Stem cells are characterized by their ability to self-renew and differentiate into the different cell lineages of their tissue of origin. The discovery of stem cells in adult tissues, together with the description of specific markers for their isolation, has opened up new lines of investigation, expanding the horizons of biomedical research and raising new hope in the treatment of many diseases.
In this article, we review in detail the main characteristics of the stem cells that produce the specialized cells of the skin (epidermal, mesenchymal, and melanocyte stem cells) and their potential implications and applications in diseases affecting the skin.
Part I deals with the principal characteristics and potential applications of epidermal stem cells in dermatology.
Las células madre son células que se caracterizan por su capacidad para autorrenovarse y diferenciarse hacia células de todos los linajes que constituyen su tejido de origen. El descubrimiento de las células madre en un organismo adulto, y la descripción de los marcadores que han permitido aislar de forma específica estas células, han abierto nuevas perspectivas y nuevos horizontes en la investigación biomédica y también nuevas esperanzas en el tratamiento de muchas enfermedades. En este artículo se revisan de forma detallada las principales características de las células madre que dan origen a las distintas células de la piel humana, incluyendo las células madre epidérmicas, mesenquimales y melanocíticas, y sus potenciales implicaciones y aplicaciones en las enfermedades cutáneas. La primera parte de este artículo revisa las células madre epidérmicas, con sus principales características y sus potenciales aplicaciones en dermatología.
Stem cells are defined by 2 fundamental characteristics: their capacity for self-renewal and for differentiation into all cell lines within their tissue of origin.1
In adults, stem cells have been identified in different organs, including the skin, intestine, muscle, hematopoietic system, and even the human brain.2 These cells are responsible for maintaining tissue homeostasis where they reside and also for repairing damage when it occurs.
The discovery of stem cells in adult organisms and characterization of their markers to enable isolation of specific cells have opened up new perspectives and new horizons in biomedical research, with new hopes for treatment in an range of diseases. Epidermal stem cells are of particular interest as they are relatively numerous and also accessible, making them easy to obtain. In the first part of this review, we have aimed to summarize the main findings of basic research in the field of epidermal stem cells. We then discuss their potential applications in clinical dermatology.
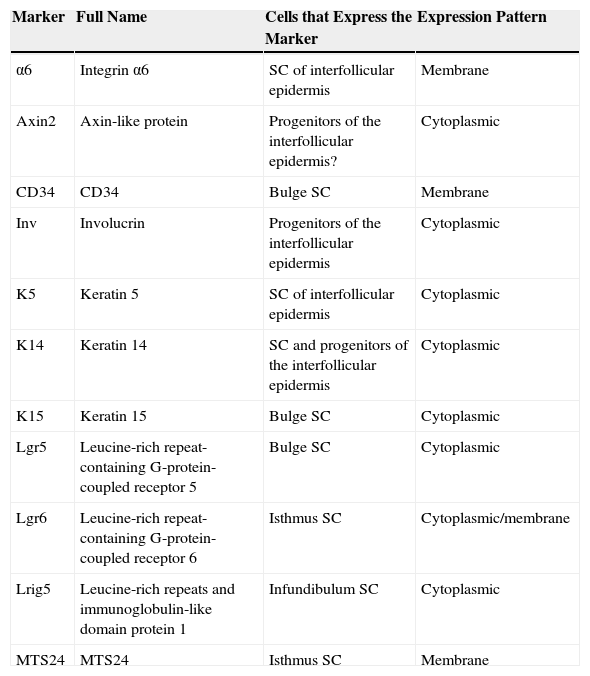
Different Stem Cell Populations in the EpidermisTwo types of progenitor cell are present in the basal layer of the interfollicular epidermis: α6+CD34– stem cells, characterized by slow division rates (4-6 times a year) and a long lifespan, and basal layer K14+Inv+ progenitor cells,3 as well as possibly Axin2+ cells,4 characterized by faster division rates (once a week) and shorter lifespan. After a certain number of divisions these cells undergo terminal differentiation into differentiated keratinocytes, thereby losing their capacity for division. Table 1 summarizes the main characteristics of the markers.
