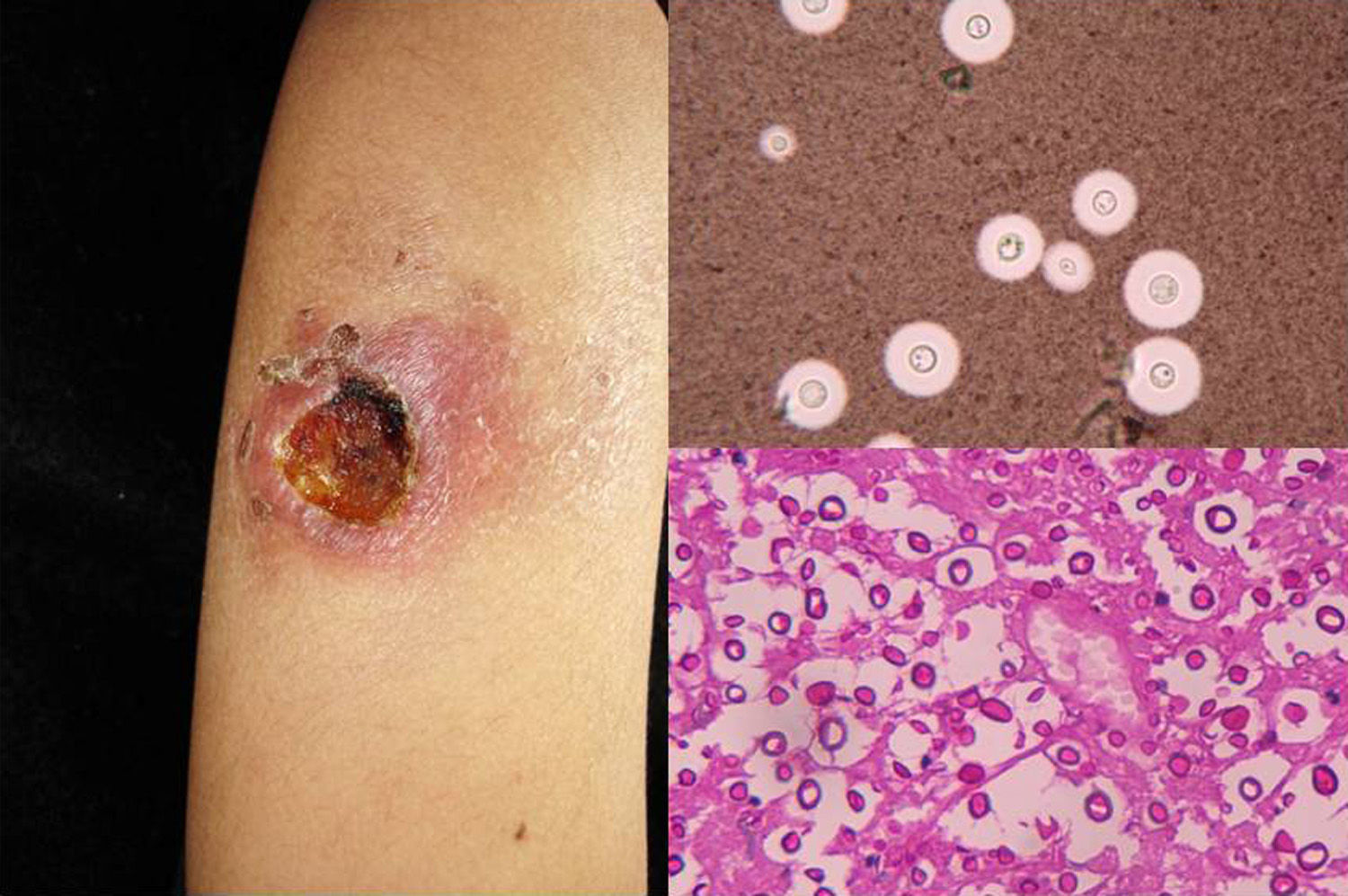
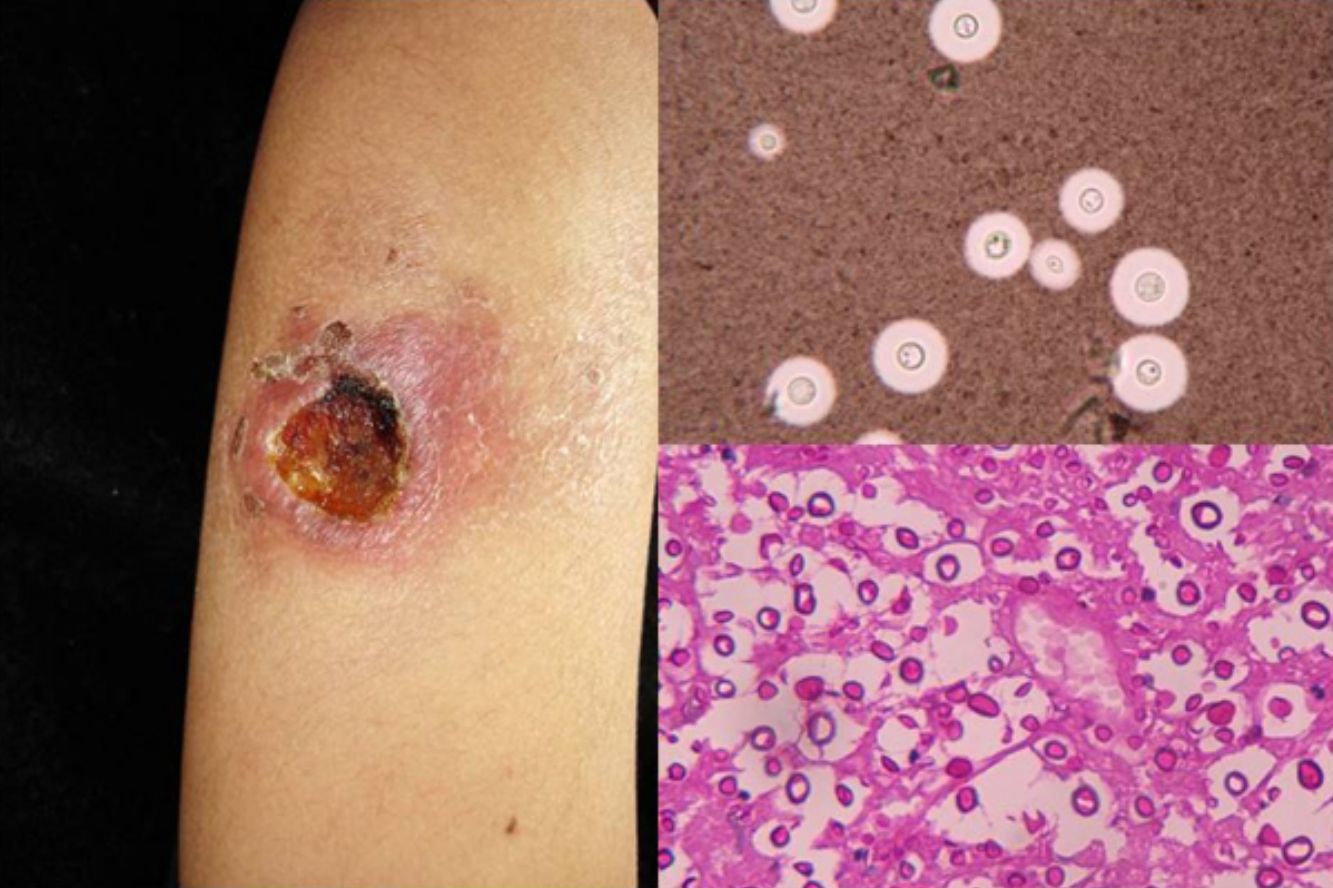
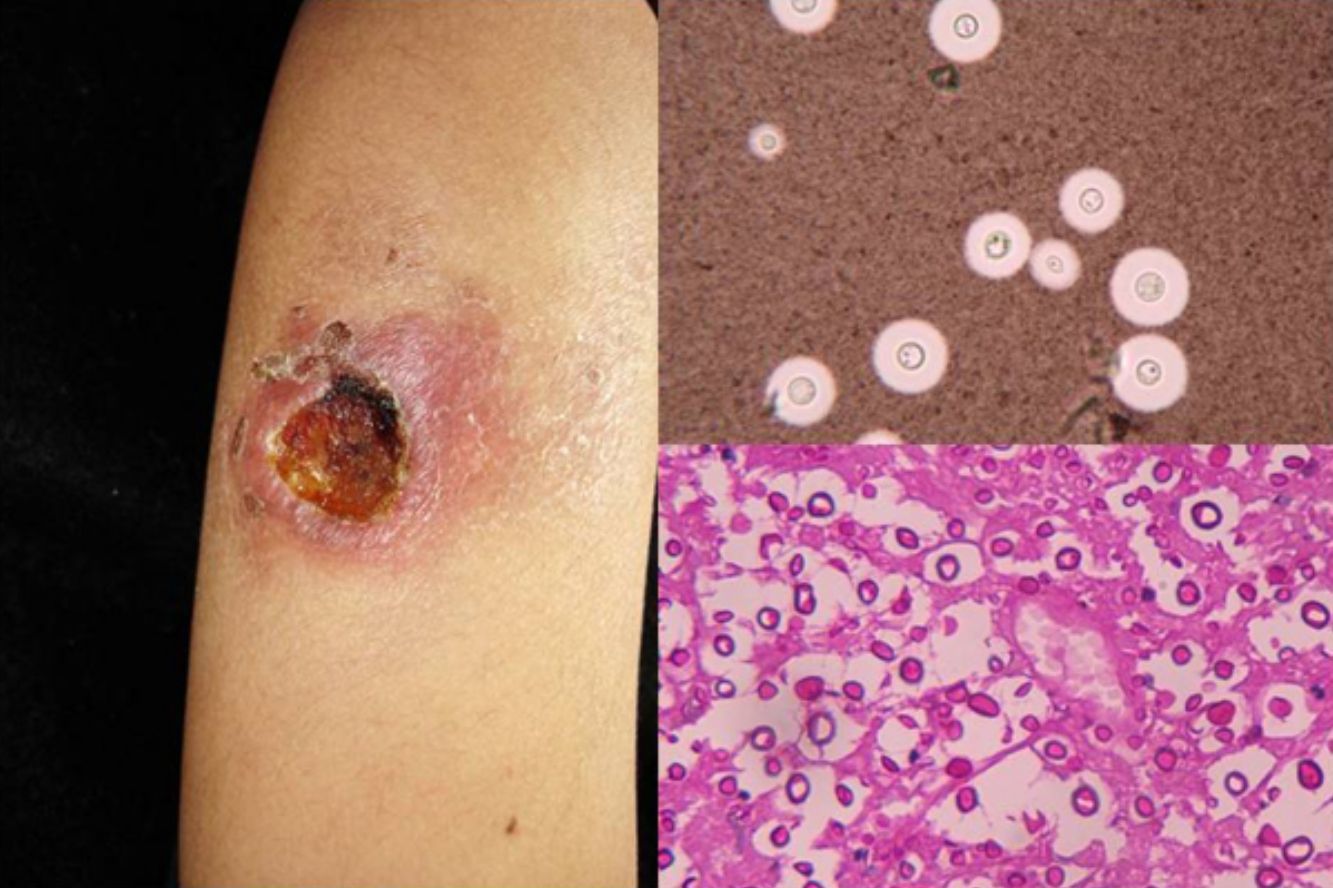
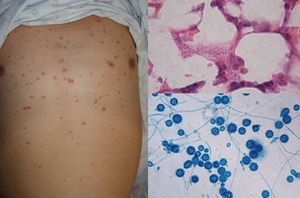
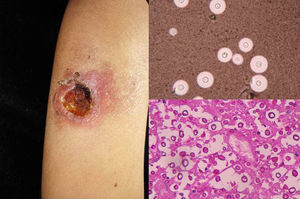
Clinical FormsNinety-five percent of people infected with histoplasmosis do not show clinical manifestations. In the acute form of disease, symptoms range from flu-like symptoms to more complex manifestations with radiological images showing disseminated calcifications and findings similar to those seen in tuberculosis.16 Acute forms can progress to chronic forms, which preferentially affect men aged over 50 years. Acute disease is characterized by cough and expectoration but it is