Immunotherapy is emerging as a new and promising treatment for a great variety of tumors, including nonmelanoma skin cancer. Checkpoint inhibitors —antibodies that block proteins that regulate the immune system—mainly target the surface protein CTLA-4 (cytotoxic T-lymphocyte-associated antigen 4) and the PD-1/PD-L1 (programmed cell death protein 1/PD-ligand 1) axis. We review the CTLA-4 and PD-1/PD-L1 pathways and current evidence supporting checkpoint inhibitor therapy in the main types of nonmelanoma skin cancer.
La inmunoterapia en el cáncer emerge como un tratamiento novedoso y prometedor en una gran variedad de tumores, incluido el cáncer cutáneo no melanoma. Los anticuerpos inhibidores de proteínas de control inmunitario están dirigidos fundamentalmente a las moléculas de superficie CTLA-4 (antígeno citotóxico de los linfocitos T) y PD-1 (molécula de muerte programada 1). En el presente artículo se revisan las vías de CTLA-4 y PD-1/PD-L1 (PD-1/ligando de la PD-1) y las evidencias actuales de tratamiento con inhibidores de puntos de control inmunitario en los principales tipos de cáncer cutáneo no melanoma.
The interaction between tumor initiation and propagation strategies and host antitumor mechanisms is key in the development of cancer. The concept of immune surveillance refers to the mechanism by which the immune system can recognize and eliminate tumor cells. However, tumors use a range of mechanisms to evade detection and so persist.
The most important antitumor immune activity is that mediated by cell response. The process of T-cell activation requires 2 signals, an initial one mediated by recognition of an antigen presented by the major histocompatibility complex to the T cell receptor (TCR), and a second signal, known as a coregulatory signal, which modulates clonal expansion and a specific effector response. The coregulatory molecules are essential glycoproteins for communication between T cells and other immune cells; there