Summary of the Main Characteristics of Markers for Epidermal Stem Cells of Different Compartments.
| Marker | Full Name | Cells that Express the Marker | Expression Pattern |
|---|---|---|---|
| α6 | Integrin α6 | SC of interfollicular epidermis | Membrane |
| Axin2 | Axin-like protein | Progenitors of the interfollicular epidermis? | Cytoplasmic |
| CD34 | CD34 | Bulge SC | Membrane |
| Inv | Involucrin | Progenitors of the interfollicular epidermis | Cytoplasmic |
| K5 | Keratin 5 | SC of interfollicular epidermis | Cytoplasmic |
| K14 | Keratin 14 | SC and progenitors of the interfollicular epidermis | Cytoplasmic |
| K15 | Keratin 15 | Bulge SC | Cytoplasmic |
| Lgr5 | Leucine-rich repeat-containing G-protein-coupled receptor 5 | Bulge SC | Cytoplasmic |
| Lgr6 | Leucine-rich repeat-containing G-protein-coupled receptor 6 | Isthmus SC | Cytoplasmic/membrane |
| Lrig5 | Leucine-rich repeats and immunoglobulin-like domain protein 1 | Infundibulum SC | Cytoplasmic |
| MTS24 | MTS24 | Isthmus SC | Membrane |
Abbreviation: SC, cell.
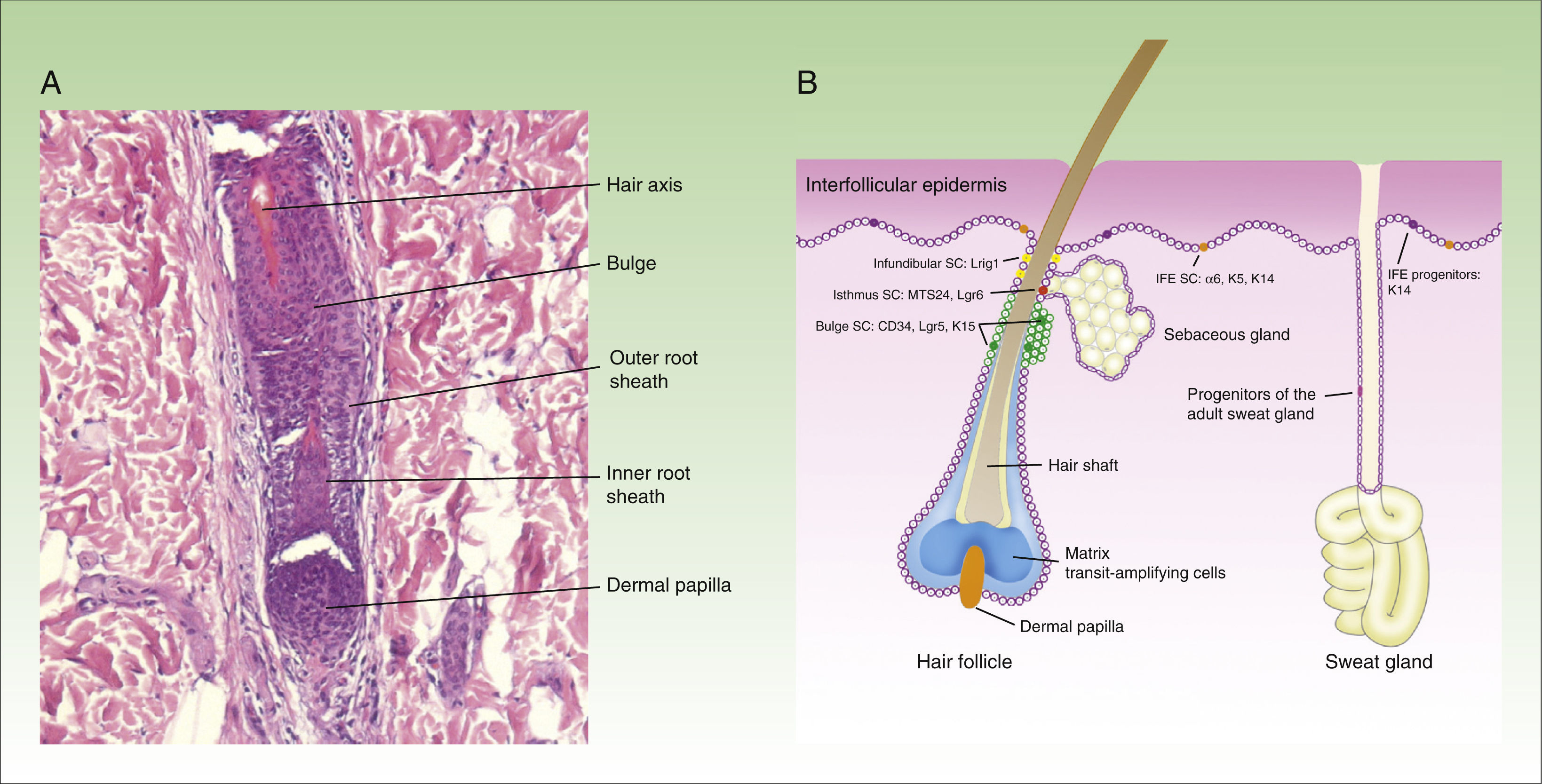
The hair follicle has 3 phases: anagen or growth phase (lasting on average 3 years), catagen or involuting phase (which lasts several weeks), and telogen or resting phase (which lasts several months). The stem cells responsible for hair follicle regeneration during anagen reside in the bulge (the lower part of the permanent portion of the hair follicle) and are characterized by expression of the markers CD34, Lgr5, and K15. The cells are multipotent,5 as they can differentiate into all cell lines present in the hair follicle unit. During anagen, the stem cells in the bulge give rise to transit-amplifying cells, which reside in the hair follicle. These rapidly and transiently proliferate before embarking on 7 differentiation programs which finally give rise to the mature hair follicle. When the matrix cells have exhausted their proliferative capacity, hair growth stops and the follicle enters catagen,6 leading to degeneration of the lower two-thirds of the follicle while the bulge region remains intact.
