Chronic spontaneous urticaria, also known as chronic idiopathic urticaria or simply chronic urticaria, is a common disorder that has a prevalence in the general population that ranges between 0.5% and 1%. This condition negatively affects the patient's quality of life and has considerable impact on direct and indirect health-related costs. Chronic urticaria is difficult to manage. Nonsedating H1 antihistamines are the first line of therapy, but fewer than 50% of patients experience relief at recommended dosages. Although guidelines call for increasing the dosage when response is inadequate, some patients still do not achieve adequate control of symptoms. New treatment alternatives, with proven efficacy under the standards of evidence-based medical practice, must therefore be developed.
La urticaria crónica espontánea, también conocida como urticaria crónica idiopática o urticaria crónica, es un proceso frecuente con una prevalencia estimada de entre el 0,5 y el 1% de la población general. Es un proceso que interfiere en la calidad de vida del paciente y ocasiona un notable impacto en los costes sanitarios directos e indirectos. La urticaria crónica es una entidad que plantea dificultades de manejo terapéutico. Se considera que los fármacos antihistamínicos H1 no sedantes son el tratamiento de primera elección. Su prescripción a las dosis recomendadas solo consigue una reducción de los síntomas en menos del 50% de los pacientes. Aunque las guías terapéuticas recomiendan incrementar las dosis en casos de respuestas no adecuadas, persiste un grupo de pacientes en los que no se consigue controlar la sintomatología. Existe, pues, la necesidad del desarrollo de nuevas alternativas terapéuticas cuya eficacia se establezca bajo criterios de medicina basada en la evidencia.
A large number of factors can trigger the onset of urticaria and/or angioedema, including physical stimuli (cold, heat, pressure, vibration, UV light), drugs (nonsteroidal anti-inflammatory drugs [NSAIDs], angiotensin-converting enzyme inhibitors, opioids), infections (Helicobacter pylori, intestinal parasites), foods, hypocomplementemia, and autoimmune disorders. To date, in contrast to urticarial vasculitis, no relationship has been detected between urticaria and malignancy. Due to the lack of consensus on the definition of the causative criteria, notable differences exist between authors regarding identification of the etiological factors implicated in chronic spontaneous urticaria. In some series, an etiological factor is identified in up to 40% to 50% of cases. The traditional term chronic idiopathic urticaria should be avoided or reserved only for those cases in which no etiological factor can be identified.1–5
The main effector cell in the production of the wheal is the cutaneous mast cell, which, after its activation, undergoes degranulation with the release of histamine and other vasoactive and proinflammatory mediators. Potential mast-cell activators include opioids, immunoglobulin (Ig) E (mast cells have a high-affinity IgE receptor [FC¿RI] on their cell membranes), complement factors (C5A), and substanceP. Once activated, mast cells release granules that mainly contain histamine, though other inflammatory mediators are also present, such as platelet activating factor (PAF), tumor necrosis factor (TNF), interleukin (IL) 3, IL-4, IL-5, IL-6, IL-8, IL-13, granulocyte-macrophage colony stimulating factor (GM-CSF), prostaglandin (PG) D-2, and leukotrienes (LT) (LTC4, LTD4, LTEA). Histamine, TNF, and IL-8 also stimulate endothelial adhesion molecules, which favor eosinophil, monocyte, and neutrophil migration from the bloodstream into the skin.3,4 It has recently been suggested that thrombin could play a role in the pathogenesis of chronic urticaria (CU), as this molecule has been found to increase vascular permeability, to act on the complement pathway, and to promote mast-cell degranulation.6
The chronic form of urticaria (wheals and/or angioedema lasting more than 6 weeks) is a skin disease with a prevalence in the general population between 0.1% and 3%.7 Many patients with CU report a considerable reduction in quality of life as a result of the pruritus, sleep disturbances, fatigue, social isolation, and emotional disorders; its impact on quality of life is comparable to that suffered by patients with severe coronary artery disease.8,9 Chronic spontaneous urticaria accounts for around 70% of all cases of CU and can persist for several years.
It has been shown that approximately a third of patients with chronic spontaneous urticaria present a positive response to the intradermal injection of their own serum (serum autoreactivity) in the autologous serum skin test (ASST). However, only a minority of these patients have detectable antibodies to the FC¿RI or to autologous IgE (autoimmunity). Chronic autoimmune urticaria accounts for 30% to 50% of all cases of CU.
In chronic autoimmune urticaria the cutaneous mast cells become permanently activated due to the presence of functional IgG antibodies to the alfa subunit of the FC¿RI (30%-50%) or to the IgE of the mast cell directly (5%-10%). Basophils also express FC¿RI, and thus a fall in basophil levels is usually detected in patients with chronic autoimmune urticaria. The capacity of the patient's serum to induce the degranulation of healthy basophils can be demonstrated by the in vitro detection of functional antibodies. It has recently been reported that IgE antithyroperoxidase antibodies can also induce mast-cell degranulation. Mast-cell degranulation is also thought to be induced by the classic complement pathway, particularly factor C5A, which, apart from favoring mast-cell and basophil degranulation, is involved in chemotaxis to recruit neutrophils, eosinophils, and monocytes. Antithyroid antibodies are detected in 25% of cases of chronic autoimmune urticaria (in contrast to 6% of the general population) and this form of urticaria has frequently been associated with diseases such as Hashimoto thyroiditis and, less commonly, with Graves disease; its association with other autoimmune diseases, such as diabetes mellitus and vitiligo, has also been reported.
General Bases for the Treatment of Chronic UrticariaGeneral MeasuresIn the treatment of urticaria it is important to avoid possible nonspecific triggers or aggravating factors such as heat, stress, alcohol, and certain drugs, including acetylsalicylic acid, NSAIDs, angiotensin-converting enzyme (ACE) inhibitors (particularly if the urticaria presents with angioedema, with or without wheals) and codeine. Cooling, antipruritic lotions such as calamine or 1% menthol in aqueous cream can be applied.2,3,10,11
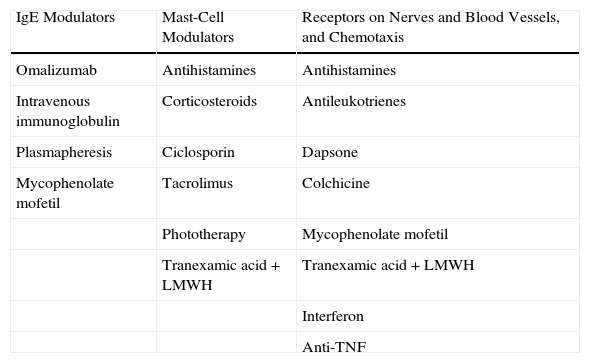
Pharmacological TreatmentThe treatments of CU can be classified according to their different functional effects. The majority of drugs modulate the capacity of mast cells to undergo degranulation and release their mediators. This group includes certain antihistamines, the corticosteroids, ciclosporin A (CsA), tacrolimus, methotrexate, and phototherapy. Drugs routinely used in the treatment of CU act by blocking the receptors and modulating the mediators that, situated on the nerve fibers and blood vessels, induce pruritus, vasodilatation, and chemotaxis. These are the most widely used drugs in the management of CU and include the antihistamines (H1 and H2 blocking agents), antileukotrienes, and dapsone. Some drugs act prior to activation of the effector cell or mast cell. This group includes omalizumab (a monoclonal antibody with the ability to bind IgE), plasmapheresis, and intravenous immunoglobulins (Table 1).