are costimulatory molecules that trigger T-cell activation and other coinhibitory ones that minimize the damage to healthy tissue (peripheral tolerance mechanism).1 Among these coinhibitory molecules, cytotoxic T lymphocyte associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and its corresponding ligand (PD-L1) are of particular note.
One of the mechanisms of action of immune therapies for cancer is recruitment of immune cells to the tumor microenvironment and/or block of coinhibitory molecules, thereby enhancing antitumor cell response. Drugs that target the CTLA-4 and PD-1/PD-L1 axes are thus gaining traction in the treatment of a range of skin tumors such as malignant melanoma (MM), Merkel cell carcinoma (MCC), and, more recently, squamous cell carcinoma (SCC) and basal cell carcinoma (BCC).
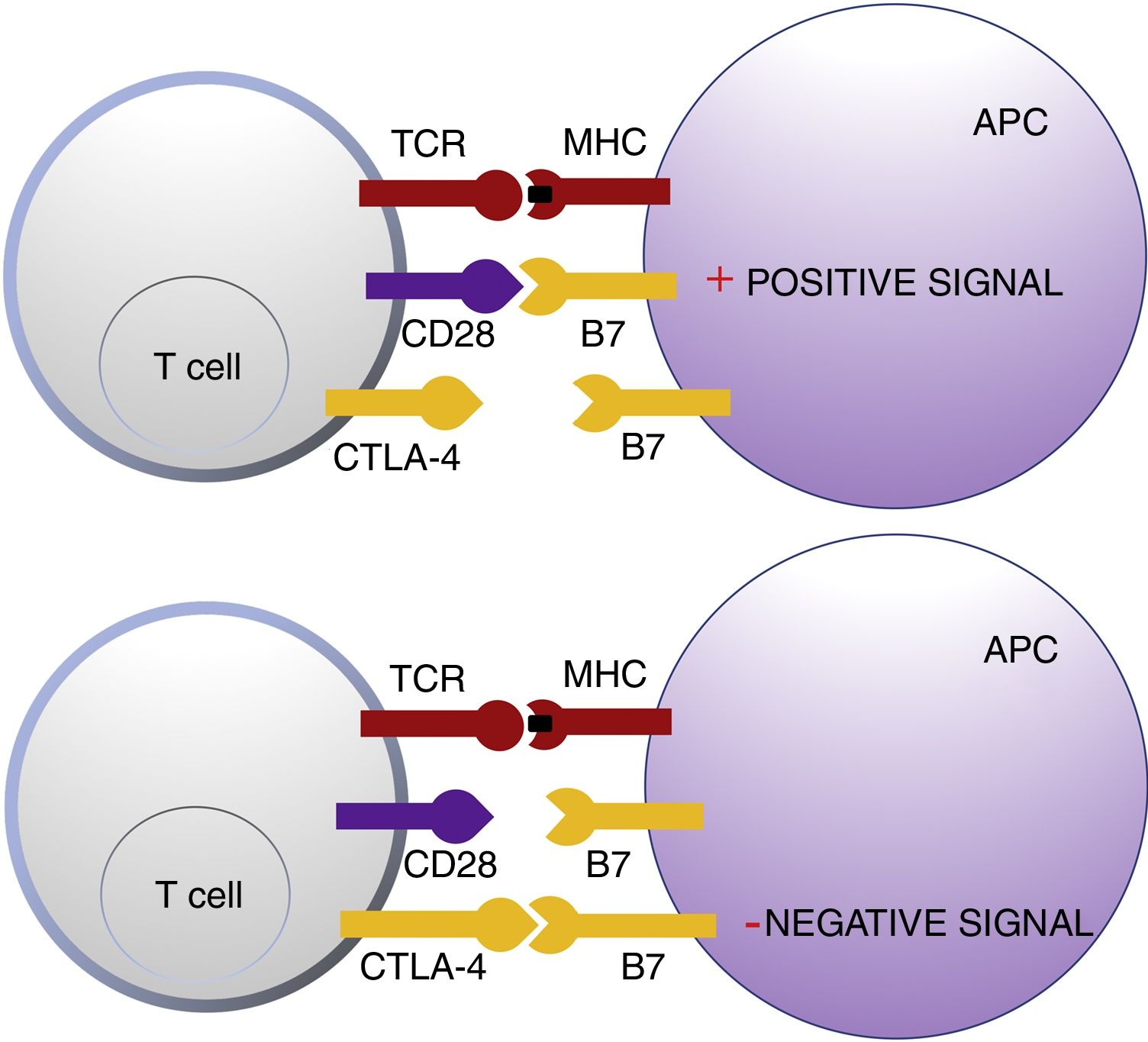
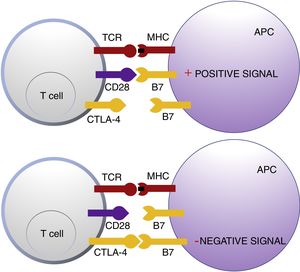
Cytotoxic T Lymphocyte AntigenCTLA-4 belongs to the immunoglobin superfamily of coinhibitory receptors. It is expressed on the surface of T cells between 24 and 48h after activation. In a first phase of immune response, antigen presenting cells (APCs) expose tumor antigens to T cells in lymphoid tissue. The T-cell receptors, CTLA-4 (inhibitory molecule) and CD28 (stimulatory molecule) compete to bind with the B7-1 (CD80) and B7-2 (CD86) molecules of the APCs. Binding of CTLA-4 to its B7 ligand triggers an inhibitory signal that blocks T-cell response in early phases of lymph node priming (Fig. 1).2,3
Schematic representation of T cell activation after interaction between the T-cell receptor and major histocompatibility complex and between B7 costimulatory molecules and CD28 (top panel). Inhibition of response after binding of cytotoxic T lymphocyte antigen to B7 (bottom panel).
Abbreviations APC: antigen presenting cells; CD28 cluster of differentiation 28; CTLA-4: cytotoxic T lymphocyte antigen; TCR: T-cell receptor; MHC: major histocompatibility complex.