The hair bulb is found in the lower part of the hair follicle. This structure, which rests on the dermal papilla, is made up of differentiated progeny of stem cells from the bulge. The dermal papilla contains specialized dermal fibroblasts, nerve fibers, and a capillary loop. It plays a fundamental role in the development of the hair follicle and control of the hair cycle in adults.7 Cells in the dermal papilla can differentiate into neuronal and mesodermal cell lines.8,9 In a recent study, Rahmani et al.10 eliminated stem cells from the dermal papilla and observed a delay in the regeneration of the hair follicle, along with change in hair type, suggesting that the dermal papilla plays a fundamental role in restoring hair growth after damage, disease, or aging.
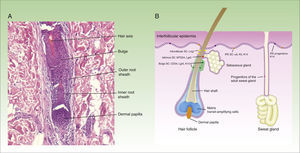
At least 3 types of epithelial stem cells have been identified recently: these types reside in the sebaceous glands, the infundibulum, and sweat glands. The sebaceous glands are maintained by unipotent Lgr6+ stem cells, which originate from Blimp1+ progenitor cells.11 In addition, stem cells from the isthmus express the MTS234 marker,12 and if transplanted to an immunodeficient mouse, are surprisingly able to give rise to epidermal, follicular, and sebaceous cell lines, suggesting that these might be multipotent cells.13 The stem cells in the infundibulum are characterized by expression of the Lrig1 marker and their multipotent capacity.14 It is also thought that they may contribute to the homeostasis of the sebaceous glands.15 Finally, although sweat glands have traditionally be considered as quiescent in adults, a study published recently suggests 4 different types of progenitor cells are present in the epithelium of these structures.16Figure 1 shows a microphotograph of the hair follicle (Fig. 1A) and a schematic of the different compartments in the epithelium of the skin and where the stem cells reside, as well as a summary of their markers (Fig. 1B).
Structure of the hair follicle and different types of epidermal stem cells.
The figure shows (A) microphotography of the hair follicle (hematoxylin and eosin, ×20) and (B) illustration of the different types of stem cells and progenitor cells in the epidermis, as well as their specific markers. IFE indicates interfollicular dermis, SC, stem cells.