Mechanism of Action of the Treatments for Chronic Urticaria.
| IgE Modulators | Mast-Cell Modulators | Receptors on Nerves and Blood Vessels, and Chemotaxis |
| Omalizumab | Antihistamines | Antihistamines |
| Intravenous immunoglobulin | Corticosteroids | Antileukotrienes |
| Plasmapheresis | Ciclosporin | Dapsone |
| Mycophenolate mofetil | Tacrolimus | Colchicine |
| Phototherapy | Mycophenolate mofetil | |
| Tranexamic acid+LMWH | Tranexamic acid+LMWH | |
| Interferon | ||
| Anti-TNF |
Abbreviations: LMWH, low-molecular-weight heparin; TNF, tumor necrosis factor.
Histamine plays a key role in the formation of the typical lesion of urticaria. It has been observed that patients with CU have higher local levels of histamine than healthy individuals both in affected skin and in healthy skin.12 There are 4 types of histamine receptor in the skin: the H1 receptors, found mainly in the endothelium, smooth muscle, and central nervous system; the H2 receptors, located also on the parietal cells of the digestive tract; the H3 receptors, present in the central nervous system and on the bronchial smooth muscle; and the H4 receptors, found on dendritic cells in the skin, and in the thymus, spleen, small bowel, and colon, and which are currently a subject of therapeutic research. The antihistamines are effective in the treatment of urticaria because of their antagonism of the H1 receptors.
Antihistamines have been used to treat CU for more than 60 years and continue to be the treatment of first choice. The majority of symptoms of urticaria are the result of the actions of histamine on the H1 receptors located on the endothelial cells (wheal) and on the sensory nerve endings (pruritus). The antihistamines act on the endothelium of the postcapillary venules, reducing extravasation and wheal formation, and on the afferent C nerve fibers of the skin, reducing pruritus. In addition, the effect of the antihistamines on cutaneous axonal reflexes reduces erythema. Many of the antihistamines have anti-inflammatory activity as they reduce the levels of preformed or newly formed cytokine mediators and cell adhesion molecules, leading to a reduction in the recruitment of inflammatory cells (lymphocytes, monocytes, neutrophils, eosinophils). These actions occur mainly through 2 mechanisms: stabilization of the basophil and mast-cell membranes by the H1-antihistamines and the inhibition of cytoplasmic transcription factors such as nuclear factor kappa-B.13
The antihistamines can be classified into anti-H1, anti-H2, anti-H3, and anti-H4 according to the receptors on which they act. The antihistamines currently used for the treatment of CU are the first-generation H1-antihistamines, the second-generation H1-antihistamines, and the H2 antihistamines.
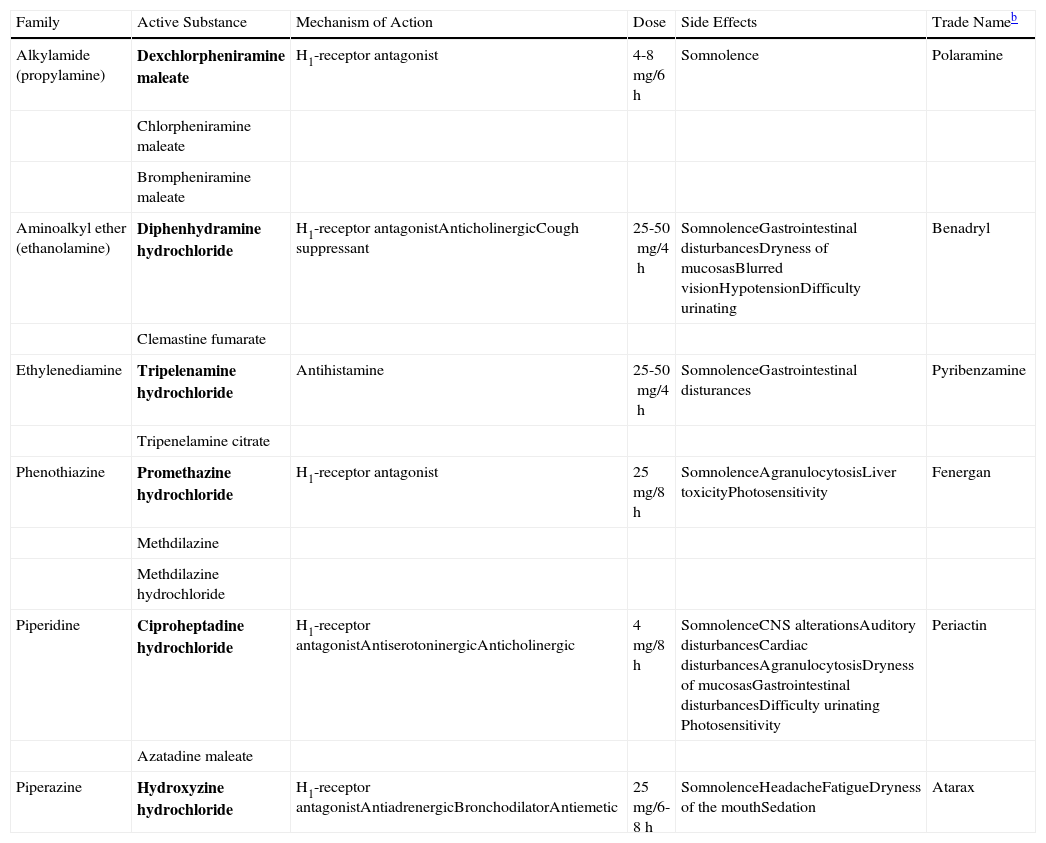
The first-generation H1-antihistamines have been used for many years to treat CU and other allergic disorders. They have anticholinergic effects and cross the blood-brain barrier, leading to sedative effects. These side effects can limit adherence to treatment, and such drugs are therefore usually reserved for cases in which the pruritic symptoms occur mainly at night (interference with sleep initiation) and in patients who do not respond to second-generation antihistamines. The first-generation H1-antihistamines include hydroxyzine, diphenhydramine, ciproheptadine, and dexchlorpheniramine (Table 2).