CTLA-4 is also implicated in other immune surveillance processes. Regulatory T cells (Treg) are a T-cell subpopulation that play a key role in the prevention of autoimmunity, promoting immune tolerance to autoantigens. They constitutively express CTLA-4 and exercise their suppressor function on CD4+an CD8+autoreactive T cells through a range of mechanisms, including secretion of regulatory cytokines.2 However, their presence in peritumoral and intratumoral infiltrate can suppress the effector function of tumor-specific CD4+and CD8+T-cells, enabling tumor development.
Ipilimumab (Yervoy®) and tremelimumab were the first antibodies used, in 2000, to block the CTLA-4 receptor and maintain the activity of specific T-cells in patients with advanced cancer, including MM.4 Since its introduction, CTLA-4 blockade in patients with metastatic MM has achieved radiological response in 15% and lasting response of more than 10 years in some patients.4,5 Later, ipilimumab demonstrated longer survival in patients treated with this agent compared with those on conventional chemotherapy with dacarbazine and glycoprotein 100 peptide vaccine in 2 phase 3 studies.6,7 It was approved by the American Food and Drug Administration (FDA) in 2011 for the treatment of advanced MM and a few months later, the European Medicines Agency (EMA) also granted approval. In the phase 3 study of tremelimumab, no significant differences were found compared with chemotherapy, and so this agent is not available for use in MM, but its application in other types of tumors is under investigation.
Programmed Cell Death 1 Protein and Its LigandIn healthy tissue, the PD-1 pathway in T cells regulates immune response to minimize damage to adjacent tissue and prevent autoimmunity from developing by enhancing autoallergen tolerance. PD-1 (or CD279) is a cosignalling immune receptor expressed by activated T cells. It operates essentially in peripheral tissues where T cells are brought into contact with their immunosuppressive ligands, PD-L1 (or B7-H1 or CD274) and PD-L2 (programmed cell death 1 protein ligand 2 or B7-DC). PD-L2 is expressed by APCs and epithelial cells, whereas PD-L1 is expressed by a wide range of immune and nonimmune cells, including some tumor cells, as a response to certain proinflammatory cytokines (interferon γ) or oncogenic processes caused by activating mutations. Binding of PD-1 to PD-L1 inhibits the production of several cytokines (interferon γ, tumor necrosis factor α, interleukin 2) and T-cell proliferation, and triggers T-cell apoptosis.2,8–11
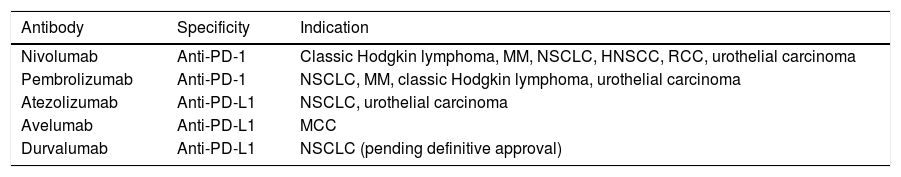
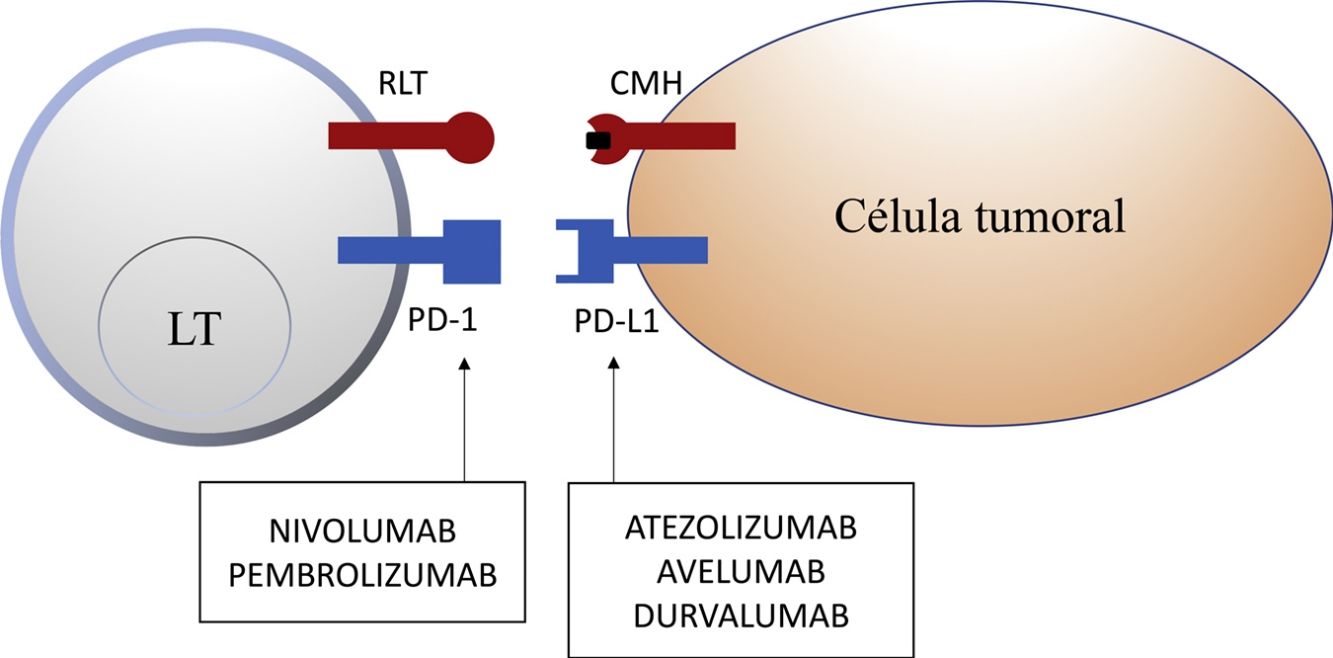
Inhibition of this pathway can occur through block of PD-1, such that binding to both PD-L1 and PD-L2 is inhibited, or through block of PD-L1, which would only block interaction with PD-1. Currently, there are 5 anti-PD-1 or anti-PD-L1 antibodies approved by the FDA for treatment of 11 types cancer: nivolumab (Opdivo®), pembrolizumab (Keytruda®), atezolizumab (Tecentriq®), durvalumab (Imfinzi®), and avelumab (Bavencio®). The first ones to be approved in 2014 were nivolumab and pembrolizumab for the treatment of patients with advanced MM (unresectable or metastatic). In 2017, avelumab was approved for the treatment of patients with metastatic MCC (Table 1, Fig. 2).
Anti-PD-1 or Anti-PD-L1 Agents Approved by the European Medicines Agency.
| Antibody | Specificity | Indication |
|---|---|---|
| Nivolumab | Anti-PD-1 | Classic Hodgkin lymphoma, MM, NSCLC, HNSCC, RCC, urothelial carcinoma |
| Pembrolizumab | Anti-PD-1 | NSCLC, MM, classic Hodgkin lymphoma, urothelial carcinoma |
| Atezolizumab | Anti-PD-L1 | NSCLC, urothelial carcinoma |
| Avelumab | Anti-PD-L1 | MCC |
| Durvalumab | Anti-PD-L1 | NSCLC (pending definitive approval) |
Abbreviations: HNSCC, head and neck squamous cell carcinoma; MCC, Merkel cell carcinoma; MM, malignant melanoma; NSCLC, nonsmall cell lung cancer; RCC, renal cell carcinoma; PD-1, programmed cell death 1 protein; PD-L1, programmed death ligand 1.