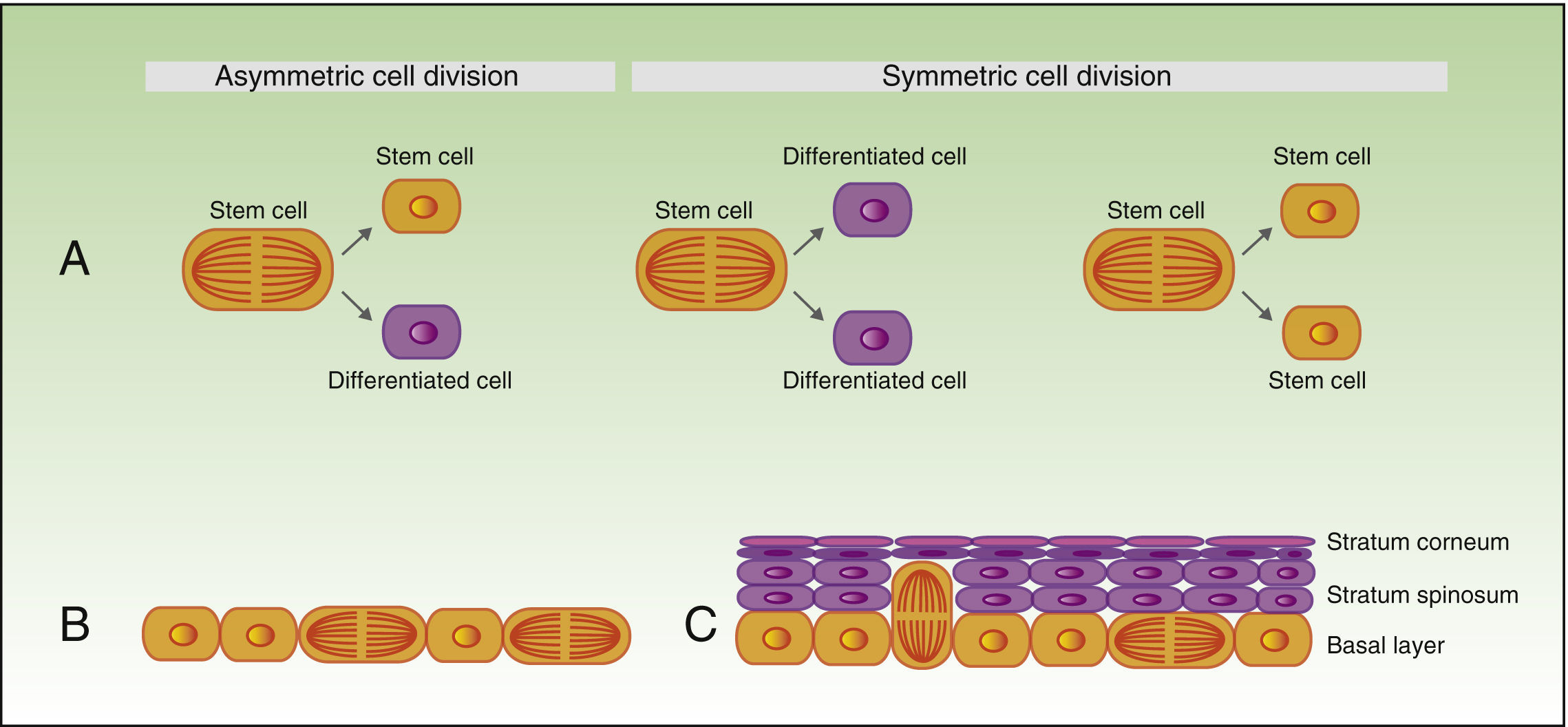
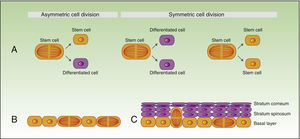
As mentioned earlier, stem cells can give rise to differentiated cells, but they also propagate to maintain a constant pool of stem cells, and can divide symmetrically or asymmetrically. During the process of asymmetric division, a stem cell gives rise to a daughter cell with the same phenotype, and another daughter cell which will differentiate. Symmetric division of a stem cell gives rise to 2 identical daughter cells, both with a differentiated or somewhat differentiated phenotype (Fig. 2A).
During the development of the embryo, most basal cell divisions are symmetric and parallel to the axis of the basal membrane (Fig. 2B), thereby allowing growth of the embryo surface and ensuring that the epithelium remains as a single layer. In contrast, during stratification of the epithelium, approximately 70% of divisions are asymmetric (Fig. 2C),6,17 thereby allowing development of suprabasal cells and establishment of the skin barrier during development and epidermal homeostasis in adulthood.
Self-renewal of stem cells.
A) Concept of symmetric and asymmetric cell division.
During the development of the embryo (B), most of the divisions are symmetric and the axis of division is parallel to the basal membrane, thereby allowing extension of the embryo surface during growth. During stratification of the epithelium, which occurs during morphogenesis and in adulthood (C), most of the divisions are asymmetric. During asymmetric division, the axis can be perpendicular to the basal membrane (on division, a daughter cell on losing contact with integrins and growth factor secreted by the basal membrane, undergoes differentiation, and the second daughter cell, on remaining in contact with the basal membrane, maintains the characteristics of the stem cell). Division can also be parallel to the basal membrane (in this case, differentiation of one of the daughter cells is induced by another mechanism).
Homeostasis is a physiological process whereby the number of cells with capacity for regeneration in a given organ remains constant. In skin homeostasis, the stem cells in each compartment are those responsible for replacing the differentiated cells that die in that compartment. However, during the evolutionary process, stem cells have acquired the capacity to participate in the repair of neighboring compartments in the event that stem cells in those compartments become damaged.