Table Comparing the First-Generation H1 Antihistaminesa
| Family | Active Substance | Mechanism of Action | Dose | Side Effects | Trade Nameb |
| Alkylamide (propylamine) | Dexchlorpheniramine maleate | H1-receptor antagonist | 4-8mg/6h | Somnolence | Polaramine |
| Chlorpheniramine maleate | |||||
| Brompheniramine maleate | |||||
| Aminoalkyl ether (ethanolamine) | Diphenhydramine hydrochloride | H1-receptor antagonistAnticholinergicCough suppressant | 25-50mg/4h | SomnolenceGastrointestinal disturbancesDryness of mucosasBlurred visionHypotensionDifficulty urinating | Benadryl |
| Clemastine fumarate | |||||
| Ethylenediamine | Tripelenamine hydrochloride | Antihistamine | 25-50mg/4h | SomnolenceGastrointestinal disturances | Pyribenzamine |
| Tripenelamine citrate | |||||
| Phenothiazine | Promethazine hydrochloride | H1-receptor antagonist | 25mg/8h | SomnolenceAgranulocytosisLiver toxicityPhotosensitivity | Fenergan |
| Methdilazine | |||||
| Methdilazine hydrochloride | |||||
| Piperidine | Ciproheptadine hydrochloride | H1-receptor antagonistAntiserotoninergicAnticholinergic | 4mg/8h | SomnolenceCNS alterationsAuditory disturbancesCardiac disturbancesAgranulocytosisDryness of mucosasGastrointestinal disturbancesDifficulty urinating Photosensitivity | Periactin |
| Azatadine maleate | |||||
| Piperazine | Hydroxyzine hydrochloride | H1-receptor antagonistAntiadrenergicBronchodilatorAntiemetic | 25mg/6-8h | SomnolenceHeadacheFatigueDryness of the mouthSedation | Atarax |
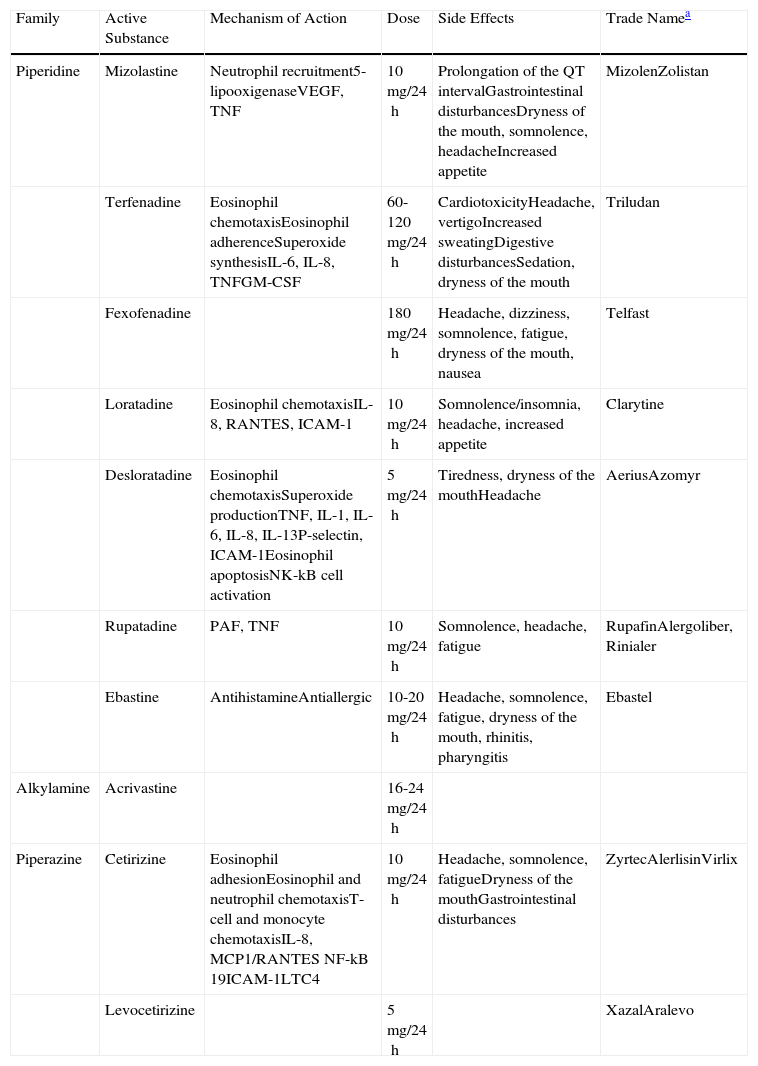
The efficacy of the second-generation H1-antihistamines has also been demonstrated in the symptomatic treatment of urticaria. Some, such as rupatadine, have even been found to have greater affinity for binding to the histamine receptors than the first-generation H1-antihistamines.14 They do not cross the blood-brain barrier and therefore do not give rise to sedative effects and they usually have no anticholinergic side effects. They are the only treatment with level 1 evidence and grade A recommendation. The second-generation H1-antihistamines are currently the drugs of choice for the treatment of CU. However, symptoms persist in a large proportion of patients despite treatment with antihistamines at the recommended doses. In a recent study performed by Maurer et al.,5 up to 50% of patients with CU did not respond to the recommended doses of antihistamines, probably because those doses are based on the treatment of other allergic diseases, such as seasonal rhinitis. In nonresponders, it has been suggested that the dose of antihistamine be increased up to 4 times the recommended dose. Other mediators released by the mast cells, such as cytokines, eicosanoids, proteases, and PAF, have been implicated in the formation of wheals in patients with CU.15 It has recently been observed that PAF can induce mast-cell degranulation in vitro.16 The authors of this paper have been able to study the specific characteristics of the wheal induced by PAF in healthy volunteers; in this context, the wheal developed independently of mast-cell degranulation.17
Two potent second-generation H1-antihistamines, terfenadine and astemizole, were withdrawn from the market due to the appearance of cardiac side effects (ventricular arrhythmias-torsade de pointes). Since that time, both the Food and Drug Administration and the European Medicines Agency require cardiotoxicity studies to be performed during the development of any new antihistamine.
Numerous clinical trials have been performed to compare the different second-generation antihistamines against each other and against placebo. No statistically significant differences were detected with regard to symptom control, safety profile, or quality of life.13,18–22 A description of the second-generation H1-antihistamines is given below.
AcrivastineAcrivastine has a short half-life and must therefore be administered 3 times a day (the other antihistamines are usually recommended once a day). It has a rapid onset of action. Acrivastine is excreted intact in the urine and its use must therefore be avoided in patients with moderate kidney failure.23
CetirizineCetirizine is the active metabolite of hydroxyzine and it can therefore present sedative effects, particularly at high doses. Cetirizine reaches its maximum plasma concentration very quickly, which can be an important clinical advantage due to its rapid bioavailability. It has antiallergic effects on inflammatory mediators released by mast cells, particularly at high doses. Cetirizine must be avoided in cases of severe kidney failure.24
LevocetirizineLevocetirizine is the active enantiomer of cetirizine. It is more potent.13 It has a higher potency than other antihistamines for the inhibition of wheal and of erythema after the intradermal injection of histamine.25
LoratadineLoratadine undergoes first-pass metabolism in the liver, where it is transformed into its active molecule, desloratadine. It has antiallergic properties.26
DesloratadineDesloratadine is the active metabolite of loratadine, and it is therefore more potent. It has a longer elimination half-life than loratadine (27hours), and its administration must therefore be stopped 6 days before performing skin prick tests. It binds selectively and with high affinity to the H1 receptors. Desloratadine also has anti-inflammatory and antiallergic activity as it acts on cytokines and cell adhesion molecules. It inhibits other mediators implicated in the appearance of wheals, including cytokines IL-4, IL-13, IL-6, TNF, and GM-CSF, chemokines such as IL-8 and RANTES (regulated on activation, normal T-cell expressed and secreted), and adhesion molecules such as P-selectin and intercellular adhesion molecule (ICAM) 1. It reduces eosinophil chemotaxis and activation in vitro.27,28 Both desloratadine and loratadine must be used with caution in patients with severe kidney failure.