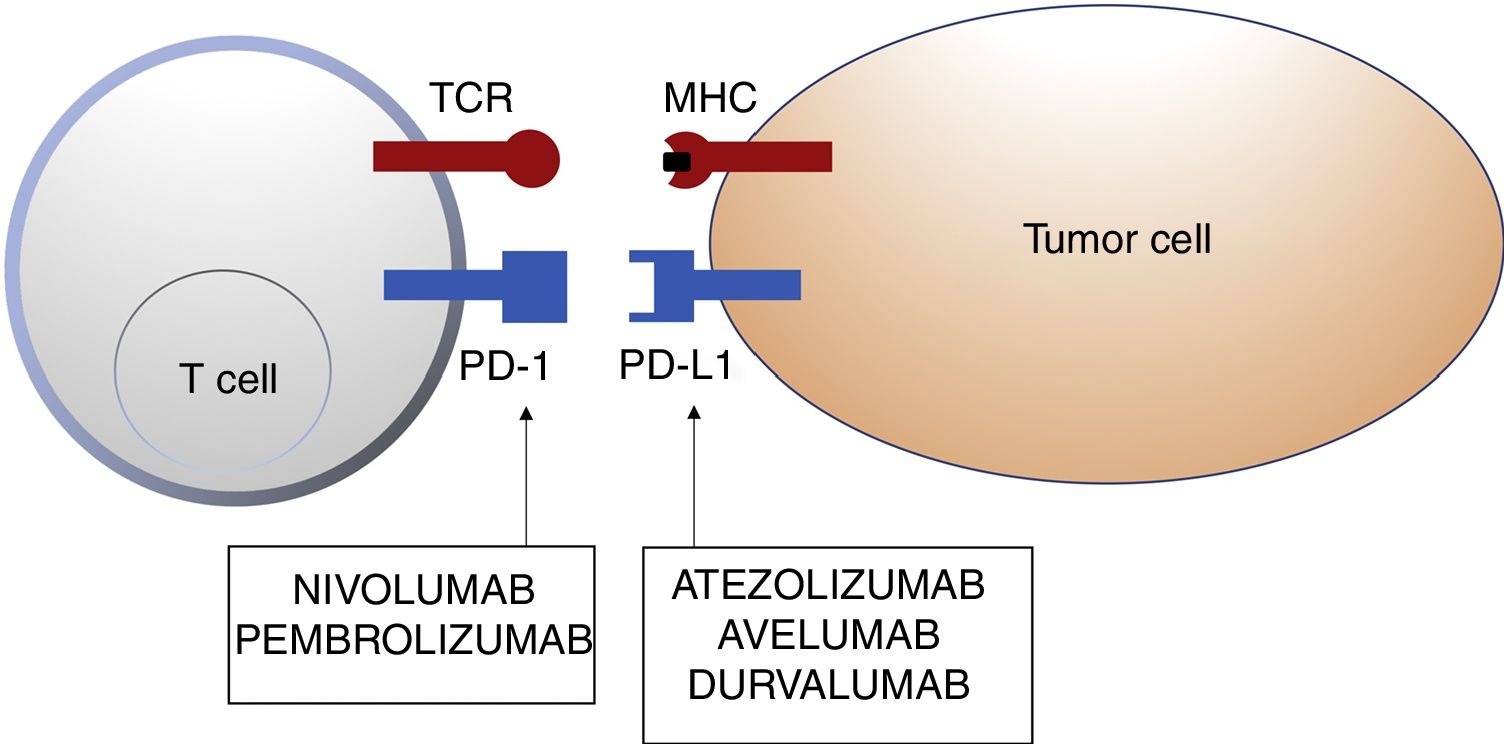
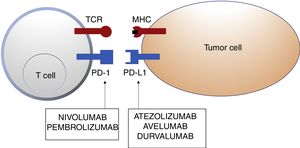
Schematic representation of T cell inhibition mediated by programmed cell death 1 protein ligand. Binding of programmed cell death 1 protein to its corresponding ligand inhibits the positive signal mediated by interaction between the T cell receptor and major histocompatibility complex.
Nivolumab and pembrolizumab bind to PD-1. Atezolizumab, avelumab, and durvalumab bind to PD-L1.
Abbreviations MHC: major histocompatibility complex; PD-1: programmed cell death 1 protein; PD-L1: programmed death ligand 1; TCR: T cell receptor.