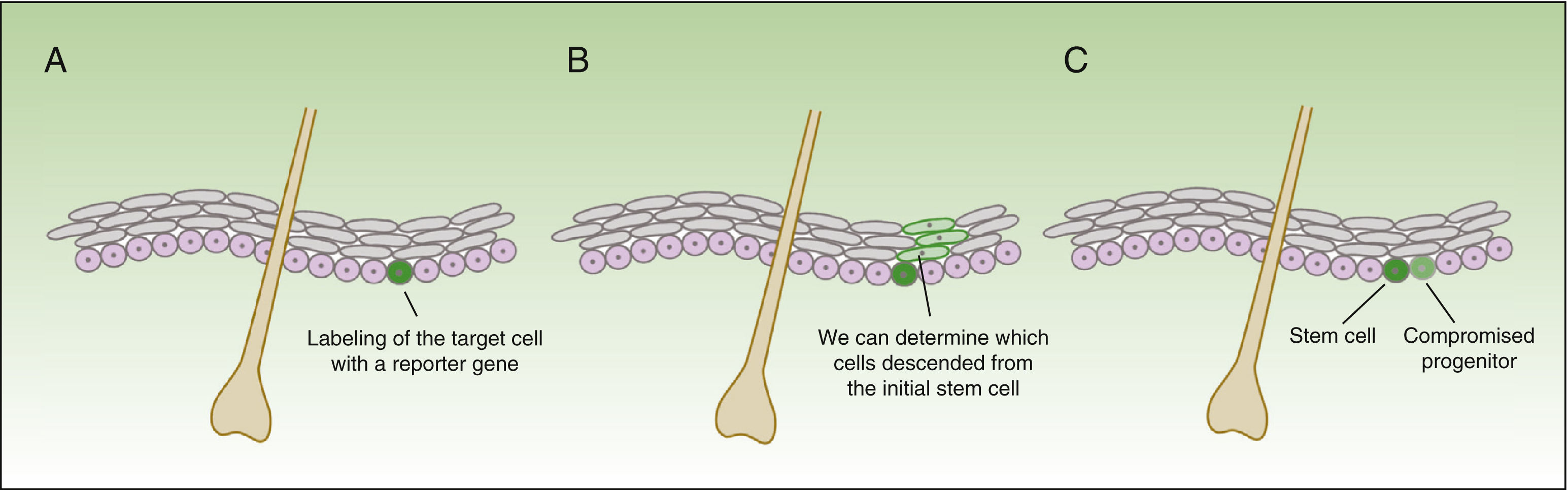
The form in which stem cells respond to damage varies drastically, depending not only on the compartment where these reside, but also how close they are to the wound.18 The technique of in vivo lineage tracing offers valuable functional information on the behavior of stem cells in homeostasis and during tissue repair, given that cell development can be followed over time in the natural environment (Fig. 3).
Concept of lineage tracing.
The figure shows a schematic of the information obtained from lineage tracing experiments. The technique consists of introducing a reporter gene associated with the marker of the cells of interest (A), thereby obtaining a fluorescent signal in the subgroup of cells of interest. All daughter cells of the labelled cells also have a fluorescent signal (B), but the intensity of the signal will decrease as the cells continue to divide. Thus, on the one hand, we can identify cells descended from the initially labelled cells, and on the other, assess the rate of division (the cells that maintain an intense signal over time will be the quiescent cells that divide very slowly—the stem cells—whereas the cells that lose color intensity will be the compromised progenitor cells [C]).