MizolastineMizolastine is contraindicated in clinically significant heart disease as it can cause prolongation of the QT interval. It must not be administered concomitantly with drugs that inhibit liver metabolism via the cytochrome P450 pathway (including macrolide antibiotics and imidazole antifungal drugs) or with potentially arrhythmogenic drugs (including tricyclic antidepressants such as doxepin). Its use is contraindicated in cases of liver failure.
EbastineEbastine is a second-generation H1-antihistamine with a structure based on oxopiperidine. It undergoes first-pass metabolism in the liver; its active form is carebastine. It is mainly excreted in the urine. Ebastine produces a dose-dependent inhibition of histamine release by cutaneous mast cells. It has activity on H1 receptors and also inhibits the release of inflammatory mediators by mast cells.22,29 The usual dose of 10mg must be adjusted in patients with kidney or liver failure.
RupatadineRupatadine is a new second-generation antihistamine with a long half-life. It is a dual antagonist of the peripheral H1 receptors and of PAF. Rupatadine competitively inhibits PAF-induced platelet aggregation in vitro, although to a lesser extent than its specific antagonist. Rupatadine also has anti-inflammatory properties: it inhibits eosinophil chemotaxis in vivo and in vitro, it suppresses the synthesis of inflammatory cytokines by activated T lymphocytes in vitro, and it interferes with cutaneous mast-cell degranulation and the release of histamine and other cytokines.14,15,22,30,31 It is indicated for the treatment of CU and allergic rhinitis. The usual dose is 10mg. The proportion of patients with CU who respond to rupatadine rises significantly if the dose is doubled to 20mg. It is a safe and well-tolerated drug.
FexofenadineFexofenadine is a potent antihistamine that is selective for the peripheral H1 receptors. It is an effective drug for the treatment of both CU and allergic rhinitis32 (Table 3).
Table Comparing the Second-Generation H1 Antihistamines.
| Family | Active Substance | Mechanism of Action | Dose | Side Effects | Trade Namea |
| Piperidine | Mizolastine | Neutrophil recruitment5-lipooxigenaseVEGF, TNF | 10mg/24h | Prolongation of the QT intervalGastrointestinal disturbancesDryness of the mouth, somnolence, headacheIncreased appetite | MizolenZolistan |
| Terfenadine | Eosinophil chemotaxisEosinophil adherenceSuperoxide synthesisIL-6, IL-8, TNFGM-CSF | 60-120mg/24h | CardiotoxicityHeadache, vertigoIncreased sweatingDigestive disturbancesSedation, dryness of the mouth | Triludan | |
| Fexofenadine | 180mg/24h | Headache, dizziness, somnolence, fatigue, dryness of the mouth, nausea | Telfast | ||
| Loratadine | Eosinophil chemotaxisIL-8, RANTES, ICAM-1 | 10mg/24h | Somnolence/insomnia, headache, increased appetite | Clarytine | |
| Desloratadine | Eosinophil chemotaxisSuperoxide productionTNF, IL-1, IL-6, IL-8, IL-13P-selectin, ICAM-1Eosinophil apoptosisNK-kB cell activation | 5mg/24h | Tiredness, dryness of the mouthHeadache | AeriusAzomyr | |
| Rupatadine | PAF, TNF | 10mg/24h | Somnolence, headache, fatigue | RupafinAlergoliber, Rinialer | |
| Ebastine | AntihistamineAntiallergic | 10-20mg/24h | Headache, somnolence, fatigue, dryness of the mouth, rhinitis, pharyngitis | Ebastel | |
| Alkylamine | Acrivastine | 16-24mg/24h | |||
| Piperazine | Cetirizine | Eosinophil adhesionEosinophil and neutrophil chemotaxisT-cell and monocyte chemotaxisIL-8, MCP1/RANTES NF-kB 19ICAM-1LTC4 | 10mg/24h | Headache, somnolence, fatigueDryness of the mouthGastrointestinal disturbances | ZyrtecAlerlisinVirlix |
| Levocetirizine | 5mg/24h | XazalAralevo |
Abbreviations: GM-CSF, granulocyte-macrophage colony stimulating factor; ICAM, intercellular adhesion molecule; IL, interleukin; LTC4, leukotriene C4; MCP1, monocyte chemoattractant protein-1; NF-kB, nuclear factor–kappa B; PAF, platelet activation factor; RANTES, regulated and normal T-cell expressed and secreted; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
There is controversy concerning the role of the H2-antihistamines in the treatment of CU. The cutaneous blood vessels have both H1 and H2 receptors and the activation of either type will induce the formation of wheal and erythema, although activation of the H2 receptors has a limited pruritic effect. The efficacy of combinations of H1-antihistamines with H2-antihistamines has been evaluated in a number of studies and a possible synergic effect has been reported. However, this association does not appear to be justified for the treatment of CU as efficacy has not been shown to be significantly better. The H2-antihistamines include cimetidine, ranitidine, nizatidine, and famotidine.13,18
Special Situations2–4,10,11,13,18,23Pregnancy and BreastfeedingThe use of any drug, including the antihistamines, should be avoided if possible during pregnancy, especially during the first trimester. The antihistamines are in pregnancy categories B and C. The second-generation antihistamines sertraline and loratadine are in category B (no evidence of fetal damage during pregnancy). Because of the greater cumulative experience, the prescription of first-generation H1-antihistamines such as chlorpheniramine is usually recommended. If an antihistamine has to be used during breastfeeding, once again cetirizine and loratadine are recommended, at the lowest possible dose.
ChildhoodAll the antihistamines can be used in children over 12 years of age. Cetirizine, loratadine, and desloratadine are authorized for the treatment of CU in children over 2 years of age.
Kidney and Liver FailureThe antihistamines that are excreted mainly in the urine are cetirizine (60%) and levocetirizine (85%). The urinary excretion of the other second-generation antihistamines is only of the order of 10% to 20%. The metabolism of the majority of these drugs usually involves first-pass metabolism in the liver via the cytochrome P-450 or CYP pathway, with the exception of cetirizine, levocetirizine, fexofenadine, and desloratadine. In any case, a dose reduction of all second-generation H1-antihistamines is recommended in patients with severe kidney or liver disease.
Tricyclic AntidepressantsThe tricyclic antidepressants such as amitriptyline and, in particular, doxepin, have been widely used in the treatment of CU for their potent H1- and H2-antihistamine effect. However, their use is limited by their sedative and anticholinergic effects, which are potentiated by the use of alcohol, and by the relative risk of cardiac arrhythmias secondary to prolongation of the QT interval. Administration at night is usually recommended because of their sedative effects, and they are particularly useful in patients with concomitant depression. Doxepin is used at doses of 10-30mg for the treatment of CU.13,18
AntileukotrienesThe most widely used antileukotriene is montelukast, a leukotriene-receptor antagonist that has been used in the treatment of asthma and urticaria. There is very little evidence of its efficacy in monotherapy.2,3,10,33,34 These drugs can be combined with antihistamines in treatment-resistant urticaria. Although the response to these drugs is not highly predictable, there are studies that suggest that montelukast may be effective in the treatment of autoimmune urticaria, delayed pressure urticaria, and in urticaria complicated by intolerance to acetylsalicylic acid or to food coloring agents, but it has no or almost no demonstrated efficacy in chronic idiopathic urticaria. Montelukast has been used to treat eosinophilic urticaria (eosinophilic infiltrate present on skin biopsy) as activated eosinophils induce the synthesis of eicosanoid mediators generated from arachidonic acid and leukotrienes, particularly LTC4, LTD4, and LTE3. The leukotrienes act by attracting cells to sites of inflammation and thus intensifying the inflammatory response.