Although these treatments provide hope for patients with advanced tumors, many do not respond. Strategies are being designed to improve response to prolong disease-free survival by testing combinations of drugs.12–14 Block of the PD-1/PD-L1 axis leads to a greater antitumor effect and lower toxicity than block of the CTLA-4 axis, given that this latter axis plays a role in the first phase of immune response. Among the most frequent side effects, of note are those related to autoimmunity in nontumor cells. In most cases, the effects are self-limiting or resolve with immunosuppressive treatment such as corticosteroids. It appears that the type of tumor, associated diseases, and prior treatments administered influence the profile of side effects arising with these therapies.15 The combination of inhibitory treatments targeting these immune checkpoints to enhance the antitumor effect leads to a higher number of immune-mediated adverse effects, some of which are potentially serious.12 Currently, the combination of nivolumab and ipilimumab is approved by the EMA for the treatment of advanced MM in adults.
Immunotherapy in Nonmelanoma Skin CancerImmunotherapy for nonmelanoma skin cancer (NMSC) is emerging as a promising therapeutic tool. This type of therapy is used in the treatment of locally advanced and metastatic MCC, SCC, BCC, and occasionally in cutaneous angiosarcoma.16
Immunotherapy in Merkel Cell CarcinomaMCC is an uncommon and aggressive neuroendocrine tumor, with a higher mortality rate (30%) than MM.17 It is associated with a polyomavirus in 80% of cases and has a low mutational load, unlike ultraviolet-induced MCC, which has no relationship with polyomavirus and many more genetic abnormalities.18 Traditionally, patients with disseminated disease receive chemotherapy. According to published results, nivolumab, avelumab, and pembrolizumab are currently preferred alternatives in patients with metastatic diseases. Longer-lasting response than that offered by conventional chemotherapy (in which disease-free survival is only 3 months) has been demonstrated.18–20
Pembrolizumab was the first inhibitor of an immune checkpoint to show objective tumor regression in patients with advanced MCC in a phase 2, uncontrolled, multicenter study that included 26 patients who had not received chemotherapy. The objective response rate was 56% and complete response was reported in 16%. Among responders, the duration of response was variable, ranging from 2.2 and 9.7 months, and progression-free survival was 67% at 6 months. Interestingly, it was effective in polyomavirus-positive and -negative tumors and immunohistochemical expression of PD-L1 was not correlated with a greater probability of response to treatment.18 According to the findings of a retrospective study presented at the American Society of Clinical Oncology (ASCO) this year, the presence of a high mutational load and prolonged exposure to UV are associated with a higher response rate to immunotherapy, but no association was found with presence or absence of Merkel cell polyomavirus.21
In March 2017, the FDA approved the first anti-PD-L1 monoclonal antibody, avelumab (Bavencio®), for the treatment of patients with metastatic MCC, on the basis of results from the JAVELIN Merkel 200 clinical trial.19
Immunotherapy in Squamous Cell CarcinomaAs with MCC, SCC is also an immunogenic tumor considered a good candidate for immunotherapy treatment. There is evidence that PD-L1 and PD-L2 play an important role in tumor progression, and so they are considered possible therapeutic targets.22,23 Experience with the use of immunotherapy in oncology in this tumor is limited to anecdotal observations and the preliminary results of some clinical trials. All these encourage further study.24,25
Of note is an article published in April 2017 in which 38 biopsies of patients with SCC were evaluated with NanoString technology, which allows study of messenger RNA (mRNA) expression in paraffin-embedded tumor samples. Ten samples of SCC with perineural invasion, 12 infiltrating tumors, 6 superficial tumors, 10 tumors from solid organ transplant recipients (SOTR) and 7 samples from normal skin as control were selected. Of all the subgroups, those with greatest expression of PD-1 and PD-L2 compared to controls were those SCC samples with perineural invasion. Greater expression of PD-1 was also observed in samples from stage 2B SCC (2 of the 3 high-risk factors