During repair of damage to the interfollicular epidermis, massive recruitment of interfollicular stem cells to the area of the wound occurs.3 Clones derived from these cells are observed migrating from the periphery of the wound towards the center, and remaining there for a long time after healing. However, much fewer Inv+ cells (short-lived progenitors) migrate towards the damaged zone, the clones are much smaller, and 35 days after injury, most of these have disappeared.3 That is, it seems that the short-lived progenitors are responsible for maintaining homeostasis of the epidermis in normal conditions, whereas stem cells, which are normally in a quiescent state, are activated when an injury occurs (wounds or drug administration).19
In addition, stem cells both in the bulge and in the infundibulum can migrate towards the epidermis in response to a wound stimulus, and participate in repair of the damage. Surprisingly, and through mechanisms that are still not well understood, when these cells migrate towards the epidermis, they lose their specific hair follicle markers and adopt a phenotype more similar to those of the stem cells of the interfollicular epidermis. However, once in the epidermis, these cells are short-lived and disappear soon after repair of the damaged tissue.20,21
Another phenomenon of great interest, which was observed on elimination of stem cells of a specific component of the epidermis with laser light, was that the empty niches were able to recruit normal differentiated cells from that compartment and induce cell proliferation and undifferentiation towards a state similar to that of stem cells.22,23
Epidermal Stem Cells as the Origin of Nonmelanoma Skin Cancer CellsThe identification of the cells that give rise to cancer is still a challenge in most malignant tumors. Theoretically, these cells acquire the first genetic or epigenetic abnormalities that culminate in the initiation of the malignant process.24 It follows that, given their characteristics (capacity for self-renewal over a long period of time), stem cells are at an increased risk of accumulating oncogenic mutations, and so they may be the cells that initiate the cancer.25,26
Using mice modified genetically with the inducible CreER-LoxP system (CreER refers to the estrogen receptor [ER] Cre-recombinase enzyme, such that administration of tamoxifen induces recombination of Cre, with the subsequent activation or deletion of the target gene), it has been shown that activation of the SmoM2 mutation in stem cells of the bulge or in transit-amplifying cells of the hair follicle did not induce tumor formation in basal cell carcinoma.27,28 In fact, the authors demonstrated that 90% of surface-spreading basal cell carcinomas originate from the interfollicular epidermis and the remaining 10% originate in the infundibulum.
In the case of spindle-cell carcinoma in mice, expression of the KRas mutation in stem cells in the bulge and in the interfollicular epidermis, but not in transit-amplifying cells, induces the formation of benign tumors but combination of the KRas mutation and deletion of the tumor suppressor gene p53 is necessary for progression to carcinoma.29
Potential Applications of Stem Cells in Clinical DermatologyBurnsFor the treatment of extensive skin burns, successful outcomes have been obtained with in vitro epidermis generated using autologous epidermal stem cells from a biopsy of undamaged patient's skin.30 The skin biopsy sample is dissociated with tripsin31 and the epidermal stem cells are isolated. These are then expanded on a base of irradiated fibroblasts, which secrete extracellular matrix and growth factors, making the environment particularly conducive to keratinocyte proliferation.32 The keratinocytes are cultured until formation of a stratified epithelium that can be used to cover the wound. However, there are 2 main drawbacks with this technique: the first is the long time needed for in vitro expansion of keratinocytes and the second is the high cost of the procedure.33
Of note is that the skin obtained with this method does not have any appendages (hair follicles or sweat glands). In a third-degree burn, the disappearance of hair follicles is due not only to destruction of multipotent stem cells in the follicle but also to the destruction of the dermis (the papillary dermis). To achieve regeneration of the hair follicles, cells from the papillary dermis need to be transplanted along with keratinocytes.34 In a recently published study, researchers managed to transplant human cells from the papillary dermis to a nude mouse (immunodeficient and without fur) and reported the regeneration of hair follicles.35 To achieve a similar hair color to the original color of the patient, a group of Swiss investigatorss added melanocytes isolated from skin biopsy to a culture of keratinocytes, achieving favorable results, both in patients with clear phototypes and with dark phototypes.36
A culture of keratinocytes on a bed of human fibroblasts embedded with a plasma matrix has been used as an alternative to transplantation of layers of keratinocytes cultured on a bed of irradiated fibroblasts.37 This approach enables restoration of both the epidermal and dermal compartment. After 24-26 days of culture, the authors achieved a 1000-fold cultured-area expansion, and successfully transplanted artificial skin from 2 patients with severe burns. Researchers from the University of Granada in Spain used fibrin-agarose biomaterials as a base to generate skin substitutes from human fibroblasts and keratinocytes. The artificial skin obtained was transplanted to nude mice and samples were taken for histological analysis and electron microscopy after 10, 20, 30, and 40 days.38 The results of these analyses showed that the structure of the skin obtained through tissue engineering was very similar to that of normal mouse skin.