difficult to isolate H capsulatum in sputum. Mucosal involvement is highly characteristic in chronic disseminated forms, and ulcerative granulomatous lesions are common on the oral mucosa, tongue, nasal septum, and larynx. Finally, meningoencephalitis and focal osteolysis in the metaphysis of long bones may be observed in acute disseminated histoplasmosis, which typically affects patients with AIDS. Around 11% of patients with AIDS develop skin manifestations,15 which tend to be disseminated and include papular lesions on the face and the trunk (Fig. 4) and, sometimes, ulcerative lesions on the mucosa.14
Papular lesions in a patient with disseminated cutaneous histoplasmosis and AIDS. Intracellular yeast forms in biopsy sample (hematoxylin-eosin, original magnification ×100) and direct examination of needle-shaped Histoplasma capsulatum conidia (lactophenol cotton blue, original magnification ×40).
The fungus can be isolated in blood, bone marrow, cerebrospinal fluid, bone marrow aspirate, or biopsy specimens of infected tissues. Specimens can be inoculated on SDA with or without antibiotics. Identification is also possible using molecular biology techniques such as PCR analysis of fungal DNA (ITS or 18S rDNA).17,18
Direct examination of Giemsa-stained specimens shows characteristic intracellular yeast forms surrounded by a halo simulating a capsule (Fig. 1).