for metastasis, recurrence, or death) and stage 3 SCC (4 additional high-risk factors for metastasis, recurrence, or death) according to the Brigham and Women's Hospital Tumor Staging. The findings from study of mRNA coding for PD-1 and PD-L2 were correlated with their immunohistochemical expression in tumor samples. The authors concluded that SCC samples express higher levels of PD-1 and their ligands (PD-L1 and PD-L2) than samples of normal skin. Perhaps blockade of PD-L1 with monoclonal antibodies is useful for activating host immune response to the tumor and offers a therapeutic alternative to patients with advanced and/or metastatic SCC without any other treatment options. Considering the above, immunohistochemical expression of PD-L1 could be a biomarker to identify those patients who may stand to benefit from these drugs.26
At this year's ASCO meeting, the preliminary results were presented of a phase 2 study in which 59 patients with metastatic SCC had been treated with cemiplimab (anti-PD-1). The objective response rate was 47.5% (28 out of 59 patients), with a response duration of more than 6 months in 57% of responders and an acceptable safety profile.27 These results have been published recently.28
Immunotherapy in Basal Cell CarcinomaAlthough the different subtypes of BCC have aberrant activation of the Hedgehog signaling pathway in common, the immune system may play an important role in the development of some of these tumors. There are several observations that point to the importance of antitumoral immunity in BCC. First, RUV-B, one of the main pathogenic factors, has long-term immunosuppressive effects, mainly T-cell-mediated immune reactions.29 It also induces the production of CD4/CD25+T regulatory cells able to inhibit antitumor effector functions and favor an immunosuppressive microenvironment.29–31 Second, SOTRs are at 10 to 16 times higher risk of developing BCC than the general population. These figures increase with increasing extent and duration of immunosuppressive therapy. These are the most aggressive, invasive, and recurrent tumors.29,32 Third, partial spontaneous regression is a relatively common phenomenon in BCC, but one that has not been extensively studied. It appears that secretion of certain cytokines by activated CD4+T-cells could induce an antitumor response.33–35 Fourth, we often find accompanying peritumoral infiltrate, formed mainly of T cells, although little is known of the significance of this finding. Finally, topical immunomodulators are curative therapeutic options in some cases of BCC.
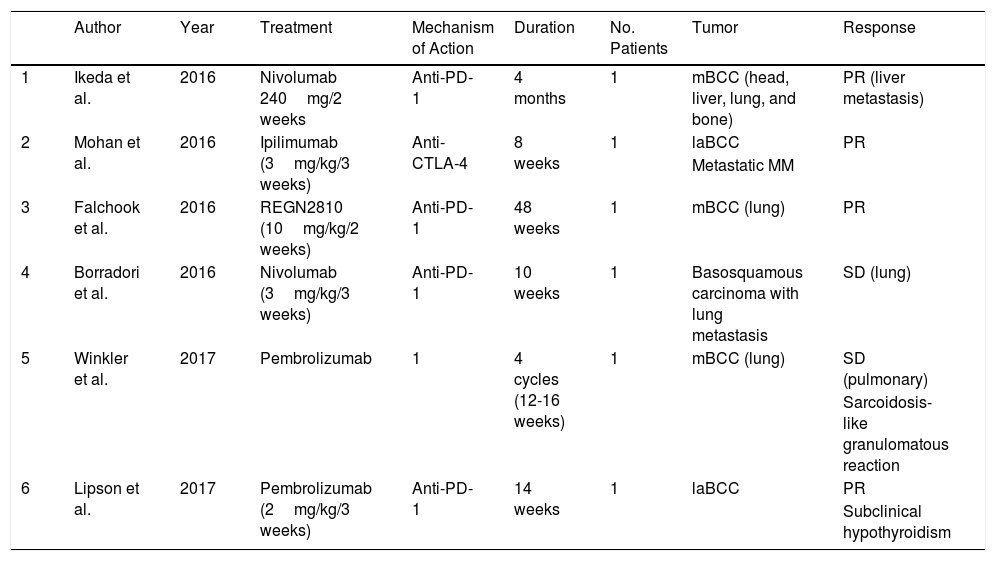
There are few publications on the role of antibodies against immunological molecular targets for the treatment of patients with locally advanced and metastatic BCC. In January 2016, Mohan et al.36 reported the case of a patient with metastatic MM treated with ipilimumab in whom incidental regression of locally advanced BCC was observed. Later, Ikeda et al.37 published a similar case. The patient was a 58-year-old man with metastatic BCC, with bone, lung, brain, and hepatic involvement, who had received several treatments (vismodegib, cisplatin, paclitaxel, sonidegib, and buparlisib), all discontinued due to disease progression or toxicity. Intravenous nivolumab was administered every 2 weeks and after 4 months, almost complete resolution of the hepatic metastases was observed. In another patient with metastatic BCC in treatment with pembrolizumab, the disease remained stable for months.38 In November 2016, Falchook et al.39 published the case of another metastatic BCC treated with REGN2810, a monoclonal anti-PD-1 antibody in a phase 1 study (NCT02383212), with partial and sustained response. Shortly afterwards, Borradori et al.40 presented the case of a patient with basosquamous carcinoma with lung metastases treated with nivolumab who maintained stable disease for a few months. Finally, Lipson et al.41 published the case of a patient with locally advanced BCC for whom surgery was not an option and who had been treated previously with radiotherapy and a Hedgehog pathway inhibitor. She started treatment with intravenous pembrolizumab 2mg/kg every 3 weeks for 14 weeks, with partial sustained response. It is worth noting that the immunohistochemical study before starting treatment only showed PD-L1 expression in immune cells, not in tumor cells, and that approximately 50% of T cells expressed PD-1 (Table 2).