Two studies of murine models published recently pointed to a possible alternative to the method used traditionally. Stimulation of the stem cells from the bulge of the hair follicle in third-degree burns in mice induced with human α-defensin-5 derived from the intestine accelerated wound healing and, notably, induced hair regeneration.39 Similarly, transplantation of Lgr6+ stem cells isolated by fluorescence-activated cell sorting and administered by injections into the wound promoted reepithelization, hair growth, and angiogenesis.40
Therapeutic Correction of the Epidermal Stem Cell Genome in Genetic DiseasesIn recent years, the cost of genetic sequencing has decreased significantly. As a result, the volume of data on the human genome in different contexts and diseases has increased exponentially. These advances in knowledge have generated high expectations around the potential therapeutic applications in genetic diseases. Although gene therapy is not applied in everyday clinical practice by dermatologists, in recent years, large steps forward have been made and the field shows great promise.
To date, the 2 most powerful therapeutic genetic techniques are gene therapy, which enables the reestablishment of the lost function of a given gene through expression of a transgene that is incorporated into the genome by viral vectors,41 and interference RNA, which can suppress expression of the defective gene through inhibition of messenger RNA.42 In recent years, moreover, new technologies have been developed with a promising future potential in gene therapy. These are based on the use of programmable nucleases, which are able to edit the genome of cells in damaged tissues, resulting in the elimination or correction of harmful mutations or the insertion of protective mutations.43–46
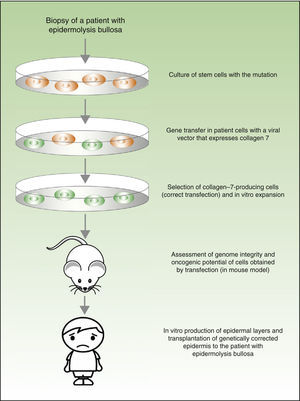
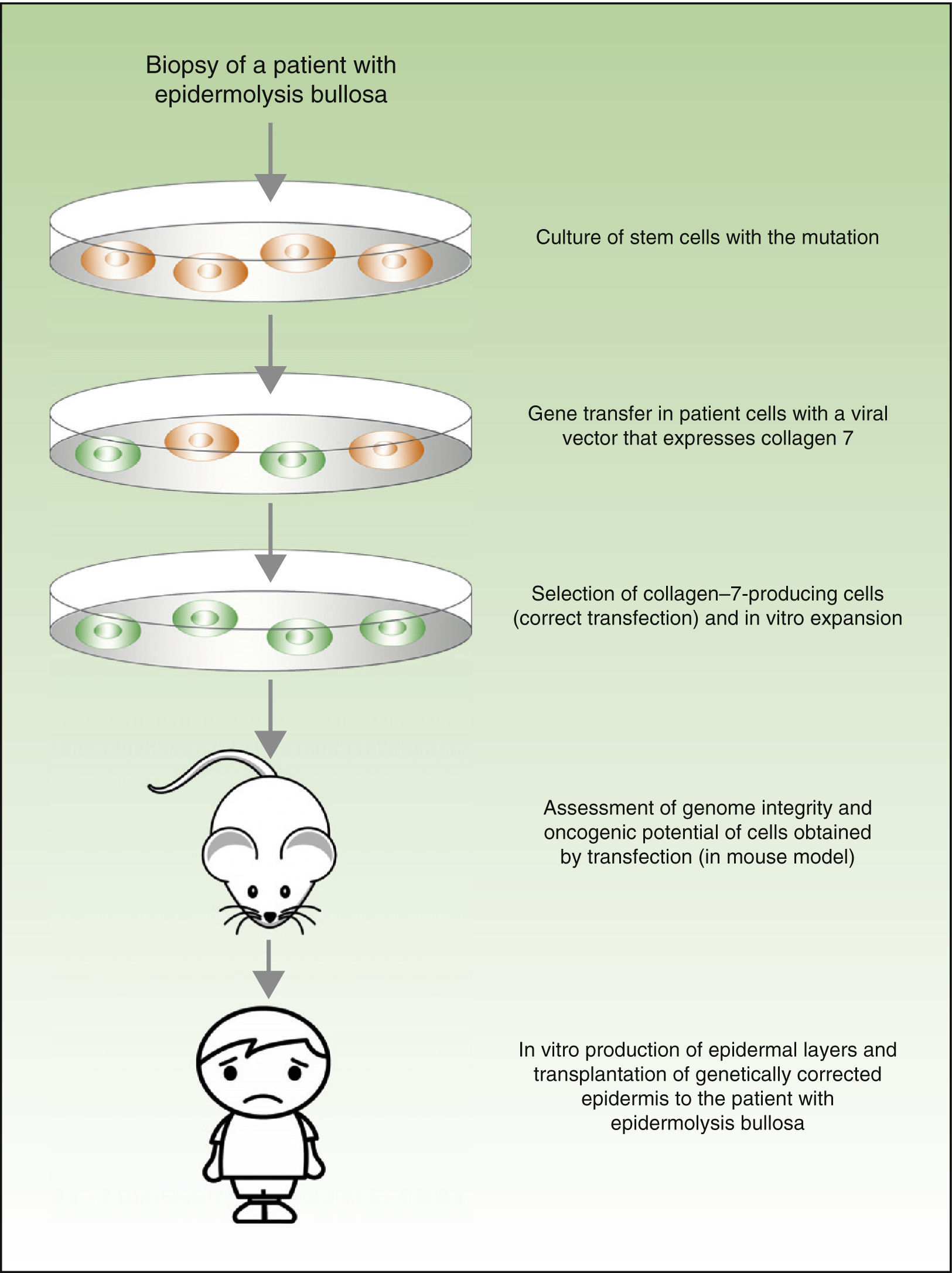
From a theoretical point of view, there are 2 possibilities for the treatment of genetic skin diseases (Fig. 4). Ex vivo therapy consists of extracting epidermal stem cells with a genetic abnormality (such as for example, laminin 5 deficit47 or collagen 7 deficit48 in the case of patients with epidermolysis bullosa) by skin biopsy, correcting the mutation in vitro by gene transfer, selecting cells in which the transfer has been successfully accomplished and the defect has been corrected, and transplanting keratinocytes once again in the skin of the patient. In a recently published study, before transplanting the corrected epidermal stem cells back into patients with epidermolysis bullosa, the authors assessed the integrity of the genome and also the oncogenic potential of these cells.49,50 They demonstrated that the procedure is safe (given that this is a transfection using viral vectors, one of the main concerns and drawbacks is the possibility of mutagenesis). In vivo therapy consists of delivery of viral or programmable nuclease vectors directly to the affected cells in their natural environment through intravenous drug administration or injection into the organ itself. This approach has several technical difficulties and safety concerns, which we will not discuss in this review but which have impeded the use of these techniques in patients with genetic skin diseases.
Conclusions and PerspectivesThe characteristics of stem cells, whereby they are able to self-renew and give rise to different cells types, along with the startling developments in bioengineering, make for a promising future. Epidermal stem cells are particularly attractive given their relatively high number proportional to the body surface and their accessibility. Although these are complex techniques with a high cost, it is likely that in the coming years, knowledge of the biology of stem cells, as well as the safety of the techniques used, will increase, and so allow a more widespread application in medicine and in dermatology in particular.
FundingThe work of I. Pastushenko was funded by the Télévie grant.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
The authors of this review would like to thank Kostiantyn Kokoriev (Kiev, Ukraine) for producing the schematics and Jesús Vera (Pathology Department, Hospital San Jorge, Huesca, Spain) for the histopathological image of the hair follicle.
Please cite this article as: Pastushenko I, Prieto-Torres L, Gilaberte Y, Blanpain C. Células madre de la piel: en la frontera entre el laboratorio y la clínica. Parte I: células madre epidérmicas. Actas Dermosifiliogr. 2015;106:725–732.


![Concept of lineage tracing. The figure shows a schematic of the information obtained from lineage tracing experiments. The technique consists of introducing a reporter gene associated with the marker of the cells of interest (A), thereby obtaining a fluorescent signal in the subgroup of cells of interest. All daughter cells of the labelled cells also have a fluorescent signal (B), but the intensity of the signal will decrease as the cells continue to divide. Thus, on the one hand, we can identify cells descended from the initially labelled cells, and on the other, assess the rate of division (the cells that maintain an intense signal over time will be the quiescent cells that divide very slowly—the stem cells—whereas the cells that lose color intensity will be the compromised progenitor cells [C]). Concept of lineage tracing. The figure shows a schematic of the information obtained from lineage tracing experiments. The technique consists of introducing a reporter gene associated with the marker of the cells of interest (A), thereby obtaining a fluorescent signal in the subgroup of cells of interest. All daughter cells of the labelled cells also have a fluorescent signal (B), but the intensity of the signal will decrease as the cells continue to divide. Thus, on the one hand, we can identify cells descended from the initially labelled cells, and on the other, assess the rate of division (the cells that maintain an intense signal over time will be the quiescent cells that divide very slowly—the stem cells—whereas the cells that lose color intensity will be the compromised progenitor cells [C]).](https://static.elsevier.es/multimedia/15782190/0000010600000009/v1_201511040157/S1578219015002450/v1_201511040157/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9/t1/zx4Q/XH5Tma1a/6fSs=)