TreatmentTreatment consists of itraconazole for 6 to 24months or amphotericinB.14
CryptococcosisCryptococcosis is a systemic mycosis caused by an encapsulated yeast of the genus Cryptococcus. The 2 most common species are Cryptococcus neoformans and Cryptococcus gatii. The lungs are the main route of entry for the pathogen. Clinical manifestations range from asymptomatic lung colonization to systemic dissemination. The main clinical manifestation is meningoencephalitis.19,20
C neoformans is typically found in soil and in pigeon and bat droppings. In urban areas, it is disseminated through domestic dust from trees.20
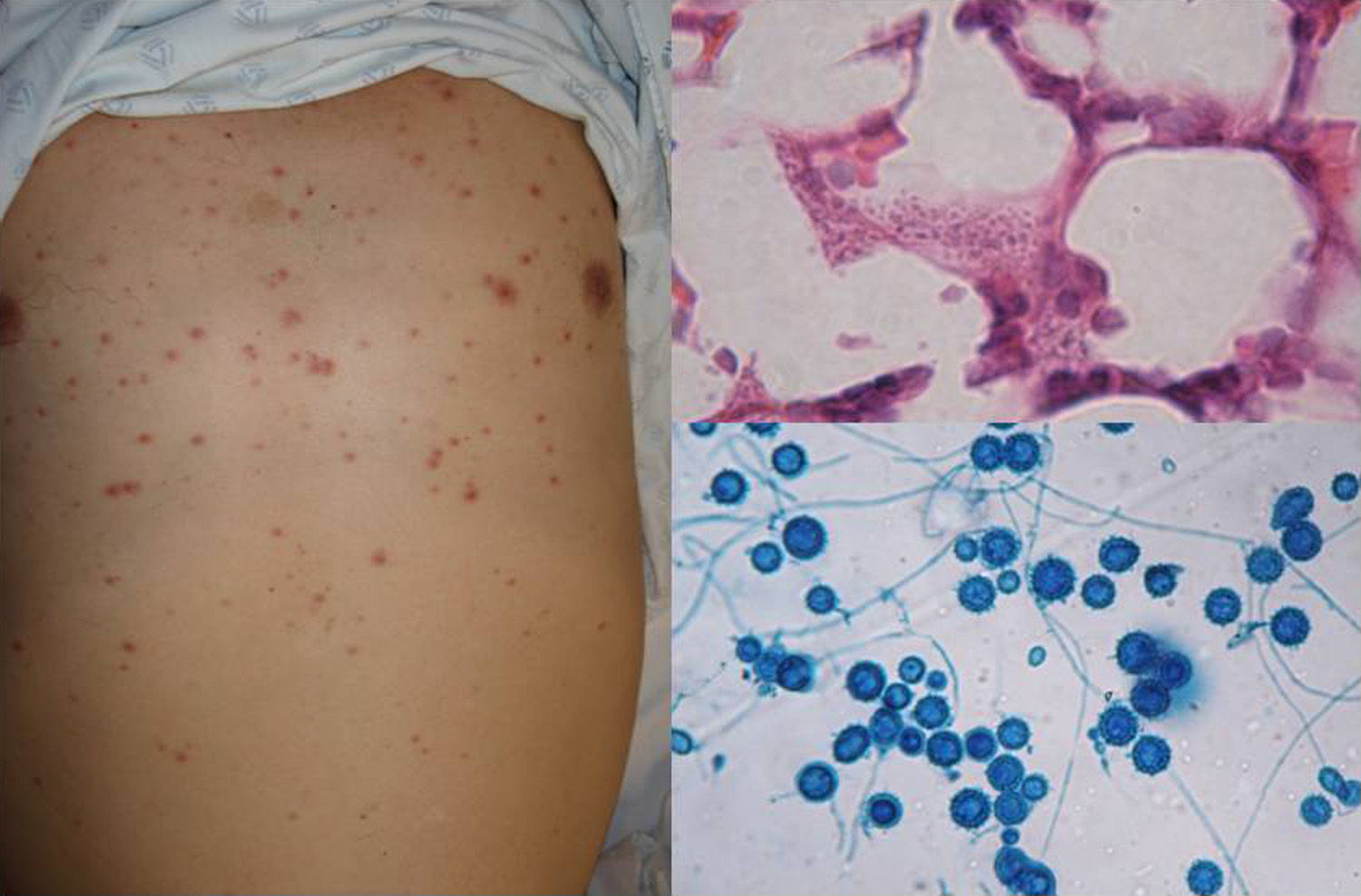
Clinical FormsInhaled yeasts and spores reach the alveolar spaces. Subsequent development of disease will depend on the phagocytic efficacy of the macrophages and the host's immune response. Clinical forms involve the lungs, the central nervous system (CNS), or the mucocutaneous structures, and may also be disseminated.20 Lung involvement tends to be asymptomatic, nonspecific, or similar to acute tuberculosis. CNS cryptococcosis is the most common clinical form and presents as chronic meningitis, meningoencephalitis, or cerebral cryptococcal granuloma. The mucocutaneous form is the result of the spread of infection from other foci in patients with disseminated disease. It presents as subcutaneous papules and nodules (Fig. 5) on the face and neck, primarily in patients with human immunodeficiency virus infection in the advanced AIDS stage.15,20
Diagnosis can be challenging given the wide range of possible lesions. The most common alternative diagnoses contemplated in the literature are molluscum contagiosum and herpes infections.20
These multiple polymorphic lesions, which are common in patients with diseases such as AIDS, lymphoma, sarcoidosis, and diabetes and in transplant recipients—and may also be due to multiple microorganisms—require an exhaustive study.