Summary of BCC Treated With Immune Control Proteins.
| Author | Year | Treatment | Mechanism of Action | Duration | No. Patients | Tumor | Response | |
|---|---|---|---|---|---|---|---|---|
| 1 | Ikeda et al. | 2016 | Nivolumab 240mg/2 weeks | Anti-PD-1 | 4 months | 1 | mBCC (head, liver, lung, and bone) | PR (liver metastasis) |
| 2 | Mohan et al. | 2016 | Ipilimumab (3mg/kg/3 weeks) | Anti-CTLA-4 | 8 weeks | 1 | laBCC | PR |
| Metastatic MM | ||||||||
| 3 | Falchook et al. | 2016 | REGN2810 (10mg/kg/2 weeks) | Anti-PD-1 | 48 weeks | 1 | mBCC (lung) | PR |
| 4 | Borradori et al. | 2016 | Nivolumab (3mg/kg/3 weeks) | Anti-PD-1 | 10 weeks | 1 | Basosquamous carcinoma with lung metastasis | SD (lung) |
| 5 | Winkler et al. | 2017 | Pembrolizumab | 1 | 4 cycles (12-16 weeks) | 1 | mBCC (lung) | SD (pulmonary) |
| Sarcoidosis-like granulomatous reaction | ||||||||
| 6 | Lipson et al. | 2017 | Pembrolizumab (2mg/kg/3 weeks) | Anti-PD-1 | 14 weeks | 1 | laBCC | PR |
| Subclinical hypothyroidism |
Abbreviations: BCC, basal cell carcinoma; CTLA-4, cytotoxic T lymphocyte antigen; laBCC, locally advanced basal cell carcinoma; mBCC, metastatic basal cell carcinoma; MM, malignant melanoma; PD-1, programmed cell death 1 protein; PR, partial response; SD, stable disease.
Currently, there are 2 ongoing studies with immune control proteins in BCC; in the first, a PD-1 inhibitor (REGN2810) is being used in patients with locally advanced or metastatic BCC not responsive or refractory to Hedgehog signaling pathway inhibitors, and in the second, nivolumab is being used in monotherapy or in combination with ipilimumab in patients with unresectable locally advanced BCC or metastatic disease.42,43
ConclusionsEnhancement of the immune system in the treatment of cancer is shaping up as an effective and promising alternative. As dermatologists, we have experience with these therapies in patients with advanced MM and, more recently, in MCC. It is worth focusing our efforts on common tumors such as BCC and SCC and conducting controlled studies to investigate their efficacy, as such agents may be a therapeutic alternative for some patients. We should also try to identify biomarkers that could enable us to select those patients who may benefit from these treatments, in monotherapy or in combination, and thus avoid side effects on those expected to be nonresponders.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Bassas Freixas P, Aparicio Español G, García-Patos Briones V. Inmunoterapia en cáncer cutáneo no melanoma. Actas Dermosifiliogr. 2019;110:353–359.