CorticosteroidsNo controlled studies have been performed on the use of corticosteroids for the treatment of urticaria or angioedema, but they are generally accepted as effective (grade D recommendation). They should be prescribed in short cycles and in tapering regimens for the treatment of severe exacerbations of CU, particularly when the condition is associated with angioedema, due to the risk of secondary respiratory difficulty. They may also be administered when difficulty is experienced in controlling symptoms with antihistamines in monotherapy or when a more rapid clinical improvement is sought. Corticosteroids have also been used for the treatment of urticarial vasculitis. The minimum effective dose should be administered and prolonged treatment should be avoided in order to avoid their side effects. Topical corticosteroids should not be used in CU.2
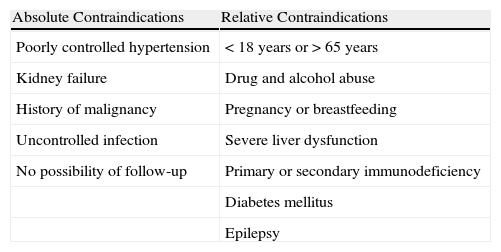
Ciclosporin ACsA is an inhibitor of calcineurin, a protein with phosphatase activity that permits nuclear factor of T cells to pass from the cytoplasm into the nucleus, with expression of the IL-2 gene that activates T cells and stimulates the secretion of interferon-γ and GM-CSF. CsA therefore acts by blocking the synthesis of IL-2, giving rise to a state of cellular and humoral immunosuppression.35,36 The main side effects of CsA are neurotoxicity and systemic hypertension (Table 4). In the management of CU, CsA is indicated in patients with severe CU with a positive ASST and who have not responded to antihistamines. It is used at doses of 3-5mg/kg/d for periods of at least 2 months. The optimal dose and duration of treatment have not yet been defined nor do we have criteria for predicting a response.37 Responses have been observed in patients with chronic spontaneous urticaria (with no evidence of functional antibodies, thus ASST negative), although this is not as well documented in the literature and the effects of treatment are less predictable than in patients with chronic autoimmune urticaria.38
Contraindications to Treatment With Ciclosporin A.
| Absolute Contraindications | Relative Contraindications |
| Poorly controlled hypertension | <18 years or>65 years |
| Kidney failure | Drug and alcohol abuse |
| History of malignancy | Pregnancy or breastfeeding |
| Uncontrolled infection | Severe liver dysfunction |
| No possibility of follow-up | Primary or secondary immunodeficiency |
| Diabetes mellitus | |
| Epilepsy |
Tacrolimus has been used successfully to treat corticosteroid-dependent CU at doses of 0.5-2mg/kg/d in 2 daily doses. It acts by inhibiting T lymphocytes and reducing the release of inflammatory cytokines.39
Mycophenolate MofetilMycophenolate mofetil is an immunomodulator drug that acts by inhibiting DNA synthesis in lymphocytes. It is a reversible inhibitor of inosine monophosphate dehydrogenase (present exclusively in lymphocytes and involved in purine synthesis). In CU, mycophenolate mofetil inhibits the production of antibodies to the high-affinity IgE receptor or to IgE itself. It also reduces the expression of adhesion molecules on in endothelial cells and inhibits lymphocyte migration towards the skin.40 In both autoimmune and idiopathic CU, mycophenolate mofetil has been found to be effective in reducing pruritus, the duration of the episode, the number of wheals, and the number of episodes of urticaria or angioedema. Complete remission has been reported in some cases. Mycophenolate mofetil has been used at doses of 500mg/12h for 2 to 4 weeks. It is a safe drug with few side-effects, which are mainly gastrointestinal at the doses used for the treatment of CU, and they can improve with time.41
CyclophosphamideGood results have been reported with intravenous cyclophosphamide; some patients with corticosteroid-dependent urticaria have achieved complete remission.42
MethotrexateMethotrexate can be useful in some cases as a corticosteroid-saving drug in patients with corticosteroid-dependent CU. It is used at doses of 10-15mg per week.43 The response does not appear to be related to the presence or absence of antibodies in the serum. The response is thought to be more closely related to its anti-inflammatory than to its immunosuppressant effect and it is therefore as successful in the treatment of chronic idiopathic urticaria as in chronic autoimmune urticaria.
Tumor Necrosis Factor InhibitorsLittle has been published in the literature about the treatment of CU with TNF inhibitors. Magerl et al.44 described the case of a patient with psoriasis and delayed pressure CU in which treatment with etanercept achieved a rapid and lasting improvement.
PhototherapyPhototherapy reduces the number of cutaneous mast cells in the superficial dermis.45 It has been used for the treatment of idiopathic CU and in symptomatic dermographism. UV-A, UV-B, or narrowband UV-B can be used for periods of 1 to 3 months. Narrowband UV-B has achieved good results as adjuvant therapy in combination with antihistamines. Long-term efficacy (up to 3 months after of the end of treatment) has been observed in some cases.46
Intravenous ImmunoglobulinThe indications for intravenous immunoglobulin approved by the Food and Drug Administration are primary immunodeficiencies, secondary immunodeficiencies, Kawasaki disease, and idiopathic thrombocytopenic purpura.47 In CU, intravenous immunoglobulin has been used mainly for chronic autoimmune urticaria refractory to other treatments. O’Donell et al.48 treated 10 patients with severe chronic autoimmune urticaria with intravenous immunoglobulin at doses of 400mg/kg/d for 5 days, achieving a clinical improvement in 9 of them—in 3 of those patients there was a long-term improvement. A good response has also been reported in some patients with delayed pressure urticaria and solar urticaria.49 The mechanism of action is not fully understood, but it has been suggested that intravenous immunoglobulin may contain anti-idiotypic antibodies that compete with endogenous IgG for the H1 receptors, blocking histamine release. Saturation of the Fc receptors by immunoglobulin prevents IgE from binding to the cutaneous mast cells and thus inhibits histamine release and the appearance of wheals. It is not known why the effect persists over time. The cost of treatment is high and it has an elevated morbidity. There are no controlled studies.
PlasmapheresisPlasmapheresis is an extracorporeal blood purification technique that consists of the separation of the plasma from the cellular components of the blood with the aim of removing the pathogenic elements causing the disease; in the case of CU, these elements are the histamine-releasing antibodies. Plasmapheresis has been used in cases of treatment-resistant CU,50 although no controlled clinical trials have been performed. The cost of plasmapheresis is high and there is potential morbidity. Recurrence is common. In monotherapy, plasmapheresis is not sufficient to prevent the re-accumulation of histamine-releasing antibodies and it must be used in combination with immunosuppressant drugs.
OmalizumabOmalizumab is a humanized recombinant monoclonal antibody that binds specifically to the C3¿ domain of the IgE heavy chain, the site that binds to the high-affinity IgE receptors on the surface of mast cells and basophils. It is currently only authorized for use in severe allergic asthma.51
There are 2 types of IgE receptors52: the FC¿RI receptor present on mast cells, basophils, monocytes, eosinophils, and Langerhans cells; and the low-affinity receptors (FC¿RII/CD23), involved in antigen presentation (FC¿RII on B lymphocytes) and in the regulation of IgE synthesis. Omalizumab reduces the levels of free circulating IgE and secondarily reduces the density of IgE receptors on basophils and cutaneous mast cells, preventing their antibody-induced activation and degranulation. IgE can modulate the level of expression of its own high-affinity and low-affinity receptors. When IgE binds to FC¿RI on mast cells and basophils, it determines the levels of expression of the surface receptors, such that a higher concentration of IgE will increase the density of its receptors (and, hence, mast cell and basophil reactivity), whilst a lower concentration will reduce receptor density. When IgE binds to FC¿RI, an interaction occurs between the C3 domain of IgE and the α-chain of the receptor.52–57 Omalizumab may also act by reducing the number of IgE antithyroperoxidase autoantibodies, producing an inhibition of mast-cell degranulation by reducing the density of IgE receptors on their surface (negative feedback).57
It has been observed that omalizumab is not only successful in the treatment of chronic autoimmune urticaria, but that it may also have a role in chronic idiopathic urticaria (ASST negative disease with no histamine release by basophils in vitro). This effect may be due to a direct action on mast cells, eosinophils, or basophils, even in the absence of autoantibodies. Another possible explanation is that omalizumab may prevent the FC¿RI-dependent secretion of cytokines and chemokines through some as yet unknown stimulus. A good response has been observed in physical urticarias (solar urticaria, heat urticaria, cold urticaria, delayed pressure urticaria, and cholinergic urticaria), although we only have isolated case reports and a larger number of studies are necessary to evaluate its potential efficacy.58–63
In the treatment of severe allergic asthma, omalizumab is administered by subcutaneous injection at doses that vary between 75mg and 375mg, depending on body weight and IgE levels in the blood. In a recent study by Saini et al.,64 a frank improvement in the urticaria activity score 7 (UAS7) with respect to baseline was observed 4 weeks after a single dose of 300mg or 600mg of omalizumab, and the treatment was more successful than placebo, with a rapid onset of effects (first week). However, no statistically significant difference with respect to placebo was observed with a dose of 75mg. Taking into account that it is necessary to wait for up to 16 weeks to observe a response after the treatment of allergic asthma, it has been suggested that omalizumab could have an effect not only on IgE, but also a direct effect on mast cells.
In several studies it has been found that omalizumab reduces the UAS and the need for rescue medication and that it increases the therapeutic response and improves the quality of life of patients with CU.52 The side effects described are mostly mild (rhinopharyngitis, diarrhea, headache, local symptoms at the site of injection) although they can be more severe in occasional cases (anaphylaxis).
Other TreatmentsNifedipineThe results on the use of nifedipine in the treatment of urticaria are contradictory. It has been reported to be effective in reducing pruritus and wheal formation in patients with CU when combined with high-dose antihistamines.65 However, in other studies, nifedipine has been found to have little effect on CU.11 Nifedipine acts by modifying calcium entry into the cutaneous mast cells. It may be a drug to consider in patients with systemic hypertension, particularly in those taking ACE inhibitors.
Anticoagulation and AntifibrinolysisIn some patients with CU, thrombin levels have been found to be elevated due to activation of the coagulation cascade via the extrinsic pathway, and signs of fibrinolysis with elevated plasma D-dimer levels were also observed. This occurred in more severe cases of CU with a poorer response to first-line treatment with antihistamines.66–69 It has been suggested that thrombin could increase vascular permeability, activating mast-cell degranulation and the complement cascade in the absence of C3. Activation of the coagulation cascade in these patients is associated with a poorer response to treatment with antihistamines at approved doses. D-dimer levels could serve as a marker to identify this group of patients with chronic idiopathic urticaria. In these patients, combined treatment with an anticoagulant (heparin) and an antifibrinolytic (tranexamic acid) could be useful, as this situation affects a subgroup of patients in whom we would be blocking a mechanism of activation of inflammation.70
DapsoneDapsone (4-49-diaminodiphenylsulfone) is a sulfone derivative that has antimicrobial and anti-inflammatory effects and has been used for many years to treat a wide variety of skin diseases with neutrophilic dermal infiltrates, including urticarial vasculitis, Behçet disease, and pyoderma gangrenosum. Dapsone alone or combined with other treatments is considered a therapeutic option for the treatment of urticaria. Anecdotally, there have been occasional reports of an improvement in chronic idiopathic urticaria and delayed pressure urticaria.71
ColchicineColchicine has been widely used for the treatment of a heterogeneous group of skin diseases typically characterized by the presence of neutrophilic dermal infiltrates, including psoriasis, leukocytoclastic vasculitis, and urticarial vasculitis. Colchicine limits the chemotactic and phagocytic activity of neutrophils and suppresses leukocyte function by increasing the levels of cyclic adenosine monophosphate in the cytoplasm and by inhibiting lysosome degranulation.72,73 The increased levels of cyclic adenosine monophosphate release prostaglandinE, which suppresses leukocyte function. Oka et al.74 demonstrated that colchicine causes the depolymerization of microtubules crucial for mast-cell degranulation. The inhibitory effect of colchicine in urticaria could be due to a dual effect: chemotactic blockade of the neutrophils and reduced mast-cell degranulation. It has occasionally been used in patients with urticarial vasculitis.
SulfasalazineSulfasalazine belongs to a group of antifolate drugs and has been used for many years in the treatment of inflammatory bowel diseases and rheumatoid arthritis. Its main side effects are liver toxicity, neurotoxicity, and myelotoxicity. The mechanism of action of sulfasalazine in CU is unknown. It may affect IgE-mediated histamine release and reduce the activity of prostaglandin synthetase. It could be useful as a corticosteroid-saving drug.75 There are few controlled studies on its use in CU.
HydroxychloroquineHydroxychloroquine has been used in the treatment of hypocomplementemic urticarial vasculitis syndrome.76
Recommendations of the European GuidelinesThe management and treatment of patients with CU is complex. For this reason, consensus guidelines based on scientific evidence and on expert opinion have been drawn up for the management of this disease. The 2 existing guidelines for the management of CU are the “BSACI guidelines for the management of chronic urticaria and angio-oedema,” drawn up by the British Society for Allergy and Clinical Immunology2 (BSACI) and published in Clinical and Experimental Allergy in 2007, and the European guidelines of the EAACI/GA2LEN/EDF/WAO, published in Allergy in 2009.10 The European guidelines are the result of the consensus reached by a discussion panel at the Third International Consensus Meeting on Urticaria in 2008 by the Dermatology Section of the European Academy of Allergology and Clinical Immunology (EAACI), the Global Allergy and Asthma European Network (GA2LEN), the European Dermatology Forum (EDF), and the World Allergy Organization (WAO).
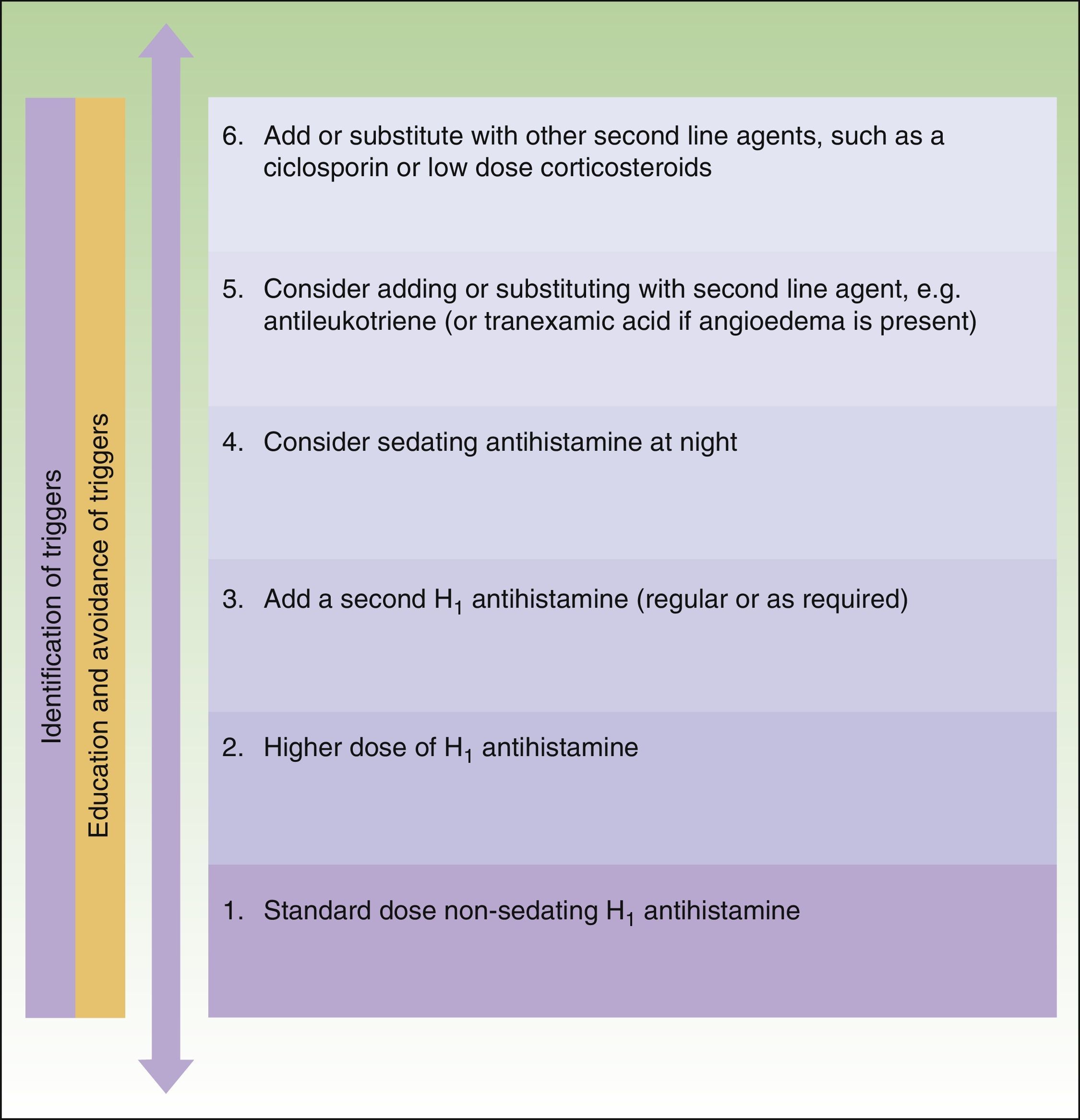
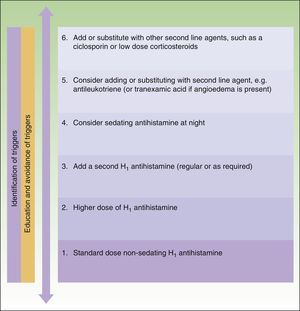
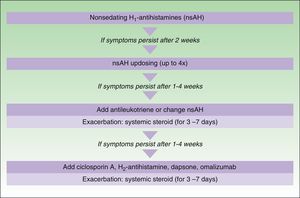
The main difference between the 2 guidelines in the treatment algorithm for CU is that the British guidelines continue to recommended the use of sedative antihistamines at night if nonsedative antihistamines have not been effective, even at high doses, whereas the European guidelines do not recommend these drugs in any case2,10 (Figs. 1 and 2).
Treatment algorithm for chronic spontaneous urticaria based on the 2009 European guidelines of the European Academy of Allergology and Clinical Immunology, the Global Allergy and Asthma European Network, the European Dermatology Forum, and the World Allergy Organization. With the permission of the authors. In the 2012 update consensus, the recommendation for the use of dapsone and H2-antihistamines was withdrawn. Increases in the dose of nonsedative H1-antihistamines of up to 4 times the approved doses was recommended and omalizumab, ciclosporin A, and antileukotrienes were maintained for second-line treatment.
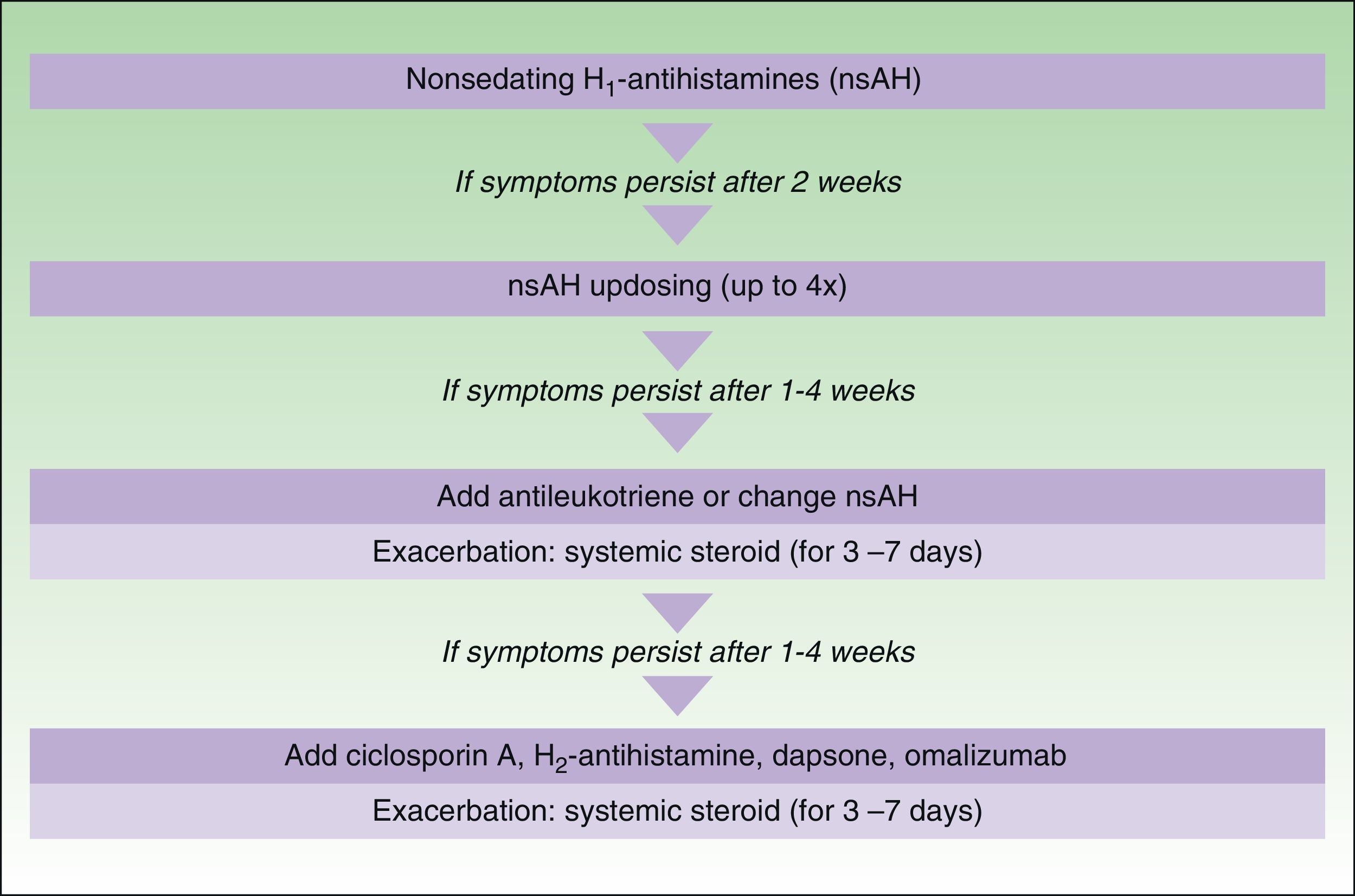
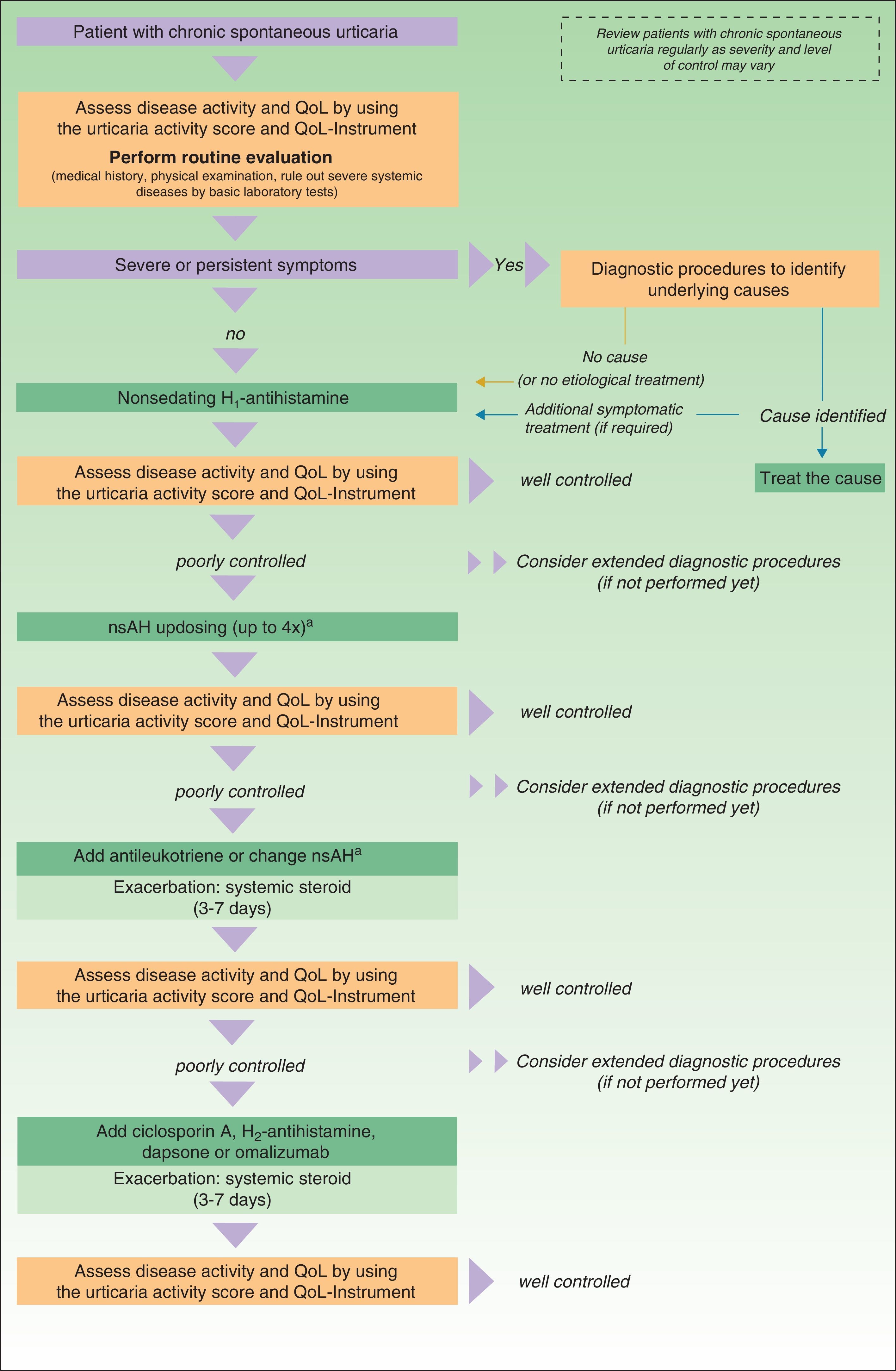
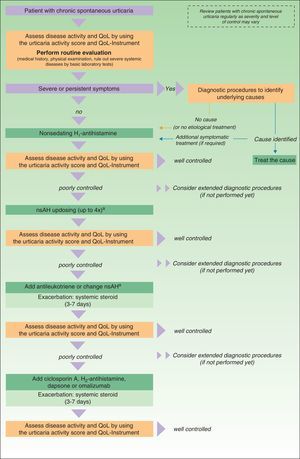
In November 2012, in Berlin, at the fourth consensus meeting to update the guidelines for chronic spontaneous urticaria, certain changes were introduced into the management algorithm for CU. As the algorithm only included those treatment options supported by a high level of recommendation, the use of dapsone and of the H2-antihistamines was withdrawn. In view of the failure of the use of the nonsedative H1-antihistamines at the approved doses, an increase of up to 4 times that dose was recommended. Omalizumab, ciclosporin A, and the antileukotrienes continue to figure as second-line treatment. These drugs are used in addition to the H1-antihistamines. However, reading of the entire guidelines is recommended as the use of other therapeutic options that do not figure in the algorithm is not rejected for certain patients (Fig. 3).
Algorithm for the management of patients with chronic spontaneous urticaria. Source: Maurer et al.,5 with the permission of the authors.
QoL indicates quality of life; nsAH, nonsedating antihistamine.
a Based on the algorithm for the management of patients with chronic spontaneous urticaria. Source: Maurer et al.,5 with permission.
The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no private patient data are disclosed in this article.
Right to privacy and informed consentThe authors declare that no private patient data are disclosed in this article.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
Please cite this article as: Curto-Barredo L, Silvestre JF, Giménez-Arnau AM. Actualización en el tratamiento de la urticaria crónica. Actas Dermosifiliogr. 2014;105:469–482.