DiagnosisCryptococcus yeasts are large encapsulated cells that are best observed under the microscope with India ink (Fig. 5) or Mayer mucicarmine stains. The microorganisms grow on SDA within 24 to 48hours or after a week. Serology is fast and specific. Immunological identification is also possible and one particularly effective test (with a sensitivity and specificity of >80%) is the latex agglutination test, which searches for the cryptococcal capsular antigen in serum.21 Detection is also possible using different PCR assays22 or MALDI-ToF mass spectrometry.23 The advantage of the latter is that positive results are identified almost immediately (10minutes) and correlate 100% with DNA sequencing results.24–26
TreatmentTreatment is with deoxycholate or liposomal amphotericin B, with or without fluorocytosine or fluconazole.20
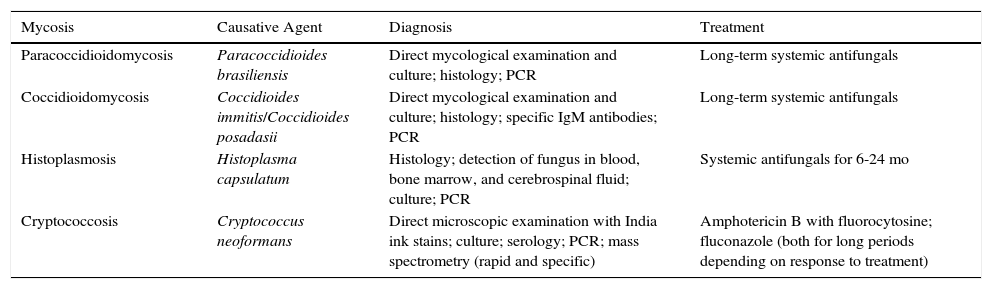
ConclusionsWe have reviewed the cutaneous manifestations of the systemic mycoses (Table 1). Recognition of these manifestations is important, as these infections are associated with high mortality. Dermatologists can play an important role in ensuring an accurate diagnosis by recognizing the clinical signs or ordering an appropriate test, such as a skin biopsy.
Summary of the Characteristics of the Systemic Mycoses.
| Mycosis | Causative Agent | Diagnosis | Treatment |
|---|---|---|---|
| Paracoccidioidomycosis | Paracoccidioides brasiliensis | Direct mycological examination and culture; histology; PCR | Long-term systemic antifungals |
| Coccidioidomycosis | Coccidioides immitis/Coccidioides posadasii | Direct mycological examination and culture; histology; specific IgM antibodies; PCR | Long-term systemic antifungals |
| Histoplasmosis | Histoplasma capsulatum | Histology; detection of fungus in blood, bone marrow, and cerebrospinal fluid; culture; PCR | Systemic antifungals for 6-24 mo |
| Cryptococcosis | Cryptococcus neoformans | Direct microscopic examination with India ink stains; culture; serology; PCR; mass spectrometry (rapid and specific) | Amphotericin B with fluorocytosine; fluconazole (both for long periods depending on response to treatment) |
Abbreviations: IgM, immunoglobulin M; PCR, polymerase chain reaction.
The authors declare that they have no conflicts of interest.
Please cite this article as: Carrasco-Zuber JE, Navarrete-Dechent C, Bonifaz A, Fich F, Vial-Letelier V, Berroeta-Mauriziano D. Afectación cutánea en las micosis profundas: una revisión de la literatura. Parte II. Micosis sistémicas. 2016;107